Abstract.
The approach to treatment of visceral leishmaniasis (VL)-HIV co-infection in East Africa has not been systematically examined. Although antiretroviral treatment (ART) should be initiated for all co-infected persons, the extent of ART prescription is not known. We conducted a retrospective cohort study including all VL–HIV co-infected adults at selected referral and district hospitals in northwest Ethiopia from 2010 to 2015. Purposes of the study were to compare the proportion of VL diagnoses made in previously diagnosed HIV-patients versus diagnosis concurrent with HIV diagnosis and to quantify utilization of ART. We included 112 patients and 58 patients at the referral and district hospital, respectively (median age: 30 years, 98% males). Of all VL cases, 56% (63/112) and 19% (11/58) occurred in known HIV patients at the referral and district hospital, respectively, with a median CD4 count at VL diagnosis of 45 cells/µL and 248 cells/µL at the referral and district hospital, respectively. Seventy-six percent (56/44) were on ART at VL diagnosis and nine (12%) started ART after VL diagnosis. The remaining 96 (56%) patients had both infections diagnosed concurrently, with a median CD4 count of 56 and 143 cells/µL at the referral and district hospital, respectively. Among cured patients, ART initiation was 67% and 36% at the referral and district hospital, respectively. A substantial proportion of VL–HIV cases occur while in HIV care, requiring further evaluation of preventive strategies. Among newly diagnosed VL–HIV co-infected patients, ART initiation was low. The reasons, including poor documentation and information exchange, should be assessed.
BACKGROUND
Visceral leishmaniasis (VL), also called kala-azar, is a vector-borne disseminated protozoan infection caused by the Leishmania donovani complex. Untreated, overt disease is universally lethal. Next to the Indian subcontinent, East Africa has the second highest VL burden. Although most of the Leishmania infections remain latent in immunocompetent individuals, HIV infection dramatically increases the risk of progression from asymptomatic Leishmania infection toward VL disease. VL in turn accelerates HIV disease progression.1 At the global level, Ethiopia has the highest burden of VL–HIV co-infection with 20% of VL patients having HIV, which is more than 10-fold the prevalence in the general population.2
Management of VL–HIV co-infection in East Africa remains unsatisfactory.3,4 Despite increased availability of antiretroviral treatment (ART) and anti-leishmanial treatment, the case fatality rate remains high. Up to 50% of patients fail to clear parasites from infected tissues with standard treatment; drug toxicity is more frequent and recurrent relapses are too common.1,3–5 Although some studies have focused on the performance of VL diagnostics or VL treatment response in VL–HIV co-infected patients, program operational aspects have been understudied.
Current guidelines recommend systematic HIV testing for all VL cases and initiation of ART for all co-infected patients, irrespective of the CD4 count.6,7 However, the impact of these guidelines largely depends on their effective implementation in routine settings. Initiation of ART might be particularly challenging in highly mobile groups such as migrant workers. There are, however, no studies that have looked into this matter within the East-African context. This is a vital issue as initiation of ART is probably one of the most important interventions needed to improve prognosis in VL–HIV co-infection.8
A complementary strategy to decrease the VL–HIV burden and associated mortality would be to prevent the onset of VL in HIV patients. With the global scale up of HIV services and ART, there are now large HIV-patient populations living in VL-endemic East African regions in HIV-care programs. Until the immune system of these patients has recovered on ART, they remain at higher risk of contracting VL and could indeed benefit from preventive strategies such as used for tuberculosis (TB) and cryptococcal infection in HIV-infected patients.9 However, there are presently no data on whether and to what extent VL is observed in individuals enrolled in HIV care in such regions or whether this risk is mitigated by ART use.
In a district hospital and a referral hospital in one of the major VL endemic areas in the northwest Ethiopia, we aimed to 1) assess the proportion and characteristics (CD4 count and ART use) of VL cases that occur in previously diagnosed HIV patients (diagnosed while in HIV care) versus those with VL and HIV diagnosed concurrently; and 2) for both groups, assess the proportion initiated on ART and cotrimoxazole secondary prophylaxis (CPT). The overall aim was to identify two potential areas for interventions to reduce VL–HIV case load and improve the associated prognosis.
