Abstract.
Yellow fever (YF) is a viral disease transmitted by mosquitoes and endemic mostly in South America and Africa with 20–50% fatality. All current licensed YF vaccines, including YF-Vax® (Sanofi-Pasteur, Lyon, France) and 17DD-YFV (Bio-Manguinhos, Rio de Janeiro, Brazil), are based on live attenuated virus produced in hens’ eggs and have been widely used. The YF vaccines are considered safe and highly effective. However, a recent increase in demand for YF vaccines and reports of rare cases of YF vaccine-associated fatal adverse events have provoked interest in developing a safer YF vaccine that can be easily scaled up to meet this increased global demand. To this point, we have engineered the YF virus envelope protein (YFE) and transiently expressed it in Nicotiana benthamiana as a stand-alone protein (YFE) or as fusion to the bacterial enzyme lichenase (YFE-LicKM). Immunogenicity and challenge studies in mice demonstrated that both YFE and YFE-LicKM elicited virus neutralizing (VN) antibodies and protected over 70% of mice from lethal challenge infection. Furthermore, these two YFE-based vaccine candidates induced VN antibody responses with high serum avidity in nonhuman primates and these VN antibody responses were further enhanced after challenge infection with the 17DD strain of YF virus. These results demonstrate partial protective efficacy in mice of YFE-based subunit vaccines expressed in N. benthamiana. However, their efficacy is inferior to that of the live attenuated 17DD vaccine, indicating that formulation development, such as incorporating a more suitable adjuvant, may be required for product development.
INTRODUCTION
Yellow fever (YF) is a mosquito-borne viral disease, most commonly affecting populations in South America and Africa. Individuals with mild cases have symptoms of fever, headaches, nausea, and vomiting, whereas cases advancing to the toxic phase (∼15%) can have heart, kidney, and liver issues along with hemorrhaging. Up to 50% of cases progressing to the toxic phase are fatal. A modeling study based on African data sources estimated the burden of YF during 2013 was 84,000–170,000 severe cases and 29,000–60,000 deaths.1 The incidence of YF has increased over the past two decades because of declining population immunity, deforestation, urbanization, population movements, and climate change.1
There is no specific antiviral therapy for YF and treatment is directed at symptomatic relief. Therefore, vaccination of populations living in or traveling to endemic areas is the best preventive measure against YF. Currently, there are four producers of YF vaccines that are prequalified by the World Health Organization to supply vaccines to international agencies: Bio-Manguinhos (17DD-YFV), Sanofi Pasteur S.A. (Stamaril®), Institute Pasteur de Dakar (Stabilized YF Vaccine, Senegal), and Chumakov Institute of Poliomyelitis and Viral Encephalitides (Russian Federation).2 In addition, Sanofi Pasteur Inc. (Swiftwater, PA) and Wuhan Institute of Biological Products (Wuhan, China) produce the YF vaccine licensed for domestic use.3,4 All these vaccines are based on live attenuated YF 17D viruses produced in hens’ eggs.2,3 The YF vaccines are highly effective, with a single dose being sufficient to confer sustained immunity and life-long protection against the disease.2,3,5 The vaccines provide effective immunity within 30 days for 99% of persons vaccinated.1 Recently, the demand for YF vaccines has increased, causing shortages in supply.5,6
Although YF vaccines are generally safe,1 they can cause allergic reactions against egg components, and in rare cases encephalitis and other neurologic syndromes.7,8 Although it is extremely rare, even more serious adverse events associated with administration of the live attenuated YF 17D vaccines have been reported, such as a severe viscerotropic disease similar to wild-type YF, resulting from active YF virus replication in a genetically predisposed host, detrimentally impacting multiple organs.9,10 Therefore, the development of alternative YF vaccines that are safe, efficacious, and cost-effective is important for continued control of the disease.
The YF virus, a member of the genus Flavivirus, is an enveloped virus with a single-stranded positive-sense RNA genome encoding three structural (envelope [E], membrane [M] and capsid [C]) and seven nonstructural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins.11 The YF virus E protein (YFE) is known to be involved in multiple stages of cell infection, including receptor-mediated binding to the cell membrane, cell membrane fusion and penetration, and fusion with the endosomal membrane.12–14 The activity of the YFE protein is dependent on its structural state. The native YFE protein is a homodimer comprising three distinct domains (DI, DII, and DIII). When it is introduced to the acidic environment of endosomes after receptor-mediated endocytosis of the virus, the dimer dissociates and converts into a fusogenic homotrimer.15 The YFE protein also plays a major role in the elicitation of virus-neutralizing antibodies and mounting protective immunity,16,17 making it a key target for subunit YF vaccine development.
There are several publications describing the recombinant YFE protein produced in different expression systems. For example, Desprès et al.18,19 successfully expressed YFE and NS1 proteins both in mammalian and insect cells. The insect cell-produced E protein did not cause syncytium formation in insect cells, likely because of the truncation of the C-terminal hydrophobic region (amino acids [a.a.] 740–778) that may be critical for oligomerization, correct folding, and biological activity of target.20 A partial genome of the YF virus expressed in insect cells by Shiu et al.21 consisting of a cDNA encoding for a.a. 109–820 of the YF virus polyprotein represented N-terminally truncated prM, the complete M protein, the E protein, and the first 42 a.a. of the NS1 protein. The resulting recombinant E protein was reported to be antigenically indistinguishable from that produced using the attenuated 17D vaccine strain of the YF virus.21 Recently, Barros et al.22 also expressed the full-length viral E protein in insect cells and demonstrated syncytium formation and specific recognition of the recombinant E protein by convalescent sera from YF virus-infected, but not dengue virus-infected, patients. Despite the importance of the E protein as a key vaccine target, only insect cell extracts containing the recombinant E protein, but not the purified target, were evaluated for immunogenicity and protective efficacy.19
During the last two decades, the potential of plants as safe, cost-effective, and highly scalable platforms for the production of recombinant vaccine antigens and therapeutic proteins has been well-documented.23–25 In addition, plant cells are capable of performing eukaryotic posttranslational modifications of target proteins, including N-linked glycosylation, which are substantially similar to those found in mammalian cells.26 Furthermore, plants can be engineered to perform mammalian-like glycosylation of recombinant proteins,27–29 or plant expression vectors can be designed to eliminate N-linked glycans from proteins that contain putative N-glycosylation sites but are not glycosylated in their native host.30
Some vaccine candidates produced in plant systems have reached clinical or advanced preclinical stages of development25 and one product, taliglucerase alfa (Protalix BioTherapeutics Inc., Carmiel, Israel), has been approved for marketing by the U.S. Food and Drug Administration.31,32
Transient expression of recombinant proteins in plants, achieved by the introduction of target genes into growing plant leaves through vacuum infiltration with Agrobacteria transformed with vectors containing genetic elements of a plant virus and/or a binary vector based on the agrobacterial Ti (tumor-inducing) plasmid (reviewed by Yusibov et al.33), has been shown to be cost-efficient and result in reduced production time.34 Fraunhofer USA Center for Molecular Biotechnology (FhCMB) has developed such a transient expression system applicable to Nicotiana benthamiana, a relative of tobacco.35 This approach allows for rapid, high-level accumulation of recombinant proteins in plants and has been used to produce multiple recombinant vaccine antigens targeting diseases caused by viruses, such as influenza,36 bacteria, such as anthrax and plague,37–39 and parasites, such as malaria and trypanosomiasis,40–42 with several advancing through Phase 1 clinical trials,43,44 using material produced under current good manufacturing practice, where they demonstrated acceptable safety and tolerability profiles and had less than 0.5 ppm nicotine. In this system, target antigens are designed to be produced either as stand-alone soluble subunits or as fusions to engineered lichenase from Clostridium thermocellum (LicKM).35–37,40,45–49
Here, we have engineered and produced YFE in N. benthamiana as a stand-alone subunit and as LicKM fusions and evaluated immunogenicity and protective efficacy of these subunit vaccine candidates in mice and nonhuman primates (NHPs).
MATERIALS AND METHODS
YFE design, cloning, and expression in plants.
The YFE protein (a.a. 286-682, AAC54267) was designed both as a stand-alone subunit (YFE-1) and as genetic fusions to LicKM (a.a. 2-224, ABG78599) by introducing YFE at three different sites: the internal loop (YFE-2E; YFE inserted between a.a. 165 and a.a. 168 of LicKM, ABG78599), C-terminus (YFE-3E), and N-terminus (YFE-4E). Sequences encoding YFE and LicKM were optimized for expression in plants (GENEART AG, Regensburg, Germany). YFE-1 and the YFE-LicKM fusion variants were engineered to contain the posttranslationally cleaved pathogenesis-related protein 1a (PR-1a) signal peptide (a.a. 1-30 of BAA14220 for YFE-1 and YFE-4E constructs and a.a. 1-32 of BAA14220 for YFE-2E and YFE-3E constructs) at the N-terminus, and a polyhistidine (His) affinity purification tag and the endoplasmic reticulum (ER) retention signal KDEL at the C-terminus. Sequences encoding these targets were subcloned into the pGR-D4 expression vector.35,48
The resulting constructs were introduced into Agrobacterium tumefaciens strain GV3101 by electroporation, the bacterial cultures were grown overnight, and bacteria were introduced into leaves of 6-week-old hydroponically grown N. benthamiana plants by vacuum infiltration as described previously.35,48,50 For analysis of target expression and target protein purification, plant biomass was harvested at 7 days post infiltration.
YFE protein purification.
All YFE vaccine candidates were produced at the 1 kg aerial plant biomass scale. Each YFE protein was extracted from plant biomass using three volumes of Tris-based extraction buffer (50 mM Tris, pH 8.0, 0.5 M NaCl) followed by the addition of Triton X-100 to the final concentration of 0.5%. Insoluble material was then clarified by centrifugation at 16,000 × g for 15 minutes at 4°C, followed by passing the supernatant through a 0.2 µm filter (Sartorius, Gottingen, Germany).
For YFE-1, clarified extract was loaded onto Ni sepharose 6FF resin (GE Life Sciences, Marlborough, MA) and eluted using 20 mM Tris, pH 8.0, containing 300 mM imidazole. Ammonium sulphate was added to the IMAC eluant to 0.5 M and the solution loaded onto phenyl sepharose HP resin (GE Life Sciences) and eluted with 20 mM Tris, pH 8.0, and 0.1 M ammonium sulphate. The protein eluent was dialyzed into 20 mM Tris, pH 8.0 before loading onto diethylaminoethyl (DEAE) sepharose resin (Tosoh, Tokyo, Japan) and the target was eluted with 20 mM Tris, pH 8.0, and 90 mM NaCl.
For YFE-2E, clarified extract was loaded onto Ni sepharose resin and eluted with 20 mM Tris, pH 8.0 containing 300 mM imidazole. NaCl was added to the IMAC eluant to 1.2 M before loading onto phenyl sepharose HP resin and eluting the target with 20 mM Tris, pH 8.0, and 0.2 M NaCl. The eluant was dialyzed into 20 mM Tris, pH 8.0 before loading onto DEAE resin and eluting target with 20 mM Tris, pH 7.0 containing 60 mM NaCl.
For YFE-3E, clarified extract was loaded onto Ni sepharose resin and eluted with 20 mM Tris, pH 8.0, and 150 mM imidazole. NaCl was added to the IMAC eluant to 1.2 M before loading onto phenyl sepharose HP resin. Bound material was washed with 20 mM Tris, pH 8.0 containing 0.2 M NaCl before elution in salt-free buffer. Eluted target was loaded onto DEAE resin and eluted with 20 mM Tris, pH 8.0 containing 125 mM NaCl.
For YFE-4E, clarified extract was loaded onto Ni sepharose resin and eluted with 20 mM Tris, pH 8.0, and 300 mM imidazole. NaCl was added to the IMAC eluant to 1.0 M before loading onto phenyl sepharose HP resin and eluting target with water. The protein eluant was loaded in 20 mM Tris, pH 8.0 onto DEAE resin and target was eluted with 20 mM Tris, and pH 8.0 containing 100 mM NaCl.
YFE protein characterization.
The protein concentration for YFE targets was estimated by measuring optical density of the preparations at 280 nm in a denaturing buffer with absorptivity coefficient calculated based on the recombinant sequence.51 Analytical size exclusion chromatography (SEC) was performed by passage over a Superdex 200 GL 10/300 column (GE Life Sciences) at 0.5 mL/minutes. The mobile phase was of the same formulation as the target molecule tested and it was composed of 20 mM Tris at pH 8.0 containing 70 mM NaCl (YFE-3E), 90 mM NaCl (YFE-1), or 100 mM NaCl (YFE-4E), respectively. The SEC mobile phase for YFE-2E was 20 mM Tris pH 7.0, 60 mM NaCl. Absolute molar mass for the SEC-separated target species were determined by multiangle light scattering (MALS) connected in tandem to the SEC column (miniDAWN TREOS®; Wyatt Technology Corp., Santa Barbara, CA). The molecular weights (MW) were determined using software supplied by the manufacturer (Astra, version 5.3.4; Wyatt Technology Corp.) and the standard refractive index increment of 0.185.
Proteins were resolved by 10% acrylamide/sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie (Gel Code Blue, Thermo Fisher Scientific, Waltham, MA). Immunoblotting was performed by wet transfer of samples to a polyvinylidene difluoride membrane and subsequently blocking with I-Block (Applied Biosystems). Target bands were detected using a tetrahistidine-specific monoclonal antibody (mAb) (Qiagen, Hilden, Germany), followed by a species-specific horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA).
Immunogenicity assessments of YFE proteins in mice.
The first immunogenicity study in mice was conducted to select candidate YFE target(s) for further evaluation. In brief, BALB/c mice (five per group) (Envigo, East Millstone, NJ) were administered intramuscularly (IM) 20 or 5 µg of each YFE vaccine candidate with 0.3% Alhydrogel (Brenntag-Biosector, Ballerup, Denmark) on study days 0, 21, and 42. The doses of the YFE-LicKM fusion proteins (YFE-2E, -3E and -4E) were calculated to contain 20 or 5 µg/dose of the YFE component in each vaccine. Blood samples were collected on study days 0, 20, 41, and 63.
Serum samples were pooled per group and evaluated for virus neutralizing (VN) activity by a plaque-reduction neutralization test (PRNT) using Vero cells (ATCC, Manassas, VA) and YF 17D virus (NR-116, GenBank: X03700; BEI Resources, Manassas, VA) as described elsewhere52 with slight modifications. Briefly, heat-treated sera were serially diluted in Dulbecco’s Modified Eagle Medium containing 2% fetal bovine serum (FBS) in 24-well plates. An equal volume (200 µL) of 17D virus diluted to ∼150 plaque-forming units (PFU)/mL was added to each plate except for cell control wells. The virus-serum mixtures were incubated for 1 hour at 37°C, after which 1.6 × 106 cells/mL of Vero cells were added to each well. After a 3-hour incubation at 37°C with 5% CO2, supernatants were removed and overlay medium (diluent with 3% carboxymethylcellulose) was added. After a 6-day incubation at 37°C with 5% CO2, cells were fixed with 10% formaldehyde and stained with 0.1% crystal violet. Plaques were counted under magnification and reciprocal serum dilutions that gave 50% plaque reduction (PRNT50 titer) were calculated through linear regression.
A second mouse study was conducted to compare the immunogenicity of YFE-1 and YFE-2E and to evaluate if a two-immunization regimen with a longer period of time between the primary and secondary immunizations could induce sufficient antibody responses in mice. In this study, BALB/c mice (10 per group) were administered IM 20, 10, 5, 2.5, and 1.25 µg/dose of YFE-1 or YFE-2E in the presence of 0.3% Alhydrogel on study days 0 and 28. Blood samples were collected prior to each immunization and 4 weeks after the second immunization (study days 0, 28, and 56). Serum samples from this study were evaluated for VN activity by PRNT as individual samples using a 96-well plate format and PRNT50 was expressed in mIU/mL (as described below). Both studies were performed by FhCMB in compliance with the U.S. Department of Agriculture’s Animal Welfare Act; the Guide for Care and Use of Laboratory Animals; and the National Institutes of Health, Office of Laboratory Animal Welfare. Whenever possible, procedures in this study were designed to avoid or minimize discomfort, distress and pain to animals. Study protocols were approved by the Institutional Animal Care and Use Committee at the University of Delaware (Newark, DE) under protocol number #1220.
Virus challenge studies in mice and NHPs.
The challenge studies in mice and NHPs were performed at Bio-Manguinhos, Fiocruz (Rio de Janeiro, Brazil). The animal ethical protocols were approved by the Institutional Committee of Animal Care and Experimentation (CEAU/FIOCRUZ, Rio de Janeiro, Brazil; protocols LW-45/11 for mice and LW-22/12 for NHPs).
For the mouse challenge study, groups of C57BL/6 mice (16 per group) (CEAU/FIOCRUZ) were inoculated with 5 µg of YFE-1 or YFE-2E IM with 0.3% Alhydrogel on study days 0 and 28. The dose of YFE-2E was prepared as described in the section “Immunogenicity assessments of YFE proteins in mice” in Materials and Methods. Animals in control groups received the 17DD vaccine (Bio-Manguinhos) IM at a dose of 2.74 log PFU/50 µL on study day 0 or phosphate buffered saline (PBS) IM on study days 0 and 28. All animals were challenged intracranially with 100 LD50 (2.78 log PFU/30 µL) of 17DD virus (grown in Vero cells at Bio-Manguinhos, Fiocruz from vaccine batch 993FB013Z) on study day 42.
For the NHP challenge study, a total of 20 captive-bred healthy rhesus monkeys (Macaca mulatta), 15 males and five females, weighing 4.12–7.62 kg, were obtained from the Primatology Division (CEAU/FIOCRUZ). All monkeys were shown to be free of YF and dengue serotypes 1, 2, 3 or 4 neutralizing antibodies as shown by PRNT before inoculation. The vaccinated monkeys (six per group) received 30 µg of YFE-1 or YFE-2E via the IM route three times on study days 0, 30, and 60 in the presence of 0.3% Alhydrogel. The dose of YFE-2E was prepared as described in the section “Immunogenicity assessments of YFE proteins in mice” in Materials and Methods. Four animals received the 17DD vaccine (Lot: 10UVFC032Z; Bio-Manguinhos) via the IM route at 4.81 log PFU/dose on study day 0 and 2 animals received a mock injection (20 mM Tris, 10 mM NaCl and 10% sucrose, pH 8.0 plus 0.3% Alhydrogel) on study days 0, 30, and 60. Two additional animals served as non-vaccinated (clean) controls. On study day 90, all animals were challenged subcutaneously with 5.85 PFU of 17DD virus per dose. The challenge studies in mice and NHPs were performed with 17DD because the studies were conducted in Rio de Janeiro, Brazil, where mosquito species, the urban vectors of YF virus, are prevalent and a BSL3 facility to work with wild type YF virus was not available.
Blood samples were collected on study days -1, 30, 60, 90, and 104 for analysis of VN antibody responses as described above, and for viremia as well as for interferon-γ (IFNγ) by enzyme-linked immunospot (ELISPOT) assay, as described below. In addition, anti-YFE antibody avidity was evaluated to further analyze the quality of the antibody responses in vaccinated NHPs.
Assessment of VN antibody responses in mouse and NHP sera from challenge studies.
Serum samples from the mouse challenge study were individually assessed for VN antibody responses in PRNT. Briefly, serum samples were heat-inactivated at 56°C for 30 minutes and serially diluted in PRNT diluent (M199 containing 5% FBS) in 96-well plates. An equal volume (50 µL) of 17D virus diluted to 600 PFU/mL was added to each plate except for cell control wells. The virus-serum mixtures were incubated for 1 hour at 37°C, after which 1.6 × 106 cells/mL of Vero cells were added to each well. After a 3-hour incubation at 37°C with 5% CO2, supernatants were removed and an overlay medium (PRNT diluent with 3% carboxymethylcellulose) was added. After a 6-day incubation at 37°C with 5% CO2, cells were fixed with 10% formaldehyde and stained with 0.04% crystal violet.
For samples from the NHP challenge study, the 6-well format of PRNT was used. Briefly, Vero cells were plated at 3.3 × 105 cells/mL into the 6-well cell culture plate 1 day before the assay. Serum samples were heat-inactivated at 56°C for 30 minutes and serially diluted in PRNT diluent in 24-well plates. An equal volume (0.5 mL) of 17D virus diluted to 150–200 PFU/mL was added to each plate except for cell control wells. The virus-serum mixtures were incubated for 1 hour at 37°C, after which supernatants were removed and an overlay medium (PRNT diluent with 3% carboxymethylcellulose) was added. After a 7-day incubation at 37°C with 5% CO2, cells were fixed and stained as described previously.
Plaques were counted under magnification and a PRNT50 value for each sample was calculated in mIU/mL using a standard antiserum with known international units (IU) as described elsewhere.53
Assessment of viremia in NHP sera after challenge infection.
Viral load in serum samples collected after challenge infection in NHPs was measured by both virus titration in Vero cells as described by Caufour et al.54 and real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) as described by Trindade et al.55 Data are expressed as log10 PFU/mL and log10 copies/mL, respectively.
Evaluation of antibody avidity.
Anti-YFE immunoglobulin G (IgG) avidity was measured by enzyme-linked immunosorbent assay (ELISA) as described by Matos et al.56 with slight modification. Briefly, plates were coated with 10 μg/mL of YF 17DD purified virus and serum samples serially diluted in PBS-Tween-20 (PBST) supplemented with bovine serum albumin were added to the plate. After 1-hour incubation at 37°C, the plates were washed with PBST and incubated with 100 µL of 3.0 M urea for 15 minutes. Control wells with serum samples were incubated with PBST without urea. Plates were then washed with PBST to remove both unbound antibodies and the chaotropic agent. The remaining bound IgG antibodies were detected using HRP-conjugated antimonkey IgG and tetramethylbenzidine as a substrate (Sigma-Aldrich, St. Louis, MO). The reaction was stopped with 1 M H2SO4. Absorbance was measured at 450 nm using a microplate reader (Sunrise™, Tecan, Germany).
The avidity index (AI) was calculated as the mean absorbance of reactions in which the antibodies were exposed to urea divided by the mean absorbance of reactions in nonurea control wells and expressed as a percentage. Samples with an AI of below 49% were considered as low avidity and samples with an AI of 50–79% or ≥ 80% were considered as medium or high avidity, respectively.
IFNγ ELISPOT assay.
The frequency of IFNγ secreting cells in peripheral blood mononuclear cells (PBMCs) from NHPs in vaccinated and control groups was analyzed using the ELISPOT assay as described elsewhere.57 Briefly, PBMCs were obtained using the Histopaque® density gradient (Sigma-Aldrich) according to the manufacturer’s suggestions and resuspended in RPMI 1640 containing 10% FBS after the lysis of residual red blood cells. The cell suspensions were plated into precoated IFNγ ELISPOT plates (Mabtech, Nacka Strand, Sweden) according to the manufacturer’s protocol and cultured for 20 hours in the presence or absence of 20 µg of YFE-1. After culture, cells were washed and incubated with a biotinylated anti-IFNγ antibody for 2 hours at room temperature (RT) followed by incubation with alkaline phosphatase-conjugated streptavidin for 1 hour at RT. The spots of IFNγ-secreting cells were visualized using the NBT/BCIP substrate and counted using the ImmunoSpot® image analyser (CTL, Cleveland, OH). The results are presented after the subtraction of background and compared using analysis of variance (GraphPad Prism 5.02). The cut-off value was determined as the mean spot number from all nonstimulated cells plus the standard deviation (125 spots/106 cells).
RESULTS
YFE subunit vaccine engineering, expression in N. benthamiana, purification, and characterization.
Four versions of YFE-based subunit vaccine candidates were engineered, produced in N. benthamiana plants and characterized: a stand-alone YFE protein (YFE-1) and three YFE-LicKM fusion variants, YFE-2E, YFE-3E, and YFE-4E, fused to the internal loop, C-terminus and N-terminus of LicKM, respectively. Schematics of the engineered constructs encoding the recombinant YFE proteins are shown in Figure 1.
Figure 1.
Yellow fever virus envelope protein (YFE) constructs schematics. YFE-1, stand-alone YFE protein; YFE-2E, YFE fusion to the surface loop of LicKM; YFE-3E, YFE fusion to the C-terminus of LicKM; and YFE-4E, YFE fusion to the N-terminus of LicKM. His = polyhistidine tag; KDEL = ER retention signal; LicKM = engineered lichenase; PR1a = signal peptide of tobacco pathogenesis-related protein 1a.
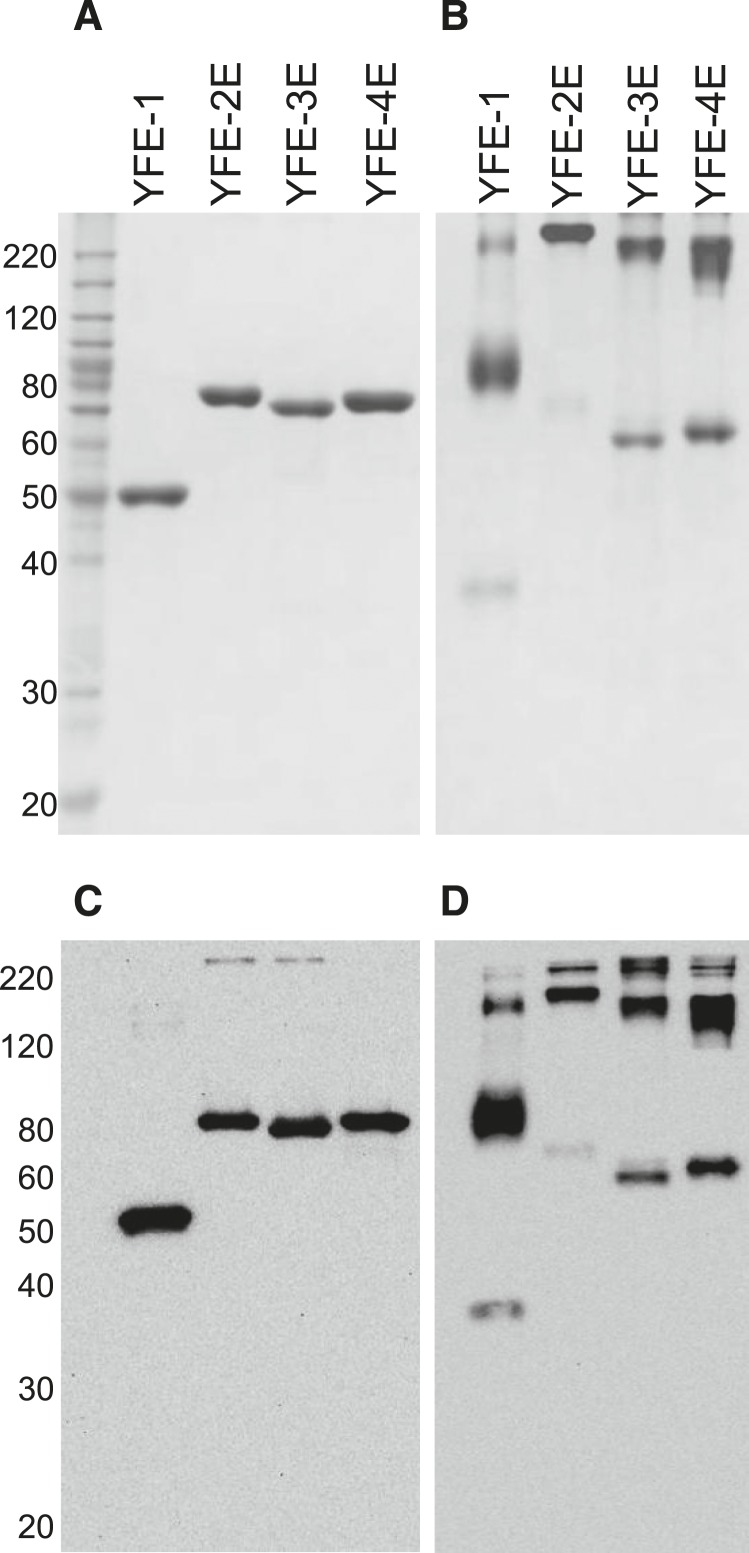
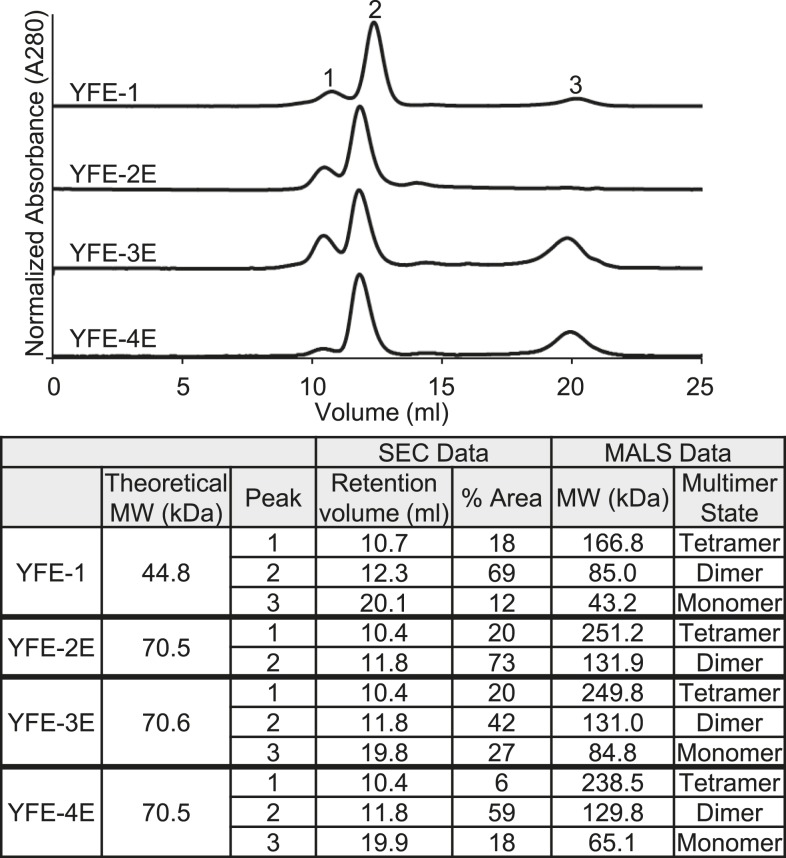
The YFE recombinant proteins were recovered using a three-step chromatography process with > 90% target purity as determined by reducing SDS-PAGE, with the purified proteins resolving as single bands of the expected MW of 45 kDa for YFE-1 and 71 kDa for YFE-2E, YFE-3E, and YFE-4E (Figure 2A). The identity of the proteins was confirmed by immunoblotting using a mAb specific to the poly-His tag (Figure 2C). Resolution of the proteins by nonreducing SDS-PAGE (Figure 2B) and immunoblotting (Figure 2D) revealed that all targets, YFE-1, YFE-2E, YFE-3E, and YFE-4E, resolved predominately at an increased MW, consistent with the presence of disulfide bond-dependent multimers. To determine the solution state of the purified proteins, SEC-MALS was performed using a Superdex 200 column. Consistent with the SDS-PAGE results, in all cases the YFE proteins resolved with a dimer being the predominant species (69% of peak area for YFE-1, 73% for YFE-2E, 45% for YFE-3E, and 59% for YFE-4E) (Figure 3), similar to the prefusion E protein found in the infected host. The MALS data were confirmed using nonreducing SDS-PAGE and immunoblot analysis of the SEC-fractionated proteins (not shown).
Figure 2.
Yellow fever virus envelope protein (YFE) recombinant proteins resolved under reducing and nonreducing conditions. Analysis of purified YFE proteins on reducing (A and C) and nonreducing (B and D) sodium dodecyl sulfate polyacrylamide gel electrophoresis. Resolved proteins (1 µg) were stained with Coomassie (A and B) or probed with an antipoly-His monoclonal antibody (mAb) (C and D). The expected molecular weight (MW) for YFE-1 is 45 and 71 kDa for YFE-2E, YFE-3E, and YFE-4E. BenchMark MW markers (Thermo Fisher) are shown in the left lane 1.
Figure 3.
Size exclusion chromatography multiangle light scattering (SEC-MALS) comparison of recombinant yellow fever virus envelope protein (YFE) proteins. YFE protein samples were analyzed using a Superdex 200 column by tandem 280 nm ultraviolet (UV) connected to a MALS detector. Upper panel, representative UV traces of resolved proteins. Lower panel, calculated MALS determination of protein molecular weight (MW) and multimer state. Peaks: 1- tetramer; 2- dimer; and 3- monomer.
Immunogenicity of four YFE subunit vaccines in mice.
The immunogenicity of the YFE vaccine candidates was evaluated in BALB/c mice in the presence of Alhydrogel adjuvant. Serum samples were pooled per group at each time point for evaluation of VN antibody response in the PRNT assay. After the primary immunization (day 0), VN antibodies were below the limit of detection (PRNT50 titer < 5) in animals that received YFE-3E or YFE-4E, regardless of the antigen dose (Table 1). Animals that received either 20 or 5 µg of YFE-1 or 20 µg of YFE-2E showed VN antibody responses just above the detection limit after the primary immunization (Table 1). PRNT50 titers in serum samples from animals after the second (day 41) and third (day 63) immunizations with YFE-1 or YFE-2E slightly increased, although immunization with 5 µg of YFE-2E did not elicit robust VN antibody responses even after the third immunization. PRNT50 titers in serum samples from animals that received YFE-3E or YFE-4E were either below the limit of detection or just above the detection limit of the assay throughout the study (Table 1). Based on the results of immunogenicity evaluation in mice, YFE-1 and YFE-2E were selected for further immunogenicity evaluation in mice and protective efficacy evaluation in mice and NHPs.
Table 1.
Virus neutralizing antibody responses (PRNT50 titers) in YFE-vaccinated mice
| Vaccine antigen (dose in µg) | PRNT50 titers | |||
|---|---|---|---|---|
| Day 0* | Day 20† | Day 41‡ | Day 63§ | |
| YFE-1 (20) | < 5 | 7.1 | 18.5 | 51.3 |
| YFE-1 (5) | < 5 | 5.2 | 17.3 | 34.2 |
| YFE-2E (20) | < 5 | 10.3 | 30 | 89 |
| YFE-2E (5) | < 5 | < 5 | 5.8 | 6 |
| YFE-3E (20) | < 5 | < 5 | < 5 | < 5 |
| YFE-3E (5) | < 5 | < 5 | < 5 | < 5 |
| YFE-4E (20) | < 5 | < 5 | < 5 | 5.2 |
| YFE-4E (5) | < 5 | < 5 | < 5 | < 5 |
| Saline | <5 | < 5 | < 5 | < 5 |
PRNT50 = plaque-reduction neutralization test; YFE = yellow fever virus envelope protein.
Pre-immune.
Post first dose.
Post second dose.
Post third dose.
VN antibody responses in mice immunized with YFE-1 or YFE-2E using a two-immunization regimen.
Two selected YFE subunit vaccines, YFE-1 and YFE-2E, were further evaluated for immunogenicity using a two dose, prime/boost regimen on study days 0 and 28. A range of antigen doses was tested to select an effective dose for the mouse challenge study. Four weeks after the primary immunization, the VN antibody values of the YFE-1 groups were significantly lower than those of the groups that received YFE-2E when groups that received the same antigen doses were compared, except for 10 µg/dose groups (Table 2). However, after the second immunization, no such statistically significant differences were observed when groups that received a same dose of YFE-1 versus YFE-2E were compared (Table 2). Interestingly, the group that received 5 µg of YFE-1 showed higher VN antibody values (P < 0.05) than the group that received the same dose of YFE-2E.
Table 2.
Geometric mean values of VN antibodies in mice immunized YFE-1 or YFE-2E
| Antigen | Dose of antigen | GM (mIU/mL) (95% CI) | |||
|---|---|---|---|---|---|
| Pre-immune | Day 28 | Day 56 | % Responder† | ||
| YFE-1 | 20 | 172 (122–282) | 464 (401–545) | 1,972 (1,094–5,017) | 80 |
| 10 | 92 (65–147) | 557 (466–681) | 2,031 (325–8,437) | 70 | |
| 5 | 123 (79–215) | 528 (458–620) | 1,865 (1,318–2,793)* | 100 | |
| 2.5 | 108 (72–173) | 351 (281–450) | 1,065 (772–1,585) | 70 | |
| 1.25 | 126 (86–204) | 239 (195–304) | 655 (367–1,279) | 30 | |
| 0 | 116 (72–201) | 161 (121–245) | 104 (81–134) | 0 | |
| YFE-2E | 20 | 88 (64–123) | 1,236 (671–2,373)** | 2,036 (1,666–2,584) | 100 |
| 10 | 191 (136–300) | 671 (533–859) | 1,267 (891–1,922) | 80 | |
| 5 | 92 (65–130) | 844 (712–1,056)** | 918 (656–1,451) | 60 | |
| 2.5 | 125 (95–172) | 537 (455–651)** | 921 (727–1,220) | 60 | |
| 1.25 | 131 (106–162) | 469 (287–876)* | 654 (520–866) | 30 | |
| 0 | 120 (137–312) | 117 (81–186) | 70 (70–70) | 0 | |
CI = confidence interval; GM = geometric mean; VN = virus neutralizing; YFE = yellow fever virus envelope protein.
P < 0.05 and **P < 0.01 when VN values of two group received same dose of YFE-1 vs. YFE-2E on the same study day (Mann–Whitney test).
Percent of animals showed VN antibody values over 794 mIU/mL on study day 56.
Immunization with YFE subunit vaccines partially protected mice against lethal viral challenge.
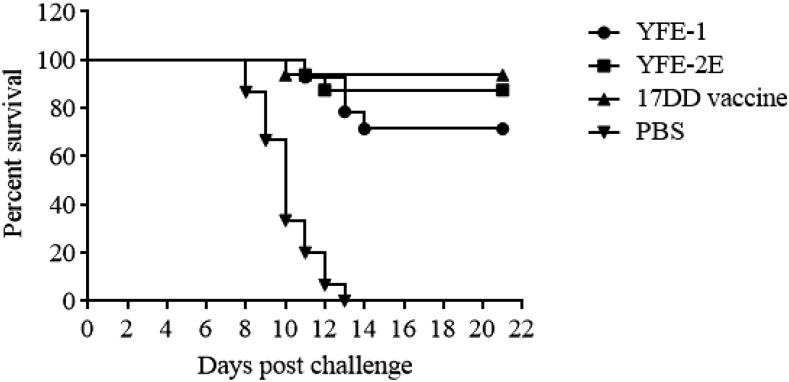
The protective efficacy of YFE-1 and YFE-2E was first evaluated in C57BL6 mice. After two immunizations with 5 µg of YFE-1 or YFE-2E plus Alhydrogel or one immunization with the 17DD live attenuated vaccine, animals were challenged intracranially with 100 LD50 of 17DD virus. Serum analysis showed that the primary immunization with YFE-1, YFE-2E or 17DD vaccine elicited VN antibody responses with geometric mean (GM) values of 470, 359, or 1,422, respectively (Table 3). The VN antibody responses in the YFE-1 group were enhanced by a second immunization and showed a GM value of 1,483 on the day of challenge (Table 3). By contrast, the VN antibody responses from the YFE-2E immunized group were not so enhanced by a second immunization showing a GM value of 459 on the day of challenge (Table 3). After a lethal challenge infection, 71% and 88% of animals immunized with YFE-1 or YFE-2E, respectively, and 94% of animals in the YF 17DD vaccine control group survived the lethal challenge infection (Figure 4). Although YF 17DD typically confers 100% protection, IM administration, as performed here, is not optimal for this vaccine. All negative control animals succumbed to the challenge infection by 13 days post-challenge (dpc) (Figure 4).
Table 3.
Virus neutralizing antibody responses (mIU/mL) in mice from challenge study
| Immunogen/vaccine formulation | Geometric mean PRNT50 value (mIU/mL) | ||
|---|---|---|---|
| Post first dose | Post second dose | Day of challenge | |
| YFE-1 5 µg/aluminum hydroxide | 470 | 722 | 1,483 |
| YFE-2E 5 µg/aluminum hydroxide | 359 | 778 | 459 |
| 17DD live attenuated vaccine | 1,422 | 950 | 905 |
| PBS | 243 | 165 | 385 |
PBS = phosphate-buffered saline; PRNT50 = plaque-reduction neutralization test; YFE = yellow fever virus envelope protein.
Figure 4.
Percent survival of mice after lethal challenge infection with yellow fever (YF) 17DD virus. Animals were observed for survival up to 3 weeks after challenge infection and the percent survival in each group was plotted.
Immunization with YFE subunit vaccines elicited VN antibody responses in NHPs and reduced viremia after viral challenge.
The protective efficacy of YFE-1 and YFE-2E, and VN serum antibody responses before and after challenge infection were also evaluated in Macaque monkeys. After the primary vaccination, on study day 30, all animals in the 17DD vaccine control group showed positive VN antibody responses with a mean (standard error of the mean, SEM) value of 13,028 (1,983) mIU/mL (Table 4). In other groups, on study day 30, VN antibody responses were low (Table 4). After the second and third vaccinations (study days 60 and 90), > 80% of animals that received YFE-1 or YFE-2E showed VN antibody responses with mean (SEM) values of 1,427 (729) or 1,534, respectively, on study day 60 and 3,316 (1,469) or 1,360 (287), respectively, on study day 90, whereas no positive VN antibody responses were observed in animals in the mock inoculated or clean control groups before the challenge infection (Table 4). The VN values in the YFE-2E group on study day 90 were significantly lower (P < 0.05, Kruskal-Wallis test) than those obtained in the group that received a single dose of the 17DD vaccine (Table 4). After the challenge infection on study day 104, all animals in all groups showed VN antibody responses with mean (SEM) values of 9,172 (2,770), 14,114 (8,054), 6,995 (3,995), 102,180 (10,136), and 16,366 (9,100) for the groups receiving YFE-1, YFE-2E, mock, 17DD, and nothing, respectively (Table 4).
Table 4.
Virus neutralizing antibody responses in sera of rhesus monkeys from challenge study
| Vaccine | PRNT50 value (mIU/mL) on study day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 30 | Day 60 | Day 90 | Day 104 | ||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| YFE-1 | 108 | 0 | 267* | 72.6 | 1,427** | 729 | 3,316 | 1,469 | 9,172* | 2,770 |
| YFE-2E | 108 | 0 | 192** | 8.4 | 1,534** | 266 | 1,360* | 286.6 | 14,114* | 8,054 |
| Mock | 108 | 0 | 199 | 20 | 170.6 | 11.9 | 162 | 0 | 6,995 | 3,995 |
| Clean | 108 | 0 | 199 | 20 | 170.6 | 11.9 | 161 | 0.8 | 16,366 | 9,100 |
| 17DD | 108 | 0 | 13,028 | 1,983 | 28,187 | 6,421 | 13,710 | 1,911 | 102,180 | 10,136 |
PRNT50 = plaque-reduction neutralization test; SEM = standard error of the mean; YFE = yellow fever virus envelope protein.
P < 0.05 and **P < 0.01 when YFE-1, YFE-2E, and 17DD groups were compared (Kruskal–Wallis test).
Viremia in serum samples collected after the challenge infection was also evaluated, using both virus titration and qRT-PCR. Samples with titers above the detection limit (0.4 log10 PFU/mL) or with ≥ 3.3 log10 copies/mL determined by qRT-PCR were considered positive. Viremia measured by virus titration (0.9 log10 PFU/mL) was observed at 3 and 4 dpc in one out of six (17%) animals in the group receiving YFE-1. In the group receiving YFE-2E, viremia (0.7 log10 PFU/mL) was observed at 3 dpc in one out of six (17%) animals. All animals in these two groups recovered from viremia by 5 dpc (Table 5). In contrast, one out of two (0.7 and 0.4 log10 PFU/mL on 3 and 5 dpc, respectively) and two out of two (0.4–1.4 log10 PFU/mL at 3–6 dpc) animals in the mock-vaccinated and clean control groups, respectively, showed viremia by virus titration. Viremia measured by qRT-PCR was observed at 2–10 dpc in two out of two (3.35–5.27 log10 copies/mL) animals in the clean control group. Only one out of two (50%) animals in the mock control group showed viremia by qRT-PCR at 4 dpc (3.55 log10 copies/mL, Table 5). Viremia by qRT-PCR was not observed in any animals in the YFE-1 and YFE-2E vaccinated groups. No viremia was detected by either of the methods in the 17DD vaccine group through 10 dpc (Table 5).
Table 5.
Viremia in NHPs after challenge infection
| Group | Vaccine | % Positive* (pos./tested) in qRT-PCR | % Positive† (pos./tested) in virus titration | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Days post challenge | Days post challenge | ||||||||
| 3 | 4 | 5 | 10 | 3 | 4 | 5 | 10 | ||
| 1 | YFE-1 | 0 (0/6) | 17 (1/6) | 0 (0/6) | 0 (0/6) | 17 (1/6) | 17 (1/6) | 0 (0/6) | 0 (0/6) |
| 2 | YFE-2E | 0 (0/6) | 0 (0/6) | 0 (0/6) | 0 (0/6) | 17 (1/6) | 0 (0/6) | 0 (0/6) | 0 (0/6) |
| 3 | Mock | 0 (0/2) | 50 (1/2) | 0 (0/2) | 0 (0/2) | 50 (1/2) | 50 (1/2) | 50 (1/2) | 0 (0/2) |
| 4 | Clean | 100 (2/2) | 100 (2/2) | 100 (2/2) | 50 (1/2) | 50 (1/2) | 100 (2/2) | 50 (1/2) | 0 (0/2) |
| 5 | 17DD | 0 (0/4) | 0 (0/4) | 0 (0/4) | 0 (0/4) | 0 (0/4) | 0 (0/4) | 0 (0/4) | 0 (0/4) |
NHP = nonhuman primates; PFU = plaque-forming units; qRT-PCR = real-time quantitative reverse transcription polymerase chain reaction; YFE = yellow fever virus envelope protein.
Above the detection limit = 0.4 log10 PFU/mL is considered positive.
Above 3.3 log10 copies/mL is considered positive.
YFE-specific IgG avidity in NHPs immunized with YFE subunit vaccines.
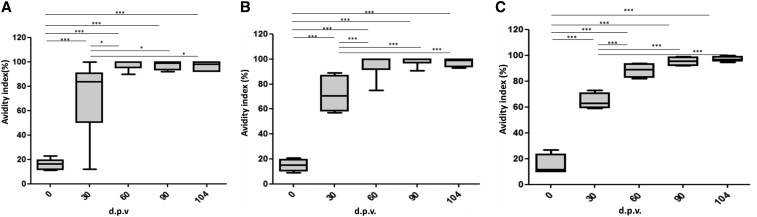
The YFE-specific IgG avidity was evaluated to further investigate the quality of antibody responses elicited by recombinant YFE vaccine candidates. There was an increased IgG avidity specific to YFE during the course of vaccination with YFE-1 or YFE-2E (Figure 5). More specifically, YFE-specific IgG avidity after the primary vaccination with YFE-1 ranged between low (AI < 50%), medium (50 ≤ AI < 80%), and high (AI ≥ 80), and these avidities increased to the highest level in all serum samples after the second and third vaccinations. In the serum samples from the group receiving YFE-2E, the IgG avidity increased to a medium-to-high level after the primary vaccination and the avidity in all serum samples reached the higher level after the second and third vaccinations. The avidity in sera from animals that received a single administration of the 17DD vaccine increased from medium on study day 30 to high on study day 90. The avidity in sera from the mock or clean animals remained low, even after challenge infection.
Figure 5.
Avidity index (AI) of YFE-specific NHP IgG after vaccination with YFE-1, YFE-2E, and YF 17DD. Groups of NHPs were immunized as indicated in the “Materials and Methods” section. Box plot graphs represent distribution of AI (%) for total anti-YFE IgG in serum from monkeys immunized with YFE-1 (A) YFE-2E, (B) YF 17DD, (C) and at different days postvaccination (d.p.v.). Error bars show outliers and horizontal bars represent means. Sera with AI lower than 49% are considered low avidity. Sera with AIs ranging from 50% to 75% are considered medium avidity and sera with AIs higher than 75% are considered high avidity. The means were compared using analysis of variance-Tukey post-hoc test. *P < 0.05, **P < 0.01, ***P < 0.001. IgG = immunoglobulin G; NHP = nonhuman primates; YFE = yellow fever virus envelope protein.
IFNγ production by PBMCs from NHPs immunized with YFE subunit vaccines.
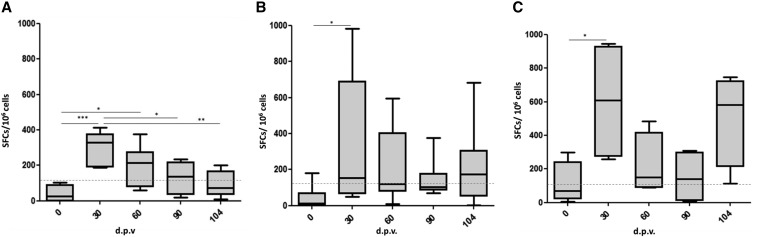
To evaluate the vaccine-induced cellular responses, the frequency of IFNγ-producing cells upon stimulation with YFE-1 was assessed using the ELISPOT assay. Thirty days after the first vaccination with YFE-1, YFE-2E, or YF 17DD, the numbers of IFNγ-secreting cells were significantly increased when compared with prevaccination samples. The second and third administrations of YFE-1 or YFE-2E did not increase the number of IFNγ-secreting cells. Increase in the number of IFNγ-secreting cells was also observed after the challenge infection in YFE-2E or YF 17DD immunized animals, although the differences were not statistically significant. The number of IFNγ-secreting cells in the group receiving YFE-1 gradually decreased after study day 30 and did not increase even after the challenge infection (Figure 6).
Figure 6.
Magnitude of YFE-specific cellular immune responses in NHPs immunized with YFE-1 (A), YFE-2E (B), or 17DD (C). Groups of NHPs were immunized as indicated in the “Materials and Methods” section. Box plots represent the distribution of interferon-γ-producing clones in response to the recombinant YFE protein in peripheral blood mononuclear cells of each animal (number of spot-forming cells [SFCs]/106 cells) at different days postvaccination (d.p.v.). Solid horizontal lines show means of each group and dotted horizontal lines represent the cut-off value. Statistical analysis was performed using analysis of variance-Tukey post-hoc test. *P < 0.05, **P < 0.01, ***P < 0.001. NHP = nonhuman primates; YFE = yellow fever virus envelope protein.
DISCUSSION
E proteins of flaviviruses play a critical role in the virus life cycle, including attachment to host cells, membrane fusion, assembly, and budding.12–14 Because of their important role in the virus life cycle and the presence of epitopes recognized by VN antibodies,58 the E proteins represent attractive targets for vaccine development. Using plants as a cost-effective and scalable platform for transient expression of vaccine antigens, we engineered and produced recombinant YFE as either a stand-alone antigen (YFE-1) or as fusions to LicKM (YFE-2E, YFE-3E and YFE-4E). LicKM is an engineered molecule based on a thermostable enzyme β-1,3-1,4-glucanase (lichenase) and has demonstrated advantages as a carrier, such as enhanced expression, stability, and immunogenicity of vaccine antigens fused to it.35,37,40,45,46 The additional advantage of LicKM as a fusion partner is its flexibility in engineering, as it has three potential sites to fuse target antigen, depending on size and structural characteristics of the molecules. In this study, we have engineered three variants of the YFE-LicKM fusion protein: 1) YFE-2E, with YFE inserted into the LicKM internal loop; 2) YFE-3E, with YFE fused to the C-terminus of LicKM; and 3) YFE-4E, with YFE fused to the N-terminus of LicKM. While all three YFE-LicKM fusion variants showed a similar size distribution of dimer, an immunogenicity study revealed that neither YFE-3E nor YFE-4E elicited VN antibodies in mice. Although biophysical characterization is required to elucidate the detailed mechanism underlying the observed weak immunogenicity of YFE-3E and YFE-4E, observation of different proportions of multimer species among YFE-LicKM fusion variants suggests that fusion to certain sites of LicKM may affect the multimeric state of YFE-LicKM fusion proteins and the overall immunogenicity of YFE antigens. Based on the results showing that YFE fused to the internal loop of LicKM (YFE-2E) had superior immunogenicity over the other two YFE-LicKM fusion variants, YFE-2E was selected for further evaluation along with the stand-alone target, YFE-1. Because the first mouse immunogenicity study was conducted with on a low and high antigen dose range and the fact that the PRNT was conducted using pooled antiserum per group without a standard antiserum, five different antigen doses were tested in a second mouse study to further compare the immunogenicity of YFE-1 and YFE-2E. In this study, a two-immunization regimen was also evaluated because VN antibody responses were not enhanced by a third vaccination in the first mouse immunogenicity study. We also wanted to evaluate the effect a longer interval between the first and second immunization may have on induction of VN antibody responses. In addition, the study was designed to allow PRNT assessment to be conducted on individual serum samples alongside a standard antiserum. In this study, immunization with YFE-2E induced superior VN antibody responses after the first immunization; however, VN antibody responses after the second immunization were comparable between groups, except at a 5 μg dose, where YFE-1 induced a superior response. This difference could be because of the different optimal dose and/or immunization regimen between stand-alone YFE (YFE-1) and a fusion protein to the internal loop of LicKM (YFE-2E).
To further investigate and compare YFE-1 and YFE-2E as potential YF vaccine antigens, mice were vaccinated with YFE-1 or YFE-2E and challenged with a lethal dose of 17D virus. In this study, mice were administered with two vaccinations, a prime and a boost, 4 weeks apart on study days 0 and 28 as tested in the second mouse study and, in fact, two vaccinations with YFE-1 or YFE-2E on study days 0 and 28 in the presence of Alhydrogel protected 71% and 88% of animals, respectively, from lethal challenge infection. A longer interval between the prime and boost doses in the presence of aluminum hydroxide adjuvant induced higher VN antibody responses in mice as was observed in a previous study with inactivated YF vaccine.59 In this article, the authors demonstrated that the inactivated YF vaccine is highly immunogenic in mice, hamsters, and cynomolgus macaques and after a single dose in hamsters and macaques; VN antibody titers were similar to those elicited by the live attenuated 17D vaccine.59 In our challenge study in mice, VN antibody responses in mice immunized with YFE-1 or YFE-2E were significantly lower than those observed in mice immunized with a single dose of 17DD live attenuated vaccine. The VN antibody responses were enhanced by a second dose in both YFE-1 and YFE-2E groups but to a greater magnitude in the YFE-1 immunized groups where the VN responses reached 1,483 mIU/mL on the day of challenge. These mouse immunogenicity studies used two mouse strains, BALB/c and C57BL/6, with different types of immune responses to evaluate the immunogenicity of the YFE subunit-based vaccines. BALB/c mice predominantly produce Th2 responses where C57BL/6 mice lean toward Th1-dominated immune responses.60,61 In both cases, a booster immunization with YFE-1 plus Alhydrogel enhanced VN antibody responses, whereas the increase in VN antibody responses in mice immunized with YFE-2E plus Alhydrogel were moderate to weak. Of note, in the C57BL/6 challenge study the VN antibody titer of the YFE-1 immunized group continued increasing after the second dose through to the day of challenge, potentially because of the depot effect of Alhydrogel adjuvant. However, the titer declined during this period for the YFE-2E immunized group that was also administered with Alhydrogel. The survival rate after the 17DD challenge infection of immunized C57BL/6 mice indicated that YFE-2E was superior to YFE-1, although the difference was not statistically significant. These differences observed between YFE-1-and YFE-2E immunized mice need to be further investigated to elucidate if the carrier protein, LicKM is involved in inducing different immune responses to the YFE antigen and/or a different folding of the YFE protein when expressed as a fusion molecule. So far, few studies have been published demonstrating in vitro authentic antigenicity of recombinant YFE proteins expressed in insect cells21,22 and only one study was published demonstrating immunogenicity and protective efficacy in mice of a soluble recombinant E protein expressed in insect cells.19 In this challenge study, mice were immunized with cell lysate containing the E protein and/or YF NS1, and the exact amount of the E protein in the vaccine preparation was not known. Thus, it is difficult to compare the results obtained in our study with the results from the literature that would allow us to hypothesize an impact of a carrier protein on the biophysical and immunological characteristics of the YFE protein. Furthermore, because Alhydrogel was the only adjuvant used in both the mouse immunogenicity and challenge studies described previously, additional in vivo studies using adjuvants with different mechanisms of action are necessary to provide a more comprehensive evaluation of the immunological profile of these recombinant YFE vaccines produced in plants.
Protective efficacy results generated in NHPs by vaccination with YFE-1 and YFE-2E are somewhat limited because of the nonlethal nature of the challenge infection and the small number of animals per group. However, over 75% of animals that were vaccinated with either antigen did seroconvert, although, even after three immunizations, VN antibody responses in these animals did not reach the level elicited by a single administration of 17DD live attenuated vaccine. Similar to what was discussed for the mouse immunogenicity studies, the use of other adjuvants could be beneficial in overcoming the weak immunogenicity seen with the Alhydrogel-based YFE subunit formulation when compared with a live attenuated vaccine. To further evaluate the immune responses induced by the YFE subunit-based vaccine candidates, we investigated the avidity of YFE-specific IgG elicited by YFE-1 or YFE-2E and demonstrated an increase in YFE-specific IgG avidity in a vaccination-dependent manner similar to that for the YF 17DD vaccinated group, reaching 100% AI before challenge. High avidity binding to the antigen is thought to contribute to antibody effector functions.62 Puschnik et al.63 reported that serum avidity correlates with neutralization capacity in the case of infection with the dengue virus, a related flavivirus to YF. In addition to the antibody responses, recent efforts to elucidate the mechanisms of protective immune responses elicited by YF 17D vaccines revealed the profound involvement of innate and cellular immune responses,64–66 particularly the role of IFNγ production in driving cellular and humoral responses against the attenuated virus in mouse and monkey models as well as in humans.67–69 Therefore, we have investigated IFNγ secretion by PBMCs from vaccinated NHPs to obtain preliminary data on cellular immune responses after vaccination with recombinant YFE antigens. We observed higher IFNγ responses in groups immunized with YF 17DD and YFE-2E when compared with YFE-1. These data suggest that YFE-2E is more efficient in inducing cellular immune responses than YFE-1, independent of the adjuvant used, in this case alum. Moreover, a higher survival rate in the mouse challenge study was observed in the YFE-2E vaccinated group despite the presence of relatively low VN antibody titers in the serum, indicating the importance of cellular immunity in the protective efficacy of a YF vaccine as described by Liu and Chambers.70 Detailed analysis of cytokine profiles and cellular immune responses and further investigation of alternative adjuvants that stimulate cellular as well as humoral immune responses will facilitate the further development of an optimal YF vaccine using recombinant YFE proteins.
In summary, recombinant YFE expressed as a stand-alone protein or as a fusion to a LicKM carrier molecule in N. benthamiana plants elicited VN antibody responses in mice and NHPs and protected over 70% of immunized mice from a lethal YF viral infection. These data indicate the potential of these YFE subunit antigens for use in the development of a noninfectious YF vaccine. However, considering that two or three immunizations of these YFE antigens in the presence of Alhydrogel in mice or NHPs, respectively, did not demonstrate equivalent immunogenicity and protective efficacy to a live attenuated 17DD vaccine, further investigation such as exploring different adjuvants, vaccine dose regimen, and detailed immunological evaluation are required before further development of a YFE-based subunit vaccine candidate produced in N. benthamiana plants.
Acknowledgments:
The following reagent was obtained through the NIH Biodefense and Emerging Infectious Research Resources Repository, NIAID, NIH: Yellow Fever Virus, 17D, NR-116. We thank the Instituto de Tecnologia em Imunobiológicos/Bio-Manguinhos/FIOCRUZ for continued interest and support to this work. We also thank Fernanda Rimolli de Castro Araujo and SheilaMaria Barbosa de Lima for the use of animal and virological facilities, respectively. We are grateful to Dr. Natasha Kushnir for editorial assistance.
REFERENCES
- 1.WHO , 2016. Yellow Fever Fact Sheet. Updated May 2016. Available at: http://www.who.int/mediacentre/factsheets/fs100/en/. Accessed February 2017.
- 2.WHO , 2017. WHO Prequalified Vaccines. Available at: https://extranet.who.int/gavi/PQ_Web/. Accessed February 2017.
- 3.Sanofi Pasteur , 2016. Yellow Fever Vaccine YF-VAX®. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM142831.pdf. Accessed February 2017.
- 4.Barrett ADT, 2016. Yellow fever in Angola and beyond—the problem of vaccine supply and demand. N Engl J Med 375: 301–303. [DOI] [PubMed] [Google Scholar]
- 5.Martins RM, et al. 2013. 17DD yellow fever vaccine: a double blind, randomized clinical trial of immunogenicity and safety on a dose-response study. Hum Vaccin Immunother 9: 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camacho LA, 2008. Yellow fever and public health in Brazil. Cad Saude Publica 24: 482–483. [DOI] [PubMed] [Google Scholar]
- 7.Campi-Azevedo AC, et al. 2014. Subdoses of 17DD yellow fever vaccine elicit equivalent virological/immunological kinetics timeline. BMC Infect Dis 14: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins Rde M, et al. 2014. Adverse events following yellow fever immunization: report and analysis of 67 neurological cases in Brazil. Vaccine 32: 6676–6682. [DOI] [PubMed] [Google Scholar]
- 9.Monath TP, 2012. Review of the risks and benefits of yellow fever vaccination including some new analyses. Expert Rev Vaccines 11: 427–448. [DOI] [PubMed] [Google Scholar]
- 10.Seligman SJ, 2014. Risk groups for yellow fever vaccine-associated viscerotropic disease (YEL-AVD). Vaccine 32: 5769–5775. [DOI] [PubMed] [Google Scholar]
- 11.Gubler DJ, Kuno G, Markoff L, 2007. Flaviviruses. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, ed. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins, 1153–1252. [Google Scholar]
- 12.Kaufmann B, Rossmann MG, 2011. Molecular mechanisms involved in the early steps of flavivirus cell entry. Microbes Infect 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smit JM, Moesker B, Rodenhuis-Zybert I, Wilschut J, 2011. Flavivirus cell entry and membrane fusion. Viruses 3: 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stiasny K, Heinz FX, 2006. Flavivirus membrane fusion. J Gen Virol 87: 2755–2766. [DOI] [PubMed] [Google Scholar]
- 15.Stiasny K, Fritz R, Pangerl K, Heinz FX, 2011. Molecular mechanisms of flavivirus membrane fusion. Amino Acids 41: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 16.Pierson TC, Diamond MS, 2008. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert Rev Mol Med 10: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS, 2008. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 4: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desprès P, Cahour A, Wychowski C, Girard M, Bouloy M, 1988. Expression of the yellow fever virus envelope protein using hybrid SV40/yellow fever viruses. Ann Inst Pasteur Virol 139: 59–67. [DOI] [PubMed] [Google Scholar]
- 19.Desprès P, Girard M, Bouloy M, 1991. Characterization of yellow fever virus proteins E and NS1 expressed in Vero and Spodoptera frugiperda cells. J Gen Virol 72: 1331–1342. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Linares A, Cahour A, Desprès P, Girard M, Bouloy M, 1989. Processing of yellow fever virus polyprotein: role of cellular proteases in maturation of the structural proteins. J Virol 63: 4199–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiu SY, Morikawa S, Buckley A, Higgs S, Karunakarannair V, Blachere C, Gould EA, 1991. 17D yellow fever vaccine virus envelope protein expressed by recombinant baculovirus is antigenically indistinguishable from authentic viral protein. J Gen Virol 72: 1451–1454. [DOI] [PubMed] [Google Scholar]
- 22.Barros MC, Galasso TG, Chaib AJ, Degallier N, Nagata T, Ribeiro BM, 2011. Yellow fever virus envelope protein expressed in insect cells is capable of syncytium formation in lepidopteran cells and could be used for immunodetection of YFV in human sera. Virol J 8: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mett V, Farrance CE, Green BJ, Yusibov V, 2008. Plants as biofactories. Biologicals 36: 354–358. [DOI] [PubMed] [Google Scholar]
- 24.Rybicki EP, 2010. Plant-made vaccines for humans and animals. Plant Biotechnol J 8: 620–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yusibov V, Streatfield SJ, Kushnir N, 2011. Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Hum Vaccin 7: 313–321. [DOI] [PubMed] [Google Scholar]
- 26.Gomord V, Faye L, 2004. Posttranslational modification of therapeutic proteins in plants. Curr Opin Plant Biol 7: 171–181. [DOI] [PubMed] [Google Scholar]
- 27.Bosch D, Castilho A, Loos A, Schots A, Steinkellner H, 2013. N-glycosylation of plant-produced recombinant proteins. Curr Pharm Des 19: 5503–5512. [DOI] [PubMed] [Google Scholar]
- 28.Gomord V, Fitchette AC, Menu-Bouaouiche L, Saint-Jore-Dupas C, Plasson C, Michaud D, Faye L, 2010. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol J 8: 564–587. [DOI] [PubMed] [Google Scholar]
- 29.Webster DE, Thomas MC, 2012. Post-translational modification of plant-made foreign proteins; glycosylation and beyond. Biotechnol Adv 30: 410–418. [DOI] [PubMed] [Google Scholar]
- 30.Mamedov T, Ghosh A, Jones RM, Mett V, Farrance CE, Musiychuk K, Horsey A, Yusibov V, 2012. Production of non-glycosylated recombinant proteins in Nicotiana benthamiana plants by co-expressing bacterial PNGase F. Plant Biotechnol J 10: 773–782. [DOI] [PubMed] [Google Scholar]
- 31.Shaaltiel Y, et al. 2007. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol J 5: 579–590. [DOI] [PubMed] [Google Scholar]
- 32.Traynor K, 2012. Taliglucerase alfa approved for Gaucher disease. Am J Health Syst Pharm 69: 1009. [DOI] [PubMed] [Google Scholar]
- 33.Yusibov V, Streatfield SJ, Kushnir N, Roy G, Padmanaban A, 2013. Hybrid viral vectors for vaccine and antibody production in plants. Curr Pharm Des 19: 5574–5586. [DOI] [PubMed] [Google Scholar]
- 34.Yusibov V, Rabindran S, 2008. Recent progress in the development of plant derived vaccines. Expert Rev Vaccines 7: 1173–1183. [DOI] [PubMed] [Google Scholar]
- 35.Musiychuk K, et al. 2007. A launch vector for the production of vaccine antigens in plants. Influenza Other Respir Viruses 1: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoji Y, et al. 2011. Plant-based rapid production of recombinant subunit hemagglutinin vaccines targeting H1N1 and H5N1 influenza. Hum Vaccin 7 (Suppl): 41–50. [DOI] [PubMed] [Google Scholar]
- 37.Chichester JA, Manceva SD, Rhee A, Coffin MV, Musiychuk K, Mett V, Shamloul M, Norikane J, Streatfield SJ, Yusibov V, 2013. A plant-produced protective antigen vaccine confers protection in rabbits against a lethal aerosolized challenge with Bacillus anthracis Ames spores. Hum Vaccin Immunother 9: 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chichester JA, Musiychuk K, Farrance CE, Mett V, Lyons J, Yusibov V, 2009. A single component two-valent LcrV-F1 vaccine protects non-human primates against pneumonic plague. Vaccine 27: 3471–3474. [DOI] [PubMed] [Google Scholar]
- 39.Mett V, Lyons J, Musiychuk K, Chichester JA, Brasil T, Couch R, Sherwood R, Palmer GA, Streatfield SJ, Yusibov V, 2007. A plant-produced plague vaccine candidate confers protection to monkeys. Vaccine 25: 3014–3017. [DOI] [PubMed] [Google Scholar]
- 40.Farrance CE, et al. 2011. Antibodies to plant-produced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. Hum Vaccin 7 (Suppl): 191–198. [DOI] [PubMed] [Google Scholar]
- 41.Jones RM, et al. 2015. A novel plant-produced Pfs25 fusion subunit vaccine induces long-lasting transmission blocking antibody responses. Hum Vaccin Immunother 11: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knapp EGL, et al. 2010. Tubulin-based vaccine candidates to combat African animal trypanosomiasis. Am J Trop Med Hyg 83 (Suppl): 206 (American Society of Tropical Medicine and Hygene 59th Annual Meeting. Abstract 691). [Google Scholar]
- 43.Chichester JA, Jones RM, Green BJ, Stow M, Miao F, Moonsammy G, Streatfield SJ, Yusibov V, 2012. Safety and immunogenicity of a plant-produced recombinant hemagglutinin-based influenza vaccine (HAI-05) derived from A/Indonesia/05/2005 (H5N1) influenza virus: a phase 1 randomized, double-blind, placebo-controlled, dose-escalation study in healthy adults. Viruses 4: 3227–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cummings JF, Guerrero ML, Moon JE, Waterman P, Nielsen RK, Jefferson S, Gross FL, Hancock K, Katz JM, Yusibov V, 2014. Safety and immunogenicity of a plant-produced recombinant monomer hemagglutinin-based influenza vaccine derived from influenza A (H1N1)pdm09 virus: a Phase 1 dose-escalation study in healthy adults. Vaccine 32: 2251–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chichester JA, Musiychuk K, de la Rosa P, Horsey A, Stevenson N, Ugulava N, Rabindran S, Palmer GA, Mett V, Yusibov V, 2007. Immunogenicity of a subunit vaccine against Bacillus anthracis. Vaccine 25: 3111–3114. [DOI] [PubMed] [Google Scholar]
- 46.Mett V, et al. 2008. A plant-produced influenza subunit vaccine protects ferrets against virus challenge. Influenza Other Respir Viruses 2: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoji Y, et al. 2009. Plant-derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine 27: 1087–1092. [DOI] [PubMed] [Google Scholar]
- 48.Shoji Y, et al. 2008. Plant-expressed HA as a seasonal influenza vaccine candidate. Vaccine 26: 2930–2934. [DOI] [PubMed] [Google Scholar]
- 49.Shoji Y, et al. 2009. Immunogenicity of hemagglutinin from A/Bar-headed Goose/Qinghai/1A/05 and A/Anhui/1/05 strains of H5N1 influenza viruses produced in Nicotiana benthamiana plants. Vaccine 27: 3467–3470. [DOI] [PubMed] [Google Scholar]
- 50.Shamloul M, Trusa J, Mett V, Yusibov V, 2014. Optimization and utilization of Agrobacterium-mediated transient protein production in Nicotiana. J Vis Exp 86: e51204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T, 1995. How to measure and predict the molar absorption coefficient of a protein. Protein Sci 4: 2411–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niedrig M, Lademann M, Emmerich P, Lafrenz M, 1999. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop Med Int Health 4: 867–871. [DOI] [PubMed] [Google Scholar]
- 53.Simoes M, Camacho LA, Yamamura AM, Miranda EH, Cajaraville AC, da Silva Freire M, 2012. Evaluation of accuracy and reliability of the plaque reduction neutralization test (micro-PRNT) in detection of yellow fever virus antibodies. Biologicals 40: 399–404. [DOI] [PubMed] [Google Scholar]
- 54.Caufour PS, Motta MC, Yamamura AM, Vazquez S, Ferreira II, Jabor AV, Bonaldo MC, Freire MS, Galler R, 2001. Construction, characterization and immunogenicity of recombinant yellow fever 17D-dengue type 2 viruses. Virus Res 79: 1–14. [DOI] [PubMed] [Google Scholar]
- 55.Trindade GF, Marchevsky RS, Fillipis AM, Nogueira RM, Bonaldo MC, Acero PC, Caride E, Freire MS, Galler R, 2008. Limited replication of yellow fever 17DD and 17D-dengue recombinant viruses in rhesus monkeys. An Acad Bras Cienc 80: 311–321. [DOI] [PubMed] [Google Scholar]
- 56.Matos DC, Silva AM, Neves PC, Martins RM, Homma A, Marcovistz R, 2009. Pattern of functional antibody activity against Haemophilus influenzae type B (Hib) in infants immunized with diphtheria-tetanus-pertussis/Hib Brazilian combination vaccine. Braz J Med Biol Res 42: 1242–1247. [DOI] [PubMed] [Google Scholar]
- 57.Santos AP, Matos DC, Bertho AL, Mendonca SC, Marcovistz R, 2008. Detection of Th1/Th2 cytokine signatures in yellow fever 17DD first-time vaccinees through ELISpot assay. Cytokine 42: 152–155. [DOI] [PubMed] [Google Scholar]
- 58.Dowd KA, Pierson TC, 2011. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology 411: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monath TP, et al. 2010. Inactivated yellow fever 17D vaccine: development and nonclinical safety, immunogenicity and protective activity. Vaccine 28: 3827–3840. [DOI] [PubMed] [Google Scholar]
- 60.Liu T, Matsuguchi T, Tsuboi N, Yajima T, Yoshikai Y, 2002. Differences in expression of toll-like receptors and their reactivities in dendritic cells in BALB/c and C57BL/6 mice. Infect Immun 70: 6638–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulte S, Sukhova GK, Libby P, 2008. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am J Pathol 172: 1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stiasny K, Aberle JH, Keller M, Grubeck-Loebenstein B, Heinz FX, 2012. Age affects quantity but not quality of antibody responses after vaccination with an inactivated flavivirus vaccine against tick-borne encephalitis. PLoS One 7: e34145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Puschnik A, Lau L, Cromwell EA, Balmaseda A, Zompi S, Harris E, 2013. Correlation between dengue-specific neutralizing antibodies and serum avidity in primary and secondary dengue virus 3 natural infections in humans. PLoS Negl Trop Dis 7: e2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barba-Spaeth G, Longman RS, Albert ML, Rice CM, 2005. Live attenuated yellow fever 17D infects human DCs and allows for presentation of endogenous and recombinant T cell epitopes. J Exp Med 202: 1179–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luiza-Silva M, et al. 2011. Cytokine signatures of innate and adaptive immunity in 17DD yellow fever vaccinated children and its association with the level of neutralizing antibody. J Infect Dis 204: 873–883. [DOI] [PubMed] [Google Scholar]
- 66.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B, 2006. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med 203: 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kohler S, Bethke N, Bothe M, Sommerick S, Frentsch M, Romagnani C, Niedrig M, Thiel A, 2012. The early cellular signatures of protective immunity induced by live viral vaccination. Eur J Immunol 42: 2363–2373. [DOI] [PubMed] [Google Scholar]
- 68.Neves PC, Rudersdorf RA, Galler R, Bonaldo MC, de Santana MG, Mudd PA, Martins MA, Rakasz EG, Wilson NA, Watkins DI, 2010. CD8+ gamma-delta TCR+ and CD4+ T cells produce IFN-gamma at 5–7 days after yellow fever vaccination in Indian rhesus macaques, before the induction of classical antigen-specific T cell responses. Vaccine 28: 8183–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neves PC, Santos JR, Tubarao LN, Bonaldo MC, Galler R, 2013. Early IFN-gamma production after YF 17D vaccine virus immunization in mice and its association with adaptive immune responses. PLoS One 8: e81953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu T, Chambers TJ, 2001. Yellow fever virus encephalitis: properties of the brain-associated T-cell response during virus clearance in normal and gamma interferon-deficient mice and requirement for CD4+ lymphocytes. J Virol 75: 2107–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]