Abstract.
Chagas disease has the highest prevalence of any parasitic disease in the Americas, affecting 6–7 million people. Conventional diagnosis requires a well-equipped laboratory with experienced personnel. The development of new diagnostic tools that are easy to use and adapted to the reality of affected populations and health systems is still a significant challenge. The main objective of this study was to measure Trypanosoma cruzi infection status using saliva samples of infected subjects. Blood and saliva samples from 20 T. cruzi-seropositive individuals and 10 controls were tested for T. cruzi infection using two different commercial serological tests. We have shown that detection of T. cruzi infection is possible using saliva samples, supporting the potential use of saliva to diagnose Chagas disease in humans. This method could provide a simple, low-cost but effective tool for the diagnosis of T. cruzi infection. Its noninvasive nature makes it particularly well suited for endemic areas.
INTRODUCTION
Chagas disease (CD) is a major public health problem in Latin America, affecting approximately 6–7 million people. In addition, travel and immigration patterns have increased the relevance of Trypanosoma cruzi infection outside of endemic areas.1–3 Trypanosoma cruzi can be transmitted to humans by reduviid insects that inhabit housing made of mud, thatch, and other natural materials in endemic areas4; by blood transfusion, organ transplant from infected donors,5 congenital route from mother to infant;6 and by ingestion of food or drink contaminated with infected triatomines.7
The diagnosis and management of CD still face multiple challenges. There are no preventive vaccines for human or veterinary use, and the two available treatments are not optimal as they are plagued by side effects and inconsistent efficiency.8–11 In addition, the diagnosis of this infection confronts several limitations. In the acute phase of the infection, diagnosis is based on the microscopic detection of trypomastigotes in blood. However, during the chronic phase of the infection, parasite persistence is low, and the detection of T. cruzi using this method is difficult and unreliable. Serology is the gold standard for T. cruzi infection diagnosis during the chronic phase of the disease. At least two different serological methods (preferably based on different antigens) are used to detect the presence of immunoglobulin G (IgG) antibodies against T. cruzi antigens. Currently, no single assay for the detection of chronic T. cruzi infection has shown enough sensitivity and specificity to be used alone for diagnosis.12
Chagas disease particularly affects poor rural and peri-urban areas of Latin America, where health-care access is limited. Developing new diagnostic tools which are easy to use and adapted to the reality of affected populations and health systems is still a substantial need.13 The purpose of this study is to address this challenge by exploring the detection of T. cruzi infection status using saliva samples.
MATERIALS AND METHODS
Ethics statement.
The study protocol was approved by the Ethics Committee of the Hospital Clinic of Barcelona and the Scientific Committee of the Barcelona Institute for Global Health (ISGlobal). Individual written informed consent was obtained from all study participants before the collection of samples.
Design and setting.
The study was designed as a pilot project to detect T. cruzi infection status using the saliva of infected individuals. A total of 20 T. cruzi-seropositive individuals were enrolled in one group (GA) and 10 T. cruzi-seronegative individuals in another group (GB). GB was included as a negative control group. GA patients were originally from Latin American countries where CD is endemic. Among them, 10 received benznidazole treatment between 2005 and 2015 (all of them completed the treatment regimen) and 10 of them did not receive treatment during the length of the study. GB subjects came from different areas of the world.
Selection of subjects.
Inclusion criteria: adult subjects (older than 18 and less than 50 years old); weight more than 40 kg, with serologic tests confirming or excluding T. cruzi infection. All the participants were recruited at the Center for International Health at the Hospital Clinic of Barcelona, Spain.
Participants.
Subjects of both genders aged between 18 and 50 years were included in the study (Table 1).
Table 1.
Age and gender of study participants
| Age | Gender | |
|---|---|---|
| GB | ||
| European controls | 39 | Female |
| 32 | Male | |
| 31 | Male | |
| 35 | Female | |
| 26 | Female | |
| Endemic country controls | 28 | Female |
| 43 | Male | |
| 30 | Female | |
| 39 | Male | |
| 28 | Female | |
| GA | ||
| Untreated patients | 38 | Female |
| 31 | Female | |
| 44 | Male | |
| 44 | Female | |
| 42 | Female | |
| 39 | Female | |
| 44 | Male | |
| 42 | Female | |
| 34 | Male | |
| 33 | Female | |
| Treated patients | 54 | Female |
| 46 | Female | |
| 43 | Female | |
| 50 | Female | |
| 33 | Female | |
| 49 | Female | |
| 40 | Female | |
| 48 | Female | |
| 50 | Female | |
| 41 | Female |
Sample collection and procedures.
Blood samples.
Per individual 10 mL blood sample was collected for diagnosis of T. cruzi infection in serum. The samples were centrifuged at 1,600 × g for 10 minutes at room temperature.
Saliva samples.
Subjects also provided 10 mL of unstimulated saliva samples. The saliva samples were centrifuged using ultrafiltration membranes (Amicon Ultra-15 devices) at 4,000 × g for 15 minutes at room temperature.
ELISA tests.
To perform the serological test, 300 µL of concentrated saliva samples and 10 µL of blood serum were used. Two different commercial serological tests were used: BioELISA CHAGAS (Biokit S.A., Lliçà d'Amunt, Barcelona, Spain) and DRG Trypanosoma cruzi IgG (DRG International Inc., Springfield, NJ).
Data analysis.
Statistical analysis was performed by analysis of variance and unpaired t test using the GraphPad PRISM 5.0 software.
RESULTS
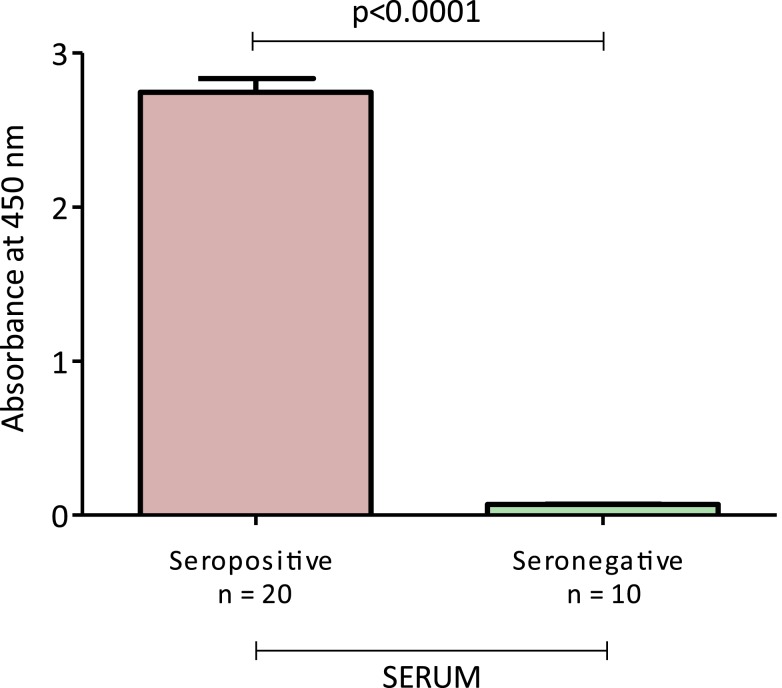
We first detected T. cruzi infection status with the current serological tests using enzyme-linked immunosorbent assay (ELISA) from Biokit. As expected, seropositive patients showed higher levels of IgG anti-T. cruzi antibodies compared with T. cruzi-seronegative subjects (Figure 1).
Figure 1.
Serum immunoglobulin G response to Trypanosoma cruzi antigen in T. cruzi-seropositive and T. cruzi-seronegative subjects. This figure appears in color at www.ajtmh.org.
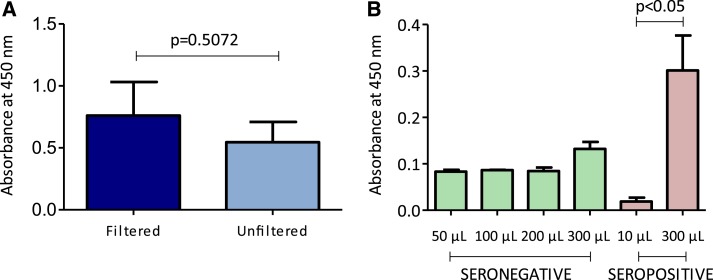
Before running ELISA on the saliva samples of T. cruzi-infected individuals, we tested whether a filtered and, therefore, a more concentrated saliva sample would exhibit higher sensitivity than an unfiltered sample. Filtered saliva samples showed higher levels of response to T. cruzi antigen than their unfiltered counterparts, although these differences were not statistically significant (Figure 2A). In addition, different volumes of filtered saliva samples were tested and the results showed higher sensitivity when using 300 μL compared with other volumes (Figure 2B).
Figure 2.
(A) Saliva immunoglobulin G (IgG) response to Trypanosoma cruzi antigen using ultrafiltration membranes. (B) Saliva IgG response to T. cruzi antigen in T. cruzi-seropositive and T. cruzi-seronegative subjects using different sample volumes. This figure appears in color at www.ajtmh.org.
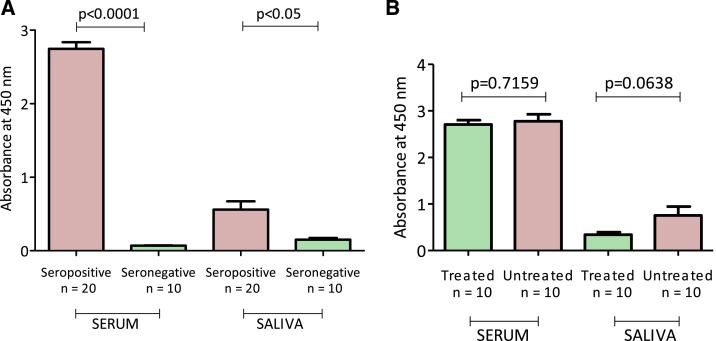
Then, we tested 20 T. cruzi-seropositive and 10 T. cruzi-seronegative serum and saliva samples using the same test. The results are summarized in Figure 3. Trypanosoma cruzi-seropositive subjects presented higher levels of IgG anti T. cruzi antibodies compared with T. cruzi-seronegative subjects, using serum or saliva samples (Figure 3A). The ELISA results demonstrated consistency between serum and saliva samples. The saliva test presented a specificity of 100%: all T. cruzi-seronegative individuals were negative using saliva as a sample. The sensitivity of the test was 70%: six of the 20 T. cruzi-seropositive individuals were negative using saliva as a source. Four of these subjects were previously treated with benznidazole and two of them were not previously treated. The ELISA’s cutoff value was computed by using the usual cutoff formula of the form “mean +3 standard deviation of negative controls.”
Figure 3.
(A) Serum and saliva immunoglobulin G (IgG) response to Trypanosoma cruzi antigen in T. cruzi-seropositive and T. cruzi-seronegative subjects. (B) Serum and saliva immunoglobulin G response to T. cruzi antigen in T. cruzi-seropositive subjects previously treated with benznidazole and T. cruzi-seropositive untreated subjects. This figure appears in color at www.ajtmh.org.
We also compared levels of T. cruzi antibodies for both saliva and serum of previously treated and untreated seropositive subjects. Differences in levels of serum IgG anti-T. cruzi antibodies were not found between treated and untreated individuals. Interestingly, we found differences in levels of T. cruzi antibodies between treated and untreated individuals using saliva (Figure 3B). However, these results were not statistically significant.
DISCUSSION
The most common method of diagnosing CD is detecting serum antibodies against parasite antigens mainly using ELISA tests. However, there are some limitations of using serum as a diagnostic sample. Blood collection is invasive, and it demands specially trained personnel.
Saliva has the potential to become a first-line diagnostic sample of choice. The use of this fluid for diagnostic purposes is increasing in popularity: it is easy to collect, store, and transport; it does not require highly trained personnel; and it is safer for medical staff to handle compared with other body fluids. In addition, it is noninvasive and the donation process is relatively stress free, minimizing donor discomfort. This can help improve access to diagnosis and treatment of people living in rural areas far from health-care centers. These characteristics make salivary diagnosis especially valuable for vulnerable populations. In addition, analysis of saliva may provide a cost-effective approach for the screening of large populations.14,15 Because of its many potential advantages, salivary diagnosis provides an attractive alternative to more invasive, time-consuming, complicated, and expensive diagnostic approaches.
Pinho et al.16 explored this area of research in 1999 for T. cruzi infection, showing that detection of T. cruzi infection was possible using saliva samples. Our data confirm these findings, and thus support saliva as a possible source for the detection of CD.
Although our results have shown consistency between serum and saliva samples of T. cruzi-seropositive and T. cruzi-seronegative subjects, the low sensitivity of the ELISA test using saliva as a source of diagnosis could present a challenge. It is common to find low concentrations of molecules in saliva compared with blood.15 This weakness could be partially overcome using ultrafiltration membranes to concentrate saliva samples.
Our results have shown differences in the levels of IgG T. cruzi antibodies between previously treated subjects and untreated patients. Presently, the gold standard for evaluating treatment efficacy is the seroconversion of conventional serological tests, which may take years to decades to assess.17 Although this is a preliminary finding, saliva could play an important role in evaluating treatment efficacy.
CONCLUSIONS
Trypanosoma cruzi-specific salivary IgG detection provides a simple, low-cost but effective tool in the diagnosis of Chagas disease. The noninvasive nature of this method makes it particularly well suited to endemic areas. The saliva test presented a specificity of 100%. Unfortunately, the sensitivity of the test was lower: 30% of T. cruzi-seropositive individuals were negative using saliva as a diagnostic sample.
In the future, saliva could play a key role in the diagnosis of T. cruzi infection and evaluation of treatment efficacy. Additional studies exploring other salivary molecules such as IgA anti-T. cruzi levels could be helpful because of the high concentration of this immunoglobulin in saliva. More research is needed with larger sample sizes to further investigate this method, which has the potential to revolutionize the diagnosis and treatment of Chagas disease.
REFERENCES
- 1.WHO , 2015. Chagas disease (American trypanosomiasis) Available at: http://www.who.int/mediacentre/factsheets/fs340/en/.
- 2.Bern C, Kjos S, Yabsley MJ, Montgomery SP, 2011. Trypanosoma cruzi and Chagas disease in the United States. Clin Microbiol Rev 24: 655–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gascon J, Bern C, Pinazo MJ, 2010. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 115: 22–27. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JE, Gurtler RE, 2001. Modeling household transmission of American trypanosomiasis. Science 293: 694–698. [DOI] [PubMed] [Google Scholar]
- 5.Young C, Losikoff P, Chawla A, Glasser L, Forman E, 2007. Transfusion-acquired Trypanosoma cruzi infection. Transfusion 47: 540–544. [DOI] [PubMed] [Google Scholar]
- 6.Gurtler RE, Segura EL, Cohen JE, 2003. Congenital transmission of Trypanosoma cruzi infection in Argentina. Emerg Infect Dis 9: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias JP, et al. 2008. Acute Chagas disease outbreak associated with oral transmission. Rev Soc Bras Med Trop 41: 296–300. [DOI] [PubMed] [Google Scholar]
- 8.Jackson Y, Alirol E, Getaz L, Wolff H, Combescure C, Chappuis F, 2010. Tolerance and safety of nifurtimox in patients with chronic chagas disease. Clin Infect Dis 51: 69–75. [DOI] [PubMed] [Google Scholar]
- 9.Pinazo MJ, Guerrero L, Posada E, Rodríguez E, Soy D, Gascon J, 2012. Benznidazole-related adverse drug reactions and their relationship to serum drug concentrations in patients with chronic chagas disease. Antimicrob Agents Chemother 57: 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Armenti A, 2009. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther 7: 157–163. [DOI] [PubMed] [Google Scholar]
- 11.Urbina JA, 2009. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115: 55–68. [DOI] [PubMed] [Google Scholar]
- 12.Sguassero Y, Cuesta CB, Roberts KN, Hicks E, Comandé D, Ciapponi A, Sosa-Estani S, 2015. Course of chronic Trypanosoma cruzi infection after treatment based on parasitological and serological tests: a systematic review of follow-up studies. PLoS One 10: e0139363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarleton RL, Reithinger R, Urbina JA, Kitron U, Gürtler RE, 2007. The challenges of Chagas disease—grim outlook or glimmer of hope? PLoS Med 4: e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C, 2011. Diagnostic potential of saliva: current state and future applications. Clin Chem 57: 675–687. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman E, Lamste I, 2002. The diagnostic applications of saliva. Crit Rev Oral Biol Med 13: 197–212. [DOI] [PubMed] [Google Scholar]
- 16.Pinho RT, Pedrosa RC, Costa-Martins P, Castello-Branco LRR, 1999. Saliva ELISA: a method for the diagnosis of chronic Chagas disease in endemic areas. Acta Trop 72: 31–38. [DOI] [PubMed] [Google Scholar]
- 17.Fabbro DL, Streiger ML, Arias ED, Bizai ML, del Barco M, Amicone NA, 2007. Trypanocide treatment among adults with chronic Chagas disease living in Santa Fe city (Argentina), over a mean follow-up of 21 years: parasitological, serological and clinical evolution. Rev Soc Bras Med Trop 40: 1–10. [DOI] [PubMed] [Google Scholar]