METHODS
Study setting, design, and population.
We conducted a retrospective cohort study including all VL–HIV co-infected adults at Metema district hospital from 2010 to 2014 and at the Leishmaniasis Research and Treatment Center (LRTC) of the University of Gondar Hospital from 2010 to 2015. Purposes of the study were to compare the proportion of VL diagnoses made in previously diagnosed HIV patients versus diagnosis concurrent with HIV diagnosis and to quantify utilization of ART. Individuals lacking information on the timing of HIV diagnosis or VL diagnosis were excluded, as this precluded determining whether VL was diagnosed concurrently with HIV or while they were enrolled in HIV care.
Metema district hospital is located in a VL endemic region in northwest Ethiopia, at the border with Sudan. The district is known for producing cash crops, with a high number of seasonal migrant workers coming from the surrounding regions. The ART program has more than 1,500 people in active follow-up.
The University of Gondar hospital is a referral hospital and hosts the LRTC, which was established with the support of the Drugs for Neglected Diseases Initiative. In addition to being a clinical trial site for VL, this center is also the main referral and treatment site for leishmaniasis patients in northwest Ethiopia. HIV patients are linked to the HIV care clinic in the hospital or referred to health centers and district hospitals near the patients’ residence to facilitate close follow-up. In 2015, there were more than 5,000 HIV patients enrolled at the HIV care and ART clinic of the hospital.
Management of HIV and VL.
HIV management follows national guidelines with HIV diagnosed using two rapid diagnostic tests (KHB and STAT-PAK). Co-trimoxazole is systematically given and ART is initiated for those with CD4 counts below 350 cells/µL (during the study period) or WHO clinical stage 4 disease, which includes VL.
At Metema hospital, diagnosis of VL relies on a positive rK39 rapid diagnostic test in patients meeting the VL clinical case definition. However, at the Gondar referral hospital, VL diagnosis relies mainly on parasitological diagnostic methods. In patients with HIV, first-line VL diagnosis is conducted parasitologically with tissue aspiration (spleen or bone marrow). Treatment of VL–HIV co-infected patients essentially relies on liposomal amphotericine B (30 mg/kg). Miltefosine is added on a case by case basis, depending on availability and preferentially used for patients with a poor prognosis. This includes patients with a very high parasite load and/or a history of (multiple) relapses, where monotherapy with liposomal amphotericine B is often insufficient. However, because of the shortage of these medications, these are often reserved for more severe cases and most patients are still treated with parenteral antimonials. Until 2013, antimonials were given as monotherapy. Since then however, the national guidelines recommend antimonials in combination with parenteral paromomycin.
Data collection and analysis.
Co-infected patients were identified by reviewing the hospitalization register and the laboratory register. The charts of all VL–HIV co-infected patients were retrieved and reviewed. Information regarding HIV and VL management was extracted using a structured data collection format. Data extracted included date of HIV diagnosis, ART initiation, VL episodes; ART and VL treatment regimens; prescription of CPT; WHO clinical stage at HIV diagnosis, and CD4 cell count values collected over time.
The main outcome of interest was ART initiation, defined as documented proof that a patient was prescribed ART (i.e., documentation that an ART prescription had been written). In addition, information on CPT prescription was also retrieved because it is also a vital component of HIV care. CPT aims to prevent opportunistic infections such as Pneumocystis pneumonia, toxoplasmosis, diarrheal diseases, and skin infections. Individuals that, at the time the patient files were evaluated for the study, were not known to have died or to be transferred-out and without any documentation of follow-up at the hospital for the previous 6 months were defined lost to follow-up (LTFU). Data were entered using Excel (Microsoft Corporation, Redmond, WA). Descriptive statistics was done using Stata (StataCorp LP, College Station, TX).
Ethical clearance.
This study was approved by the Institutional Review Boards of Institute of Tropical Medicine, Antwerp, Belgium (UZA), and University of Gondar, Gondar, Ethiopia, for use of retrospective data. No variables identifying the personal identification were collected or used in the analysis. All data was handled and managed maintaining confidentiality at all levels of data handling.
RESULTS
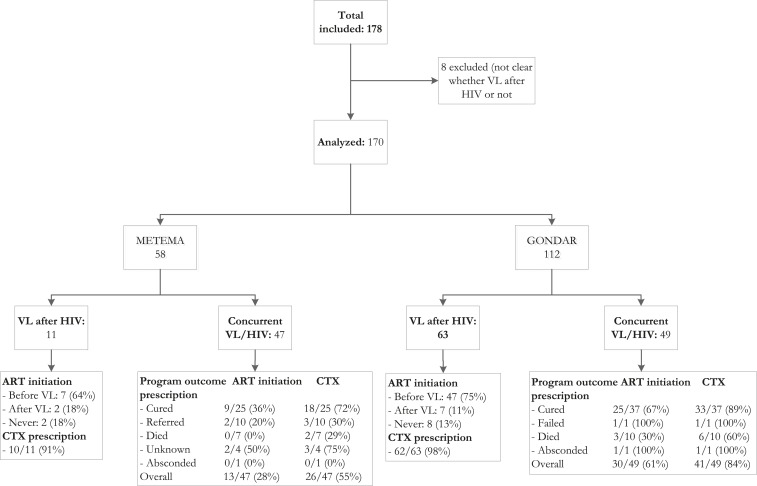
A total of 178 charts of VL–HIV co-infected adult patients were reviewed, of which eight were excluded due to incomplete data. Of the 170 included in analysis, 112 were from the Gondar referral hospital and 58 from the Metema district hospital. The vast majority (98%) were male adults, and the median age was 30 years (interquartile range 28–37), see Table 1. Antimonials were the most common drug used at both sites, but at the referral center, liposomal amphotericin B, paromomycin, and miltefosine were also available.
Table 1.
Baseline characteristics of VL–HIV co-infected patients diagnosed at the Metema district hospital (2010–2014) and the Gondar Leishmania research and treatment center (2010–2016), northwest Ethiopia
| Metema (N = 58) median (IQR) or n (%) | Gondar (N = 112) median (IQR) or n (%) | |
|---|---|---|
| Age, years; median (IQR) | 30 (27–36) | 32 (29–37) |
| Missing | 0 | 2 |
| Male sex, n (%) | 56 (98) | 109 (98) |
| Missing | 0 | 1 |
| TB at enrolment, n (%) | 7 (12) | 6 (5) |
| VL episode before study, n (%) | 6 (10) | 22 (18) |
| Total number of VL episodes during study period, n (%) | ||
| 1 | 52 (89) | 86 (70) |
| 2 | 5 (9) | 17 (14) |
| 3 or more | 1 (2) | 9 (7) |
| Treatment of current episode of VL, n (%) | ||
| SSG | 45 (78) | 55 (49) |
| SSG + PM | – | 11 (10) |
| AmBisome | 2 (3) | 26 (23) |
| AmBisome + MF | – | 12 (11) |
| Other | – | 7 (6) |
| UK | 11 (19) | 1 (1) |
| ART treatment, n (%) | 22 (78) | 84 (75) |
| TDF based | 17 (77) | 59 (70) |
| AZT based | 3 (14) | 17 (20 |
| D4T based | 2 (9) | 6 (7) |
| Other | 0 (0) | 2 (2) |
AZT = zidovudine; D4T = stavudine; IQR = interquartile range; MF = miltefosine; PM = paromomycin; SSG = sodium stibugluconate; TB = tuberculosis; TDF = tenofovir; UK = unknown; VL = visceral leishmaniasis.
The proportion of VL–HIV co-infected patients with a VL diagnosis while enrolled in HIV care was 56% (63/112) at the referral hospital and 19% (11/58) at the district hospital (Tables 2 and 3). In this patient group, the median CD4 count at VL diagnosis was 45 cells/µL at the referral hospital and 248 cells/µL at the district hospital. Ten (13%) of the patients died during admission (16% mortality at the referral hospