Abstract
Renal impairment (RI) significantly impacts the clearance of drugs through changes in glomerular filtration rate, protein binding and alterations in the expression of renal drug transport proteins and hepatic metabolizing enzymes. The objectives of this study were to quantitatively evaluate the effects of RI on the pharmacokinetics of drugs undergoing renal transporter-mediated reabsorption. We utilized a previously published semi-mechanistic kidney model incorporating physiologically-relevant fluid reabsorption and transporter-mediated active renal reabsorption (PMID: 26341876) in this study. The probe drug/ transporter pair utilized was γ-hydroxybutyric acid (GHB) and monocarboxylate transporter 1 (SCL16A1, MCT1). GHB concentrations in the blood and amount excreted into urine were simulated using ADAPT5 for the IV dose range of 200–1500 mg/kg in rats and the impact of RI on CLR and AUC was evaluated. A 90% decrease in GFR resulted in >100-fold decrease in GHB CLR. When expression of reabsorptive transporters was decreased and fu was increased, CLR approached GFR. The effect of RI on CLR was reduced when the expression of drug metabolizing enzymes (DME) was increased as a result of increased metabolic clearance; the converse held true when DME expression was decreased. In conclusion, this study quantitatively demonstrated that the effects of renal insufficiency on the clearance of drugs is modulated by transporter expression, contribution of renal clearance to overall clearance, expression of drug metabolizing enzymes, fraction unbound, and drug-drug interactions with inhibitors of renal transporters that may be increased in the presence of RI.
Keywords: Kidney disease, GFR, Renal Transport, GHB, Pharmacokinetics
Introduction
Renal impairment (RI) is a major health concern both in the US and globally. The prevalence of Chronic Kidney Disease (CKD) is 10%, and was ranked as 18th in the list of causes of total number of deaths worldwide [1]. In the U.S., the prevalence of CKD is greater than 13%, affecting over 25 million adults [2]. RI is implicated in many disease states including nephritis, glomerulonephritis, Type-II diabetes and auto-immune diseases such as lupus erythematosus [1]. One of the earliest reports of the impact of RI on pharmacokinetics (PK) of drugs was published by Dr. Gerhard Levy, who described the three key principles: (1) the quantitative contribution of each route of elimination is proportional to the clearance value of that route relative to body clearance; (2) the reduction in the glomerular filtration rate (GFR) caused by RI is, as a first approximation, an indication of the reduction in a drug’s CLR; and (3) since severe RI causes a reduction in the plasma protein binding of many drugs, the metabolic clearance of many extensively metabolized drugs will be increased [3]. The characterization of the effects of RI on PK is of vital importance to provide predictions for proper dosing of medications involved in RI, and with the disease states associated with RI [4, 5]. RI has been shown to affect the expression and activity of both drug transporters and drug metabolizing enzymes (DMEs) [6]
In rats, experimentally-induced RI resulted in lower amounts of Organic Anion Transporter 1 and 3 (Oat1 and Oat3), as well as Organic Cation Transporter 2 (Oct2) protein in the kidney, compared with controls, while the protein expression of ABC transporters (P-glycoprotein, Mrp2 and Bcrp) was largely unchanged, or increased for Mrp2 [6, 7]. Naud et al., 2011, also reported the increased protein expression of Mrp2 with RI, and also the increased expression of Mrp4 and Oatp2 in rat kidneys isolated from animals with a 5/6 nephrectomy [8]. The activity of these transporters was also shown to be inhibited by uremic toxins and endothelin-1, compounds that are associated with RI [6]. Importantly, Brandoni and Torres, 2015, have reported that in in vivo experimental models of acute kidney failure, that there was a negative correlation between uremia and renal protein expression of Oat1 and Oat3 [9].
Numerous DMEs have also been shown to be affected by RI [9]. In experimental models of end stage renal disease (ESRD), decreases in protein expression and activity of Cyp1a1, Cyp2c11, Cyp3a1, Cyp3a2, Nat1 and Nat2 were observed [2]. These changes in expression and activity have been shown to have an impact on the clinical pharmacokinetics (PK) of several drugs in patients with CKD including lidocaine, cerivastatin, cyclophosphamide, roxithromycin and others. RI has also been implicated in the alteration of protein binding for drugs such as phenytoin, warfarin and morphine [2].
Currently, the FDA recommends clinical PK studies to investigate the effect of RI on the PK of new drugs [10]. A simulation-based approach utilizing a semi-mechanistic model will enable potential prediction of the effects of RI on PK using data that has already been collected, prior to clinical studies. For these simulations we used a previously-developed PK model for GHB that includes a semi-mechanistic kidney model incorporating physiologically-relevant fractional fluid reabsorption from various nephron segments, that incorporated monocarboxylate transporter 1/sodium-dependent monocarboxylate transporter 1 (MCT1/SMCT1)-mediated renal reabsorption of γ-hydroxybutyric acid (GHB) and L-lactate, with physiologically-based disposition [11].
We hypothesize that our previously qualified mechanistic and physiologically-based PK model can be used to provide insight, through the use of simulations, on drug disposition by utilizing knowledge of drug transport and metabolism kinetic parameters, changes in expression of drug transporters and drug metabolizing enzymes, and physiological changes that occur with RI or CKD.
The overall objective is to qualitatively evaluate the effects of RI on the renal and total clearance of a drug with transporter-mediated renal drug reabsorption and saturable metabolism. Using GHB as a model substrate to illustrate the effects of RI on CLR and CL, simulations examined the effect of changes in GRF, kidney transporter expression, DME expression, protein binding and renal DDI, which may be mediated by higher concentrations of uremic toxins or other endogenous compounds present with RI.
Materials and Methods
Pharmacokinetic model
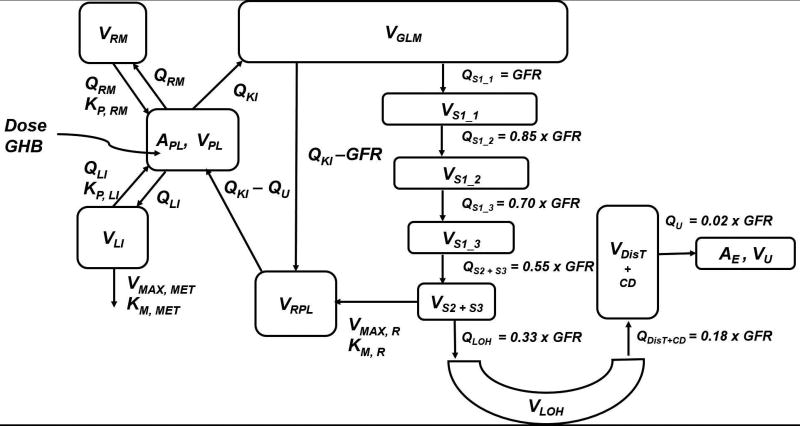
In this study, the probe drug/ transporter pair utilized was GHB and MCT1. GHB is a naturally occurring short-chain fatty acid and displays non-linear pharmacokinetics in rats [12] and humans [13, 14], including capacity-limited absorption [13, 15], capacity-limited metabolism in the liver [13], and capacity-limited renal vectorial reabsorption mediated by SMCT1 (SLC5A8, brush-border membrane) and MCT1 (SLC16A1, basolateral membrane) [16]. We utilized a previously established semi-mechanistic kidney model incorporating physiologically-relevant fluid reabsorption (67% from proximal tubules (S1–S3), 15% from loop-of-Henle, 16% from distal tubules and collecting ducts) and transporter-mediated active renal reabsorption [11]. The three key model components include (1) a semi-mechanistic kidney component incorporating physiologically-relevant fluid reabsorption and transporter-mediated active reabsorption and GHB-specific components incorporating (2) non-linear renal transport kinetics (MCT1/SMCT1) and (3) systemic saturable metabolism and distribution of GHB as illustrated in figure 1. The kidney model assumes that about 67% of the total filtrate is reabsorbed from the proximal tubule [17–19]. The proximal tubule lumen segment was sub-divided into four lumen segments, which yielded three S1 segments (S1_1, S1_2, and S1_3) and a S2+S3 segment. About 2/3 of total fluid reabsorption from proximal tubules occurs from the S1 segment [18]; therefore, subdividing the S1 segment allows for incorporating the gradual process of fluid reabsorption across the PT. This also accounts for changes in drug concentration as a result of decrease in filtrate volume and concentration of drug available for transport in subsequent segments. The fraction of fluid reabsorption from each of the three subsections of S1 is considered to be equal in magnitude. Fig. 1 and Table 1 detail the fractional decrease in flows and volumes of the filtrate, relative to GFR, with sequential fluid reabsorption. A list of all model parameters is provided in Table 1. The model equations are described in brief below and in detail in Dave and Morris [11].
Figure 1.
A semi-mechanistic and physiologically-relevant pharmacokinetic model for GHB. Symbols and their description are provided in Table 1. Model is adapted from Dave and Morris, J Pharmacokinet Pharmacodyn. Oct;42(5):497–513, 2015 PMID:26341876.
Table 1.
List of PK model parameters [references are cited]
| Parameter | Definition | Value |
|---|---|---|
| VBL (mL) | Volume of blood | 20.3 [36, 37] |
| VPL (mL) | Volume of plasma | 12.5 [36, 37] |
| VRM (mL) | Volume of remainder compartment | 264 [36, 37] |
| VLI (mL) | Volume of liver | 12.4 [36, 37] |
| QLI (mL/min) | Blood flow to liver | 11.6 [36, 37] |
| QKI (mL/min) | Blood flow to kidneys | 10.5 [36, 37] |
| QRM (mL/min) | Blood flow to remainder compartment | 27.1 [36, 37] |
| KP, LI, KP, RM | Blood-to-tissue partition coefficients for liver and remainder compartments (unpublished in vivo data) | 0.4 |
| VGLM (mL) | Volume of glomerulus | 0.08 [38] |
| VPTC (mL) | Volume of proximal tubule epithelial cells | 1.03 [38] |
| VRBL (mL) | Volume of renal blood | 0.375 [38] |
| VRPL (mL) | Volume of renal plasma | 0.206 [38] |
| GFR (mL/min) | Glomerular filtration rate | 2.2 [39] |
| VS1_1 (mL), QS1_1 (mL/min) | Volume and flow of filtrate in lumen of 1st sub-segment of S1 segment of proximal tubule | GFR |
| VS1_2 (mL), QS1_2 (mL/min) | Volume and flow of filtrate in lumen of 2nd sub-segment of S1 segment of proximal tubule | 0.85 × GFR [17–19] |
| VS1_3 (mL), QS1_3 (mL/min) | Volume and flow of filtrate in lumen of 3rd sub-segment of S1 segment of proximal tubule | 0.70 × GFR [17–19] |
| VS2+S3 (mL), QS2+S3 (mL/min) | Volume and flow of filtrate in lumen of S2 and S3 segments of proximal tubule | 0.55 × GFR [17–19] |
| VLOH (mL), QLOH (mL/min) | Volume and flow of filtrate in lumen of Loop of Henle | 0.33 × GFR [17–19] |
| VDistT+CD (mL), QDistT+CD (mL/min) | Volume and flow of filtrate in lumen of distal tubules and collecting ducts | 0.18 × GFR [17–19] |
| VU (mL), QU (mL/min) | Volume and flow of urine | 0.02 × GFR [17–19] |
| KM, MET (µg/mL) | Metabolic Michaelis-Menten affinity constant | 63 [16, 40] |
| KM, BBM ((µg/mL) | Renal reabsorption Michaelis-Menten affinity constant at BBM | 480 [29] |
| KM, BLM ((µg/mL) | Renal reabsorption Michaelis-Menten affinity constant at BLM | 1092 [29] |
| VMAX,MET (µg/min) | Maximal metabolic capacity | 670 [11] |
| VMAX,BBM (µg/min) | Maximal renal reabsorption capacity at BBM | 1950 [11] |
| VMAX,BLM (µg/min) | Maximal renal reabsorption capacity at BLM | 1101 [11] |
Blood compartment
The blood compartment (Eq. 1) is the depot for GHB input. Previous data in our lab has investigated the blood to plasma (B/P) partitioning of GHB over a wide dose-range of 400–1500 mg/kg IV dose. The highest GHB dose assessed in the present manuscript was also 1500 mg/kg IV. The B/P partitioning of GHB was 0.75 and it did not exhibit any capacity limitation over this dose range [20]. From the blood (BL), GHB distribution to the liver (LI), the kidneys (KI), and the remainder of the body (RM) as described as:
| (1) |
Liver and remainder compartments
Eq. 2 and 3 describe the distribution of GHB into the liver and the remainder compartments, respectively, with the initial condition (IC) set to 0. The saturable metabolism of GHB was incorporated as a single Michaelis-Menten equation.
| (2) |
| (3) |
Compartments incorporating physiologically-relevant fluid reabsorption and transporter-mediated renal reabsorption
The blood flow to the kidneys (QKI) carries GHB to the glomerulus (GLM), where a fraction of QKI becomes the GFR and the remaining fraction drains into the peritubular capillaries as:
| (4) |
The fluid reabsorption from the three S1 segments of proximal tubules (PT), which is 2/3 of the total fluid reabsorption from PT is described as:
| (5) |
| (6) |
| (7) |
The remaining 1/3 of the total fluid reabsorption from proximal tubules occurs from the S2 and S3 segments (S2+S3) as described in Eq. 8, where LOH is the Loop of Henle.
| (8) |
MCT1/SMCT1-mediated reabsorption of GHB from the brush border membrane (BBM) into the PT cells and MCT1-mediated transport from the basolateral membrane (BLM) into the renal blood (RBL) is described in Eqs. 8 and 9.
| (9) |
The term QKI−QU accounts for the flow balance in the system: about 98% of fluid is reabsorbed every minute, where urine flow (QU) is ~1–2% of GFR [17–19]. As urine flows through the remainder of the nephron, fluid reabsorption is incorporated in the model by defining volume and flow to each compartment as a fraction of GFR. The compartments include: Loop of Henle (LOH), Distal tubules (DisT), Collecting Ducts (CD).
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
The model outputs for the two PK endpoints, blood concentrations (CBL) and cumulative amount excreted unchanged into urine (Ae) of GHB are:
| (15) |
| (16) |
Simulation study design
GHB concentrations in the blood and amount excreted into urine were simulated for the IV dose range of 200–1500 mg/kg in rats using the model, simulations were performed assuming a 300 g rat. All simulations were performed using SIM algorithm in ADAPT5 (BMSR, Los Angeles, CA) [21]. Renal impairment was incorporated into the model by modulating the GFR parameter and perturbing its value from 2.2 mL/min (100% renal function) to 0.22 mL/min (10% renal function). Modulation of renal function was confined to decreasing GFR as the decrease in GFR is the major contributor to CLR for GHB; other physiological characteristics of the kidney were held constant. To study MCT1-mediated DDI, non-competitive and competitive inhibition of MCT1 was included in the model, using equation 17 and 18, respectively, where R is the ratio of concentration of an inhibitor administered at steady-state ([I]) and the inhibition constant (Ki):
| (17) |
| (18) |
To evaluate the effects of RI on dose-dependent PK of GHB, simulations were performed varying GFR from 10 to 100% for doses of 200, 600 and 1000 mg/kg. To evaluate the effects of renal function on CLR of GHB (1500 mg/kg dose), when expression of MCT1/SMCT1 is altered, the VMAX, BBM and VMAX, BLM parameters were altered ±2- and ±5-fold for 100, 50 and 10% GFR. The effects of DDI, examining non-competitive and competitive inhibition of GHB renal reabsorption, were also investigated along with the same alterations in MCT1/SMCT1 expression utilizing the parameter R (1, 10 and 100). The impact of protein binding (fu × GFR) was investigated with the same alterations in MCT1/SMCT1 expression. Protein binding was altered by changing fu from 0.1 to 1.0 (It should be noted that GHB is not protein bound, so these simulations are not relevant for GHB itself, but would be for other compounds undergoing active reabsorption.). Finally, the impact of altering the expression of DMEs was included by perturbing VMAX, MET ±1.5, ±2 and ±5 fold with GFR 100, 50 and 10%.
AUC0−∞ were obtained using the NCA feature in PKSolver add-on package in MS Excel. CLR was calculated as the ratio of amount of GHB excreted unchanged into urine at time infinity (Ae, ∞) and AUC0−∞.
Results
Effects of RI on the dose-dependent PK of GHB
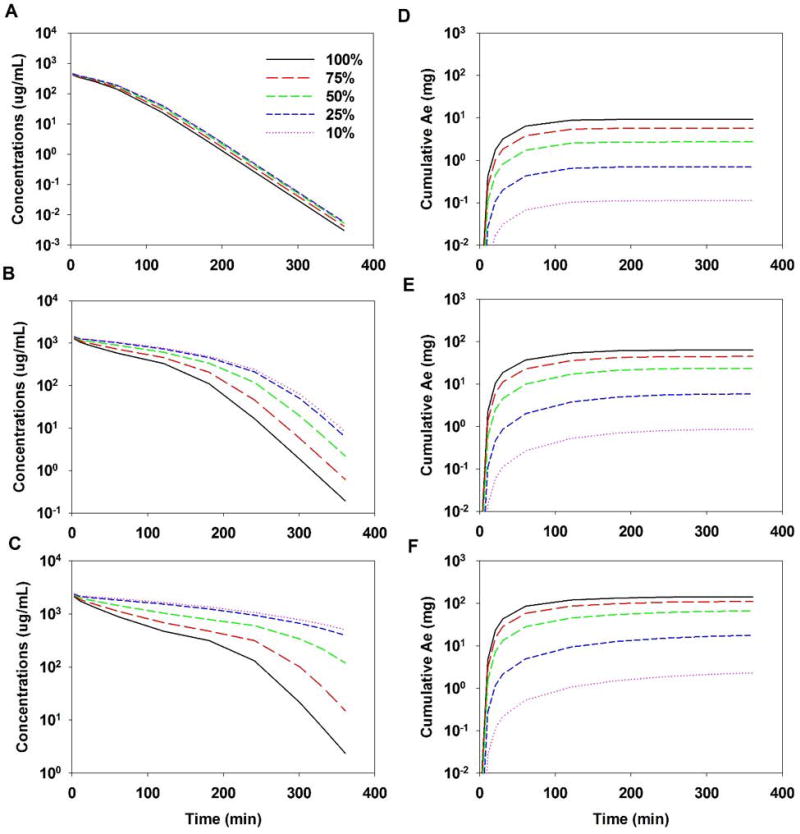
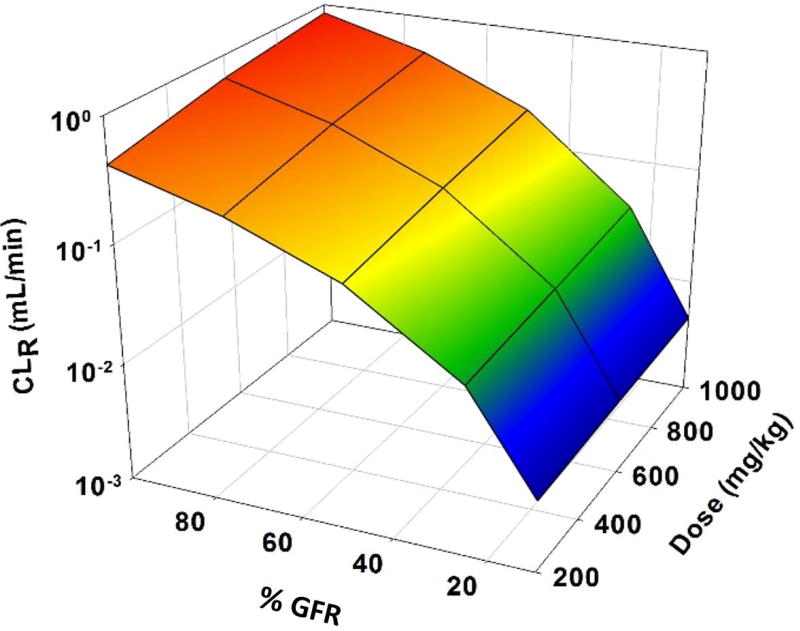
Renal impairment led to increased blood concentrations of GHB and lower values of Ae∞ (Figure 2). Figures 2A–C demonstrate that as renal function (GFR) was reduced, exposure to GHB increased for each dose tested. This effect was dose-dependent, with the greatest increase in AUC seen with the 1000 mg/kg dose (Fig. 2C). The decrease in Ae∞ and CLR was also dose dependent, with the greatest reduction in Ae∞ and the smallest reduction in CLR seen for the 200 mg/kg dose relative to 100% GFR (Fig. 2D). When GFR was reduced from 100% to 10%, there was a reduction in Ae∞ of over 55-fold for all doses (Table 2). For all doses, CLR was reduced to a similar value of 4.3 µL/min when GFR was reduced from 100% to 10%. The fold reduction in CLR for this change in GFR was over 100-fold for all doses and increased with increasing doses (Table S2). Overall CL is reduced the least for the 200 mg/kg dose (1.3-fold compared to 2- and 3.2-fold 600 and 1000 mg/kg doses) (Table 2). Figure 3 shows the direct relationship between loss of renal function and decreasing CLR for GHB across all doses tested.
Figure 2.
Model simulations for GHB concentrations in blood (A–C) and cumulative amount excreted unchanged into urine (Ae) (D–F), when renal function is altered (100%-10%): (A and D) 200 mg/kg, (B and E) 600 mg/kg, and (C and F) 1500 mg/kg doses of GHB
Table 2.
Effects of RI on the dose-dependent PK of GHB
| 200 mg/kg | ||||||
| GFR | ||||||
| parameter | units | 100% | 75% | 50% | 25% | 10% |
| AUC0-∞ | µg/ml*min (values ×103) | 22.0 | 24.2 | 26.1 | 27.6 | 28.1 |
| Ae,∞ | µg (values ×103) | 9.26 | 5.72 | 2.72 | 0.701 | 0.112 |
| CLR | mL/min | 0.420 | 0.237 | 0.104 | 0.025 | 0.004 |
| CL | mL/min | 2.72 | 2.48 | 2.30 | 2.18 | 2.13 |
| fe | 0.154 | 0.095 | 0.045 | 0.012 | 0.002 | |
| 600 mg/kg | ||||||
| GFR | ||||||
| parameter | units | 100% | 75% | 50% | 25% | 10% |
| AUC0-∞ | µg/ml*min (values ×103) | 97.5 | 124 | 158 | 190 | 200 |
| Ae,∞ | µg (values ×103) | 63.3 | 44.9 | 23.6 | 5.87 | 0.865 |
| CLR | mL/min | 0.649 | 0.363 | 0.149 | 0.031 | 0.004 |
| CL | mL/min | 1.85 | 1.46 | 1.14 | 0.947 | 0.901 |
| fe | 0.352 | 0.249 | 0.131 | 0.033 | 0.005 | |
| 1000 mg/kg | ||||||
| GFR | ||||||
| parameter | units | 100% | 75% | 50% | 25% | 10% |
| AUC0-∞ | µg/ml*min (values ×103) | 169 | 226 | 330 | 483 | 534 |
| Ae,∞ | µg (values ×103) | 143 | 111 | 67.4 | 18.5 | 2.46 |
| CLR | mL/min | 0.846 | 0.491 | 0.204 | 0.038 | 0.005 |
| CL | mL/min | 1.78 | 1.33 | 0.910 | 0.621 | 0.562 |
| fe | 0.477 | 0.370 | 0.225 | 0.062 | 0.008 | |
Figure 3.
3D mesh plot illustrating the effects of renal function (100%–10%) on renal clearance (CLR) of GHB over a wide dose-range (200–1000 mg/kg)
Effects of renal function with altered transporter expression, transporter inhibition and protein binding on the CLR and CL of GHB
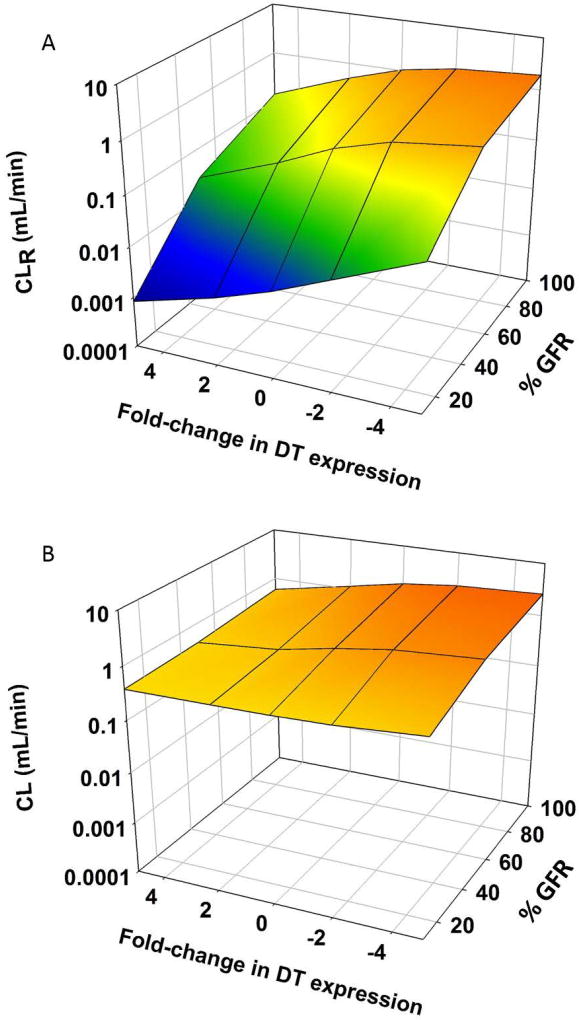
In these simulations, one dose (1500 mg/kg) was used. When the expression of reabsorptive transporters was decreased, there was an accompanying increase in CLR (Figure 4A). This led to an increase in CL, although this increase was not as pronounced as the increase in CLR (Table 2 and Figure 4B). When DT expression was increased, CLR and CL were decreased, but again the effect was less for CL than for CLR (Figure 4).
Figure 4.
3D mesh plot illustrating the effects of renal function (100%–10%) on (A) renal clearance (CLR) and (B) overall clearance (CL) of GHB (1500 mg/kg single IV bolus dose), when expression of DTs is altered (VMAX, BBM and VMAX, BLM parameters were perturbed ± 2 and 5-fold)
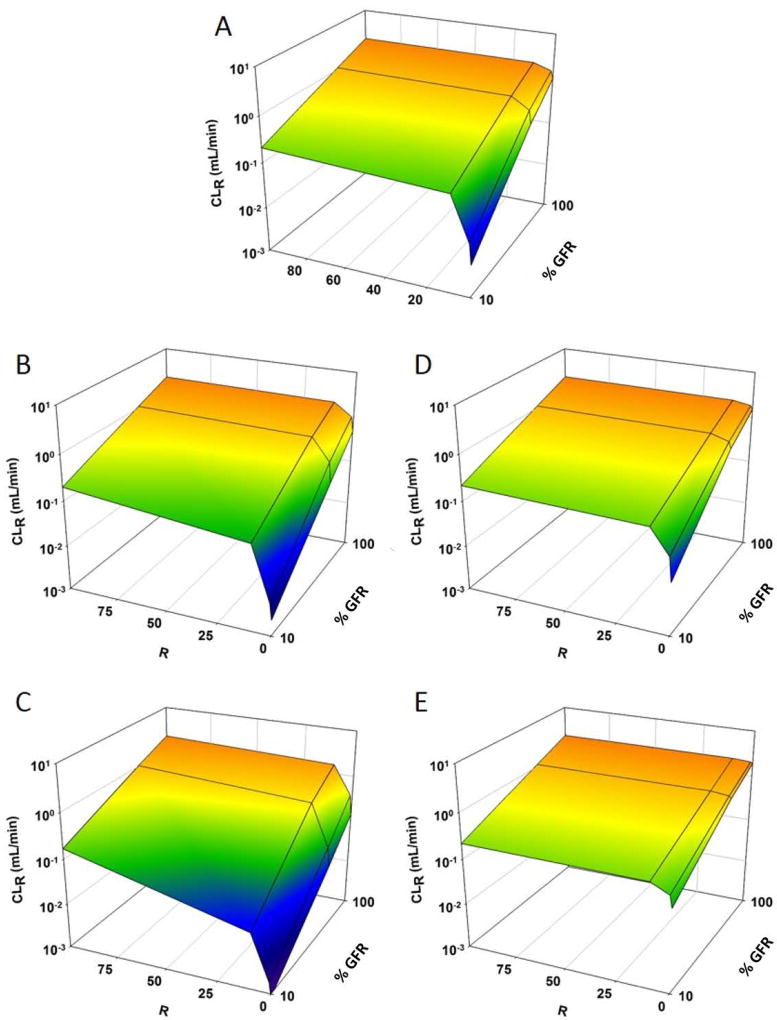
Figure 5 shows CLR for GHB with varying degrees of reabsorptive transport inhibition and changes in renal function. When inhibition of reabsorptive transport was maximal (R = 100), CLR was also maximal and approached GFR. This was true for each perturbation in transporter expression tested (Fig. 5 B–E). CLR decreased when GFR was reduced even with maximal inhibition of transport, indicating CLR was still dependent on renal function. When expression of reabsorptive transporters was increased (Fig. 5 B, C), a greater degree of inhibition (higher value of R) was needed to achieve maximal CLR. Conversely, when the expression was decreased (Fig. 5 D, E), maximal CLR was achieved with lower values of R.
Figure 5.
3D mesh plot illustrating the effects of renal function (100%–10%) on renal clearance (CLR) of GHB (1500 mg/kg single IV bolus dose), when (1) expression of renal transporters is altered (VMAX, BBM and VMAX, BLM parameters were perturbed ± 0, 2, and 5-fold) and (2) DDI is present as non-competitive inhibition of GHB renal reabsorption (R = [i]/KI = 0, 1, 10, 100): (A) No change in VMAX, (B) VMAX increases 2-fold, (C) VMAX increases 5-fold, (D) VMAX decreases 2-fold, and (E) VMAX decreases 5-fold
The same trends were observed for competitive inhibition (data not shown). The effect of competitive inhibition was not as great as that of non-competitive inhibition. Under the same conditions, CLR values ranged from 0.01- to 3.55-fold higher for non-competitive inhibition compared to competitive inhibition (Table 3 and S1). In general, the differences in CLR for non-competitive and competitive inhibition was less than 2 fold. However, there were a few incidences when the difference between CLR for non-competitive and competitive inhibition was greater than 2-fold: this generally occurred when GFR was reduced to 10% (Table S2). This is likely due to the fact that the low GFR resulted in low tubular concentrations of GHB, where inhibition of Km would be more important than decreases in the capacity for reabsorption.
Table 3.
Impact of Non-Competitive Inhibition and Drug Transporter Expression on GHB Pharmacokinetics in the Presence of Renal Impairment
| CL (mL/min) | ||||||||||||
| GFR 100% | GFR 50% | GFR 10% | ||||||||||
| R | 0 | 1 | 10 | 100 | 0 | 1 | 10 | 100 | 0 | 1 | 10 | 100 |
| 0 Fold Change | 1.79 | 2.28 | 2.72 | 2.80 | 0.863 | 1.20 | 1.56 | 1.63 | 0.388 | 0.411 | 0.594 | 0.644 |
| 2 Fold Increase | 1.14 | 1.79 | 2.62 | 2.79 | 0.555 | 0.863 | 1.48 | 1.62 | 0.383 | 0.388 | 0.535 | 0.639 |
| 5 Fold Increase | 0.590 | 0.96 | 2.33 | 2.76 | 0.425 | 0.497 | 1.24 | 1.60 | 0.381 | 0.383 | 0.419 | 0.620 |
| 2 Fold Decrease | 2.28 | 2.55 | 2.76 | 2.80 | 1.20 | 1.42 | 1.60 | 1.63 | 0.411 | 0.494 | 0.623 | 0.647 |
| 5 Fold Decrease | 2.60 | 2.71 | 2.79 | 2.80 | 1.47 | 1.55 | 1.62 | 1.63 | 0.524 | 0.588 | 0.640 | 0.648 |
| CLR (mL/min) | ||||||||||||
| GFR 100% | GFR 50% | GFR 10% | ||||||||||
| R | 0 | 1 | 10 | 100 | 0 | 1 | 10 | 100 | 0 | 1 | 10 | 100 |
| 0 Fold Change | 1.03 | 1.53 | 2.08 | 2.20 | 0.282 | 0.562 | 0.988 | 1.10 | 0.005 | 0.015 | 0.135 | 0.210 |
| 2 Fold Increase | 0.494 | 1.03 | 1.95 | 2.19 | 0.098 | 0.282 | 0.875 | 1.08 | 0.002 | 0.005 | 0.078 | 0.199 |
| 5 Fold Increase | 0.133 | 0.364 | 1.58 | 2.14 | 0.026 | 0.069 | 0.600 | 1.04 | 0.001 | 0.002 | 0.017 | 0.170 |
| 2 Fold Decrease | 1.53 | 1.85 | 2.15 | 2.21 | 0.562 | 0.798 | 1.05 | 1.10 | 0.015 | 0.051 | 0.174 | 0.215 |
| 5 Fold Decrease | 1.92 | 2.07 | 2.19 | 2.22 | 0.854 | 0.976 | 1.08 | 1.11 | 0.070 | 0.128 | 0.201 | 0.219 |
| fe | ||||||||||||
| GFR 100% | GFR 50% | GFR 10% | ||||||||||
| R | 0 | 1 | 10 | 100 | 0 | 1 | 10 | 100 | 0 | 1 | 10 | 100 |
| 0 Fold Change | 0.576 | 0.670 | 0.766 | 0.788 | 0.326 | 0.468 | 0.632 | 0.673 | 0.013 | 0.036 | 0.228 | 0.325 |
| 2 Fold Increase | 0.433 | 0.576 | 0.743 | 0.786 | 0.176 | 0.326 | 0.591 | 0.667 | 0.006 | 0.013 | 0.147 | 0.312 |
| 5 Fold Increase | 0.226 | 0.380 | 0.680 | 0.777 | 0.061 | 0.139 | 0.484 | 0.652 | 0.002 | 0.005 | 0.042 | 0.274 |
| 2 Fold Decrease | 0.670 | 0.727 | 0.778 | 0.790 | 0.468 | 0.562 | 0.654 | 0.675 | 0.036 | 0.103 | 0.279 | 0.333 |
| 5 Fold Decrease | 0.739 | 0.764 | 0.786 | 0.791 | 0.583 | 0.628 | 0.668 | 0.677 | 0.134 | 0.218 | 0.314 | 0.337 |
Fold changes indicate the changes made to the capacity (Vmax) for transport at the brush border and basolateral membranes.
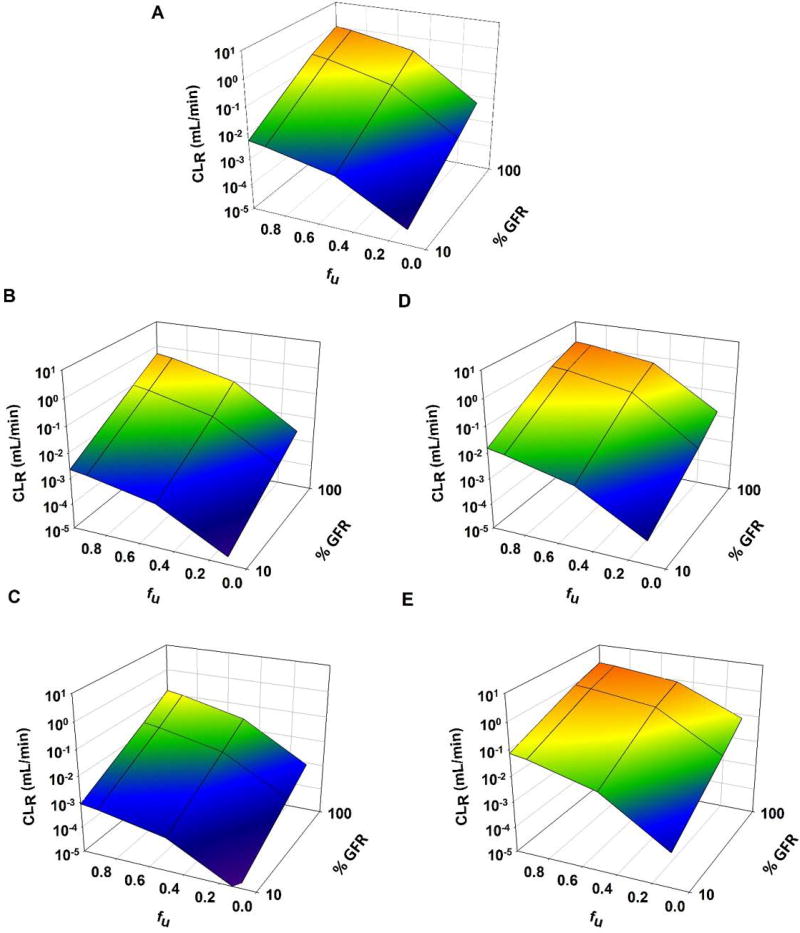
Figure 6 shows CLR for GHB with varying degrees of fraction unbound and renal function. Although GHB exhibits no protein binding, perturbations in this parameter were included in order to provide general insights for the impact of fu on CLR. As fraction unbound was increased, there was an accompanying increase in CLR, and this effect was maximized with lower expression of reabsorptive transporters (Fig. 6D, E) and minimized with increased expression (Fig. 6B, C). For all degrees of DT expression, maximal CLR was achieved when renal function and fraction unbound were also maximal. There was also an increase in CL when fu was increased for all levels of expression (Table 4). As with CLR, this effect was maximized with lower expression of reabsorptive transporters, and minimized with higher expression (Table 4). For all levels of DT expression, when fu was reduced to 0.1, CL was 0.39 ± 0.038 mL/min.
Figure 6.
3D mesh plot illustrating the effects of renal function (100%–10%) on renal clearance (CLR) of GHB (1500 mg/kg single IV bolus dose), when (1) expression of renal transporters is altered (VMAX, BBM and VMAX, BLM parameters were perturbed ± 0, 2, and 5-fold) and (2) protein binding is altered (fu = 1, 0.9, 0.5, 0.1): (A) No change in VMAX, (B) VMAX increases 2-fold, (C) VMAX increases 5-fold, (D) VMAX decreases 2-fold, and (E) VMAX decreases 5-fold
Table 4.
Impact of Protein Binding on GHB Pharmacokinetics in the Presence of Renal Impairment
| CL (mL/min) | ||||||||||||
| GFR 100% | GFR 50% | GFR 10% | ||||||||||
| fu | 1 | 0.9 | 0.5 | 0.1 | 1 | 0.9 | 0.5 | 0.1 | 1 | 0.9 | 0.5 | 0.1 |
| 0 Fold Change | 1.79 | 1.60 | 0.863 | 0.388 | 0.863 | 0.782 | 0.484 | 0.381 | 0.388 | 0.386 | 0.381 | 0.378 |
| 2 Fold Increase | 1.14 | 1.01 | 0.555 | 0.383 | 0.555 | 0.513 | 0.409 | 0.380 | 0.383 | 0.382 | 0.380 | 0.378 |
| 5 Fold Increase | 0.590 | 0.544 | 0.425 | 0.381 | 0.425 | 0.416 | 0.391 | 0.379 | 0.381 | 0.381 | 0.379 | 0.378 |
| 2 Fold Decrease | 2.28 | 2.07 | 1.20 | 0.411 | 1.20 | 1.10 | 0.677 | 0.384 | 0.411 | 0.402 | 0.384 | 0.378 |
| 5 Fold Decrease | 2.60 | 2.37 | 1.47 | 0.524 | 1.47 | 1.35 | 0.883 | 0.409 | 0.524 | 0.500 | 0.409 | 0.379 |
| CLR (mL/min) | ||||||||||||
| GFR 100% | GFR 50% | GFR 10% | ||||||||||
| fu | 1 | 0.9 | 0.5 | 0.1 | 1 | 0.9 | 0.5 | 0.1 | 1 | 0.9 | 0.5 | 0.1 |
| 0 Fold Change | 1.032 | 0.863 | 0.282 | 0.005 | 0.282 | 0.224 | 0.052 | 0.001 | 0.005 | 0.004 | 0.001 | 4.05E-05 |
| 2 Fold Increase | 0.494 | 0.394 | 0.098 | 0.002 | 0.098 | 0.075 | 0.018 | 0.001 | 0.002 | 0.002 | 0.001 | 2.00E-05 |
| 5 Fold Increase | 0.133 | 0.104 | 0.026 | 0.001 | 0.026 | 0.020 | 0.006 | 0.000 | 0.001 | 0.001 | 0.000 | 7.95E-06 |
| 2 Fold Decrease | 1.528 | 1.330 | 0.562 | 0.015 | 0.562 | 0.476 | 0.158 | 0.003 | 0.015 | 0.011 | 0.003 | 8.31E-05 |
| 5 Fold Decrease | 1.921 | 1.705 | 0.854 | 0.070 | 0.854 | 0.751 | 0.343 | 0.011 | 0.070 | 0.055 | 0.011 | 2.25E-04 |
| fe | ||||||||||||
| GFR 100% | GFR 50% | GFR 10% | ||||||||||
| fu | 1 | 0.9 | 0.5 | 0.1 | 1 | 0.9 | 0.5 | 0.1 | 1 | 0.9 | 0.5 | 0.1 |
| 0 Fold Change | 0.576 | 0.541 | 0.326 | 0.013 | 0.326 | 0.287 | 0.107 | 0.003 | 0.013 | 0.011 | 0.003 | 1.07E-04 |
| 2 Fold Increase | 0.433 | 0.391 | 0.176 | 0.006 | 0.176 | 0.146 | 0.043 | 0.001 | 0.006 | 0.005 | 0.001 | 5.29E-05 |
| 5 Fold Increase | 0.226 | 0.190 | 0.061 | 0.002 | 0.061 | 0.049 | 0.014 | 0.001 | 0.002 | 0.002 | 0.001 | 2.10E-05 |
| 2 Fold Decrease | 0.670 | 0.643 | 0.468 | 0.036 | 0.468 | 0.433 | 0.233 | 0.007 | 0.036 | 0.028 | 0.007 | 2.20E-04 |
| 5 Fold Decrease | 0.739 | 0.718 | 0.583 | 0.134 | 0.583 | 0.555 | 0.388 | 0.028 | 0.134 | 0.111 | 0.028 | 5.93E-04 |
Fold changes indicate the changes made to the capacity (Vmax) for transport at the brush border and basolateral membranes.
Effects of renal function and altered DME expression on the CLR and CL of GHB
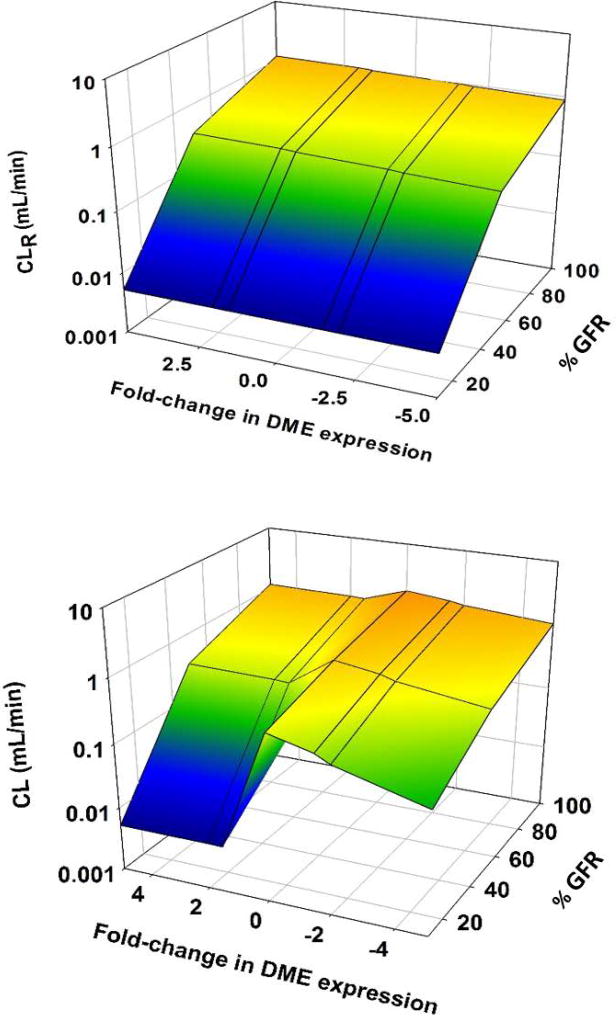
The alteration of liver DME expression had a limited effect on GHB CLR. When DME expression was increased 5 fold, there was a 13% increase in CLR; similarly, when DME expression was decreased 5 fold there was a 15% reduction in CLR (Figure 7, Table 5). When renal function was decreased by 50%, the magnitude of change in CLR increased to approximately 20% for both an increase and decrease in DME expression. However, when GFR was decreased to 10%, the changes in CLR was less than 1% for all changes in DME expression (Figure 7, Table 5).
Figure 7.
3D mesh plot illustrating the effects of renal function (100%–10%) on renal clearance (CLR) and overall clearance (CL) of GHB (1500 mg/kg single IV bolus dose), when expression of DMEs is altered (VMAX, MET parameter was perturbed ± 1.5, 2, and 5-fold)
Table 5.
Impact of Drug Metabolizing Enzyme Expression on GHB Pharmacokinetics in the Presence of Renal Impairment
| CL (mL/min) | |||
| GFR | |||
| 100% | 50% | 10% | |
| 0 Fold Change | 1.79 | 0.863 | 0.388 |
| 1.5 Fold Increase | 2.12 | 1.12 | 0.576 |
| 2 Fold Increase | 2.39 | 1.35 | 0.767 |
| 5 Fold Increase | 3.67 | 2.54 | 1.86 |
| 1.5 Fold Decrease | 1.52 | 0.680 | 0.261 |
| 2 Fold Decrease | 1.38 | 0.575 | 0.198 |
| 5 Fold Decrease | 1.08 | 0.370 | 0.083 |
| CLR (mL/min) | |||
| GFR | |||
| 100% | 50% | 10% | |
| 0 Fold Change | 1.03 | 0.282 | 5.20E-03 |
| 1.5 Fold Increase | 1.08 | 0.300 | 5.19E-03 |
| 2 Fold Increase | 1.11 | 0.312 | 5.22E-03 |
| 5 Fold Increase | 1.17 | 0.337 | 5.23E-03 |
| 1.5 Fold Decrease | 0.973 | 0.265 | 5.19E-03 |
| 2 Fold Decrease | 0.944 | 0.253 | 5.18E-03 |
| 5 Fold Decrease | 0.874 | 0.223 | 5.15E-03 |
Fold changes indicate the changes made to the capacity (Vmax) for metabolism in the liver compartment.
The effects of DME expression on CL were more pronounced. When the expression of DMEs was increased 5 fold, there was a 105% increase in CL. When the expression of DMEs was decreased 5 fold, there was a 40% decrease in CL. Unlike CLR, when GFR was decreased the effects of DME expression changes were magnified. When GFR was reduced to 10%, increasing DME expression 5-fold increased CL 380%, while decreasing expression 5-fold decreased CL by 80% (Table 5). When DME expression was decreased, the fraction excreted (fe) was increased, conversely, when DME expression was increased, fe decreased (Table 5). This effect was greatest when GFR was minimal (Table 5).
Discussion
Renal impairment is a major global health concern and the impact of this disease has been shown to impact the PK of xenobiotics. In addition to reducing GFR, RI has been shown to affect the expression of renal, hepatic and intestinal DTs and DMEs in experimental models [2, 8, 22]. Drug plasma protein binding has also been shown to be altered in RI due to uremia, hypoalbuminemia, and drug interactions with uremic toxins [23, 24]. Uremic toxins, many of which are small organic anions such as indoxyl sulfate, can accumulate in RI, and are known substrates of important DTs such as Organic Anion Transporters (OATs), as well as other SLC transporters such as OATP1B1 and ABC transporters [25, 26]. This could result in DDI interactions in RI even when no interaction is expected based on the xenobiotics administered. It is critical to analyze the impact of these factors, along with renal function to elucidate the impact of renal impairment on the PK of drugs.
This simulation based study focused on a specific compound, GHB, which is representative of an actively reabsorbed compound in the kidney. We utilized a novel PK model with a renal clearance component that incorporates active reabsorption with vectorial proximal tubule transport and reabsorption of fluid along the nephron, an important model addition that modulates concentration of drugs in the proximal tubule [27] In this model, decreases in the volumes and flows of the filtrate across the nephron segments are assumed to be constant, proportional in degree, and equal in magnitude, both, spatially and temporally. In this manner, the concentration of drug in the proximal tubule, the driving force for transport, changes along the flow path. MCT1/SMCT1-mediated reabsorption of GHB at the BBM was incorporated only from the proximal tubule S2 and S3 segments in our model, which is consistent with the observed predominant expression of SMCT1 at the S2 and S3 segments [28] and with the expression of MCT1 at the BBM [29]. The PK model also incorporated saturable metabolism of GHB in the liver and tissue distribution. The model allowed the evaluation of changes that occur with RI, namely changes in GFR, protein binding, expression of DTs and DMEs, as well as potential drug-toxin interactions, that may influence the renal clearance and total clearance of GHB and other drugs undergoing active reabsorption in the kidney.
As expected, when renal function was decreased, there was an accompanying decrease in CLR (Fig. 3). This effect was greater with increasing doses due to the dose-dependent kinetics of GHB. The dose dependent differences can be explained through the changes in overall CL. Overall CL decreases the least for the 200 mg/kg dose. The greater impact of GFR reduction on CL for the higher doses is present because with an actively reabsorbed compound, CLR becomes a more significant contributor to CL as dose increases and saturation of reabsorption is present; therefore, the impact of RI may be greater for the higher doses.
DT expression was shown to play a role in the PK of GHB in the presence of RI. When DT expression was decreased 2-fold, there was an accompanying 1.5-fold increase in CLR when renal function was unchanged (Table 3). Sodium/glucose transporter 2 (SGLT2) mRNA expression has been shown to decrease over 7-fold in 5/6 nephrectomized rats [30]. This was accompanied by a 2.3 fold reduction in Vmax for glucose transport in brush border membrane vesicles isolated from nephrectomized rats [30]. The changes in the capacity of SGLT2 glucose transport in nephrectomized rats, likely mediated by a reduction in expression as the mRNA and Vmax data suggest, resulted in a 2.4 fold increase in CLR for glucose based on glucose/creatinine ratios [30]. Therefore, decreases in DTs involved in renal reabsorption might be expected in RI, resulting in increases in CLR, as observed from our simulations with GHB.
In order to investigate the potential impact of accumulated uremic toxins, the impact of inhibition on GHB PK in the presence of RI was also included in this analysis. The effects of inhibition were augmented when transporter expression was increased (Fig. 4B, C), and diminished when expression was decreased (Fig. 4D, E). CLR for GHB was maximal with the maximal degree of inhibition, but was still dependent on renal function. Experimentally, CLR was observed to increase in rats from 0.95 to 2.1 and 2.2 mL/min when a non-competitive MCT1 inhibitor AR-C155858 was administered 5 minutes after GHB at a dose of 1 or 5 mg/kg i.v., respectively [31]. Based on the plasma concentrations of AR-C155858 after doses of 1 and 5 mg/kg IV [31] and its Ki of 2.3 nM, the R value ([I]/Ki) would be 417 for the first hour after IV administration and over 4 for the next 300 minutes. The observed change in CLR of GHB in the presence of AR-C155858 is consistent with our simulations, which show an increase in CLR from 1.03 to 1.5, 2.1, and 2.2 mL/min for R values of 1, 10, and 100, respectively.
Competitive inhibition also led to an increase in CLR but the values of CLR achieved were higher for non-competitive inhibition than for competitive inhibition, although the majority of the CLR values were within 2-fold of each other. The similar impact on CLR for both types of inhibition in RI suggests that the type of inhibition is not as critical to PK predictions as the potency and concentration of the inhibitors. Changes in GFR had a significant impact on CLR in the presence of inhibition. For a single value of GFR, increasing the degree of inhibition (R), led to an increase in CLR (Table 3 and S1). Therefore, the expected decrease in GFR and presence of DDI due to uremic toxins may result in opposing effects on CLR for actively reabsorbed compounds.
In the case of administering a transporter inhibitor to enhance the CLR of an actively reabsorbed compound, a proposed treatment option for GHB overdose, a decrease in GFR would lead to lower CLR values than expected with normal renal function. Therefore, inhibition of renal reabsorption would represent a less effective treatment option for renally impaired patients. This has been observed with SGLT2 inhibitors for the treatment of Type 2 diabetes mellitus (T2DM). SGLT2 is responsible for 90% of glucose reabsorption in the kidney, inhibition of this reabsorption leads to an increase in glucose CLR, reducing hyperglycemia in T2DM patients [32]. The efficacy of several of these compounds, including dapagliflozin, was reported to decrease with increasing degrees of RI, resulting in a reduction in glucose CLR, compared to patients without RI [32]. A single 50 mg dose of dapagliflozin resulted in a reduction in steady state glucose CLR to 58% and 16% for mild and severe RI, respectively, when compared to glucose CLR in healthy patients [33]. Our simulations were consistent with what was observed with dapagliflozin; non-competitive inhibition (R of 100) resulted in an increase in GHB CLR for all values of GFR compared to simulations without inhibition. However, when GHB CLR was compared across different degrees of renal function, while keeping inhibition consistent (R of 100), CLR was reduced to 50% and 9% for 50% and 10% renal function, respectively, when compared to simulations with 100% renal function.
Similar trends were observed with fraction unbound: maximal CLR was achieved with a fu of 1 and 100% GFR. In RI, we expect an increase in fu and a decrease in GFR. When fu was increased, there was an increase in CLR, while a decrease in GFR leads to a decrease in CLR. Due to the opposing effects of fu and GFR on CLR, it is possible that CLR could remain unchanged in RI for some compounds. It is also possible that there could also be an increase in CLR for compounds that normally have a high degree of protein binding and undergo a significant increase in fu in RI.
The magnitude of the impact of fu on CL was influenced by the contribution of CLR to total CL. At higher levels of DT expression, CLR was a smaller component of CL and the fe was smaller, due to an increased capacity for reabsorption. Therefore, the changes in CL as a result of changes in fu were decreased. When DT expression was decreased, the capacity for reabsorption was also decreased and CLR became a more significant contributor to CL. In this case, the impact of fu on CLR had a larger effect on CL. This suggests that the impact of changes in CLR that result from the physiological changes in RI will be dependent on the magnitude of the contribution of CLR to CL.
The impact of altered DME expression on CLR was minimal, but may play a significant role in the overall clearance of compounds such as GHB, which exhibits capacity-limited metabolism. When renal function was reduced to 50% which is consistent with values for CKD, reduction in the expression of DMEs from 1.5- to 5-fold resulted in a 46%–81% reduction in overall CL. This is consistent with reduction in non-renal CL reported by Nolin et al. for subjects with CKD of 30% – 67% (Nolin 2008). Although definitive comparisons are difficult to make due to the limited quantitative data on DME expression with RI, there is agreement between our simulated values and the literature reports. As expected, alterations in the expression of DMEs had a very minimal effect on CLR, since these represent independent clearance mechanisms.
There are some limitations to the model simulations performed in this study. There is the potential for changes in the fluid reabsorption along the nephron segments in CKD. Based on Bricker’s Intact Nephron Hypothesis, the fractional fluid reabsorption would be expected to be unchanged, since the hypothesis states that surviving nephrons of the diseased kidney will retain functional integrity. A series of studies utilizing variations of the canine remnant kidney model, conducted by Neal Bricker and others clearly established the legitimacy of this proposal in dogs, and these concepts have been used clinically, although there is limited clinical data [34]Secondly, protein binding in the renal tubular fluid and effects on active reabsorption was not considered in these simulations. Generally, protein binding in tubular fluid is negligible due to the negligible concentrations of albumin and other high molecular weight binding proteins present in tubular fluid. With RI, albumin and other high molecular weight proteins can be filtered, to varying extents, at the glomerulus, resulting in the potential for protein binding of drugs in the tubular fluid. This, along with the tubular fluid flow rate and residence time at the site of reabsorption, may influence renal reabsorption. Additionally, no changes in urine pH were incorporated into the simulations, since the relationship between urine pH and CKD is not yet clear. It has been shown that low urine pH (5.0 to 5.5) can be a predictor of CKD, however, the urine pH range of individuals with stage 2 CKD ranges from 5.5 to 7.0 [35], the normal range for urine pH.
In summary, this study has demonstrated that renal function is a major determinant in the clearance of drugs undergoing transporter-mediated renal reabsorption. The effect of renal function on clearance of drugs is modulated by expression of DTs and DMEs, fu, and DDIs with inhibitors of renal transporters. The potential to modify our semi-mechanistic PK model to reflect these differences among substrates, most notably changes in active transport in the kidney, provides utility for the prediction of PK under the varying conditions observed with RI. These findings highlight the importance of understanding the role of renal function and drug-kidney transporter interactions in the renal clearance of compounds. The use of simulations using a physiologically-relevant PK model provides for predictions for the impact of RI for a NME undergoing capacity-limited renal reabsorption to guide the design of selective clinical trials of NMEs in RI.
Conclusion
It has been demonstrated in the literature that in addition to a decrease in GFR, alterations in DT and DME expression and activity, as well as changes in fu are likely to occur in RI. This study has demonstrated these additional factors are likely to play a major role in determining the CLR of compounds that are actively reabsorbed, such as GHB. The utilization of a simulations based approach coupled with a semi-mechanistic kidney model enabled the prediction of the potential impact of RI on CLR. The results of the simulations agreed well with available data on GHB CLR in the presence of inhibitors, as well as with data on glucose CLR in RI populations. Further work investigating the impact of RI on other types of compounds, such as those that are actively secreted, will allow for characterization of the impact of RI on DTs, DMEs and fu and their subsequent impact on CLR.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health National Institute on Drug Abuse [grant R01DA023223]. KEF was supported in part by a fellowship from Bristol-Myers Squibb.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
Authorship contributions:
Participated in research design: Kristin E. Follman, Rutwij A. Dave and Marilyn E. Morris
Conducted experiments: Kristin E. Follman
Performed data analysis: Kristin E. Follman, Rutwij A. Dave
Wrote or contributed to the writing of the manuscript: Rutwij A Dave, Kristin E. Follman, and Marilyn E. Morris
References
- 1. [Date Accessed 2014 Accessed];Chronic Kidney Disease. https://www.kidney.org/kidneydisease/aboutckd.
- 2.Nolin TD, Naud J, Leblond FA, Pichette V. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther. 2008;83:898–903. doi: 10.1038/clpt.2008.59. [DOI] [PubMed] [Google Scholar]
- 3.Levy G. Pharmacokinetics in renal disease. Am J Med. 1977;62:461–5. doi: 10.1016/0002-9343(77)90397-7. [DOI] [PubMed] [Google Scholar]
- 4.Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol. 2009;65:757–73. doi: 10.1007/s00228-009-0678-8. [DOI] [PubMed] [Google Scholar]
- 5.Lam YW, Banerji S, Hatfield C, Talbert RL. Principles of drug administration in renal insufficiency. Clin Pharmacokinet. 1997;32:30–57. doi: 10.2165/00003088-199732010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Sun H, Frassetto L, Benet LZ. Effects of renal failure on drug transport and metabolism. Pharmacol Ther. 2006;109:1–11. doi: 10.1016/j.pharmthera.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Komazawa H, Yamaguchi H, Hidaka K, Ogura J, Kobayashi M, Iseki K. Renal uptake of substrates for organic anion transporters Oat1 and Oat3 and organic cation transporters Oct1 and Oct2 is altered in rats with adenine-induced chronic renal failure. J Pharm Sci. 2013;102:1086–94. doi: 10.1002/jps.23433. [DOI] [PubMed] [Google Scholar]
- 8.Naud J, Michaud J, Beauchemin S, Hebert MJ, Roger M, Lefrancois S, Leblond FA, Pichette V. Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab Dispos. 2011;39:1363–9. doi: 10.1124/dmd.111.039115. [DOI] [PubMed] [Google Scholar]
- 9.Brandoni A, Torres AM. Altered Renal Expression of Relevant Clinical Drug Transporters in Different Models of Acute Uremia in Rats. Role of Urea Levels. Cell Physiol Biochem. 2015;36:907–16. doi: 10.1159/000430265. [DOI] [PubMed] [Google Scholar]
- 10.(CDER) USDoHaHSFaDACfDEaR. Pharmacokinetics in Patients with Impaired Renal Function — Study Design, Data Analysis, and Impact on Dosing and Labeling Pharmacology C (ed) 2010 [Google Scholar]
- 11.Dave RA, Morris ME. Semi-mechanistic kidney model incorporating physiologically-relevant fluid reabsorption and transporter-mediated renal reabsorption: pharmacokinetics of gamma-hydroxybutyric acid and L-lactate in rats. J Pharmacokinet Pharmacodyn. 2015 doi: 10.1007/s10928-015-9441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lettieri JT, Fung HL. Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J Pharmacol Exp Ther. 1979;208:7–11. [PubMed] [Google Scholar]
- 13.Palatini P, Tedeschi L, Frison G, Padrini R, Zordan R, Orlando R, Gallimberti L, Gessa GL, Ferrara SD. Dose-dependent absorption and elimination of gamma-hydroxybutyric acid in healthy volunteers. Eur J Clin Pharmacol. 1993;45:353–6. doi: 10.1007/BF00265954. [DOI] [PubMed] [Google Scholar]
- 14.Scharf MB, Lai AA, Branigan B, Stover R, Berkowitz DB. Pharmacokinetics of gammahydroxybutyrate (GHB) in narcoleptic patients. Sleep. 1998;21:507–14. doi: 10.1093/sleep/21.5.507. [DOI] [PubMed] [Google Scholar]
- 15.Arena C, Fung HL. Absorption of sodium gamma-hydroxybutyrate and its prodrug gamma-butyrolactone: relationship between in vitro transport and in vivo absorption. J Pharm Sci. 1980;69:356–8. doi: 10.1002/jps.2600690331. [DOI] [PubMed] [Google Scholar]
- 16.Morris ME, Hu K, Wang Q. Renal clearance of gamma-hydroxybutyric acid in rats: increasing renal elimination as a detoxification strategy. J Pharmacol Exp Ther. 2005;313:1194–202. doi: 10.1124/jpet.105.083253. [DOI] [PubMed] [Google Scholar]
- 17.Khurana I. Textbook of Human Physiology for Dental Students. Second. Elsevier Health Sciences APAC; 2014. Excretory System; pp. 280–281. [Google Scholar]
- 18.Lash LH. Principles and Methods of Renal Toxicology. In: Hayes AW, editor. Principles and Methods of Toxicology. Fifth. Taylor & Francis; 2007. pp. 1513–1514. [Google Scholar]
- 19.Lote CJ. Principles of Renal Physiology. Fourth. Kluwer Academic Publishers; 2000. Summary of the principal reabsorptive and secretory processes; pp. 161–162. [Google Scholar]
- 20.Morse BL, Felmlee MA, Morris ME. gamma-Hydroxybutyrate blood/plasma partitioning: effect of physiologic pH on transport by monocarboxylate transporters. Drug Metab Dispos. 2012;40:64–9. doi: 10.1124/dmd.111.041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Argenio DZ, Schumitzky A, Wang X. ADAPT 5 User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Fourth. City: 2009. [Google Scholar]
- 22.Dreisbach AW, Lertora JJ. The effect of chronic renal failure on drug metabolism and transport. Expert Opin Drug Metab Toxicol. 2008;4:1065–74. doi: 10.1517/17425255.4.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller F, Maiga M, Neumayer HH, Lode H, Distler A. Pharmacokinetic effects of altered plasma protein binding of drugs in renal disease. Eur J Drug Metab Pharmacokinet. 1984;9:275–82. doi: 10.1007/BF03189651. [DOI] [PubMed] [Google Scholar]
- 24.Vanholder R, Van Landschoot N, De Smet R, Schoots A, Ringoir S. Drug protein binding in chronic renal failure: evaluation of nine drugs. Kidney Int. 1988;33:996–1004. doi: 10.1038/ki.1988.99. [DOI] [PubMed] [Google Scholar]
- 25.Katsube Y, Tsujimoto M, Koide H, Ochiai M, Hojyo A, Ogawa K, Kambara K, Torii N, Shima D, Furukubo T, Izumi S, Yamakawa T, Minegaki T, Nishiguchi K. Cooperative inhibitory effects of uremic toxins and other serum components on OATP1B1-mediated transport of SN-38. Cancer Chemother Pharmacol. 2017;79:783–789. doi: 10.1007/s00280-017-3276-y. [DOI] [PubMed] [Google Scholar]
- 26.Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V. Handling of Drugs, Metabolites, and Uremic Toxins by Kidney Proximal Tubule Drug Transporters. Clin J Am Soc Nephrol. 2015;10:2039–49. doi: 10.2215/CJN.02440314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dave RA, Morris ME. Semi-mechanistic kidney model incorporating physiologically-relevant fluid reabsorption and transporter-mediated renal reabsorption: pharmacokinetics of gamma-hydroxybutyric acid and L-lactate in rats. J Pharmacokinet Pharmacodyn. 2015;42:497–513. doi: 10.1007/s10928-015-9441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanase H, Takebe K, Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T. Cellular expression of a sodium-dependent monocarboxylate transporter (Slc5a8) and the MCT family in the mouse kidney. Histochem Cell Biol. 2008;130:957–66. doi: 10.1007/s00418-008-0490-z. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Darling IM, Morris ME. Transport of gamma-hydroxybutyrate in rat kidney membrane vesicles: Role of monocarboxylate transporters. J Pharmacol Exp Ther. 2006;318:751–61. doi: 10.1124/jpet.106.105965. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura N, Masuda S, Takahashi K, Saito H, Okuda M, Inui K. Decreased expression of glucose and peptide transporters in rat remnant kidney. Drug Metab Pharmacokinet. 2004;19:41–7. doi: 10.2133/dmpk.19.41. [DOI] [PubMed] [Google Scholar]
- 31.Vijay N, Morse BL, Morris ME. A Novel Monocarboxylate Transporter Inhibitor as a Potential Treatment Strategy for gamma-Hydroxybutyric Acid Overdose. Pharm Res. 2015;32:1894–906. doi: 10.1007/s11095-014-1583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheen AJ. Pharmacokinetics, Pharmacodynamics and Clinical Use of SGLT2 Inhibitors in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease. Clin Pharmacokinet. 2015;54:691–708. doi: 10.1007/s40262-015-0264-4. [DOI] [PubMed] [Google Scholar]
- 33.Kasichayanula S, Liu X, Pe Benito M, Yao M, Pfister M, LaCreta FP, Humphreys WG, Boulton DW. The influence of kidney function on dapagliflozin exposure, metabolism and pharmacodynamics in healthy subjects and in patients with type 2 diabetes mellitus. Br J Clin Pharmacol. 2013;76:432–44. doi: 10.1111/bcp.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown SA. Renal pathophysiology: lessons learned from the canine remnant kidney model. J Vet Emerg Crit Care (San Antonio) 2013;23:115–21. doi: 10.1111/vec.12030. [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi N, Fukui M, Tanaka M, Toda H, Imai S, Yamazaki M, Hasegawa G, Oda Y, Nakamura N. Low urine pH Is a predictor of chronic kidney disease. Kidney Blood Press Res. 2012;35:77–81. doi: 10.1159/000330487. [DOI] [PubMed] [Google Scholar]
- 36.Meno-Tetang GM, Li H, Mis S, Pyszczynski N, Heining P, Lowe P, Jusko WJ. Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(-4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos. 2006;34:1480–7. doi: 10.1124/dmd.105.009001. [DOI] [PubMed] [Google Scholar]
- 37.Peters SA. Physiologically-Based Pharmacokinetic (PBPK) Modeling and Simulations: Principles, Methods, and Applications in the Pharmaceutical Industry. Vol. 407. Wiley; 2012. Appendices. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niederalt C, Wendl T, Kuepfer L, Claassen K, Loosen R, Willmann S, Lippert J, Schultze-Mosgau M, Winkler J, Burghaus R, Brautigam M, Pietsch H, Lengsfeld P. Development of a physiologically based computational kidney model to describe the renal excretion of hydrophilic agents in rats. Front Physiol. 2012;3:494. doi: 10.3389/fphys.2012.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jobin J, Bonjour JP. Measurement of glomerular filtration rate in conscious unrestrained rats with inulin infused by implanted osmotic pumps. Am J Physiol. 1985;248:F734–8. doi: 10.1152/ajprenal.1985.248.5.F734. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Wang Q, Morris ME. Pharmacokinetic interaction between the flavonoid luteolin and gamma-hydroxybutyrate in rats: potential involvement of monocarboxylate transporters. AAPS J. 2008;10:47–55. doi: 10.1208/s12248-007-9001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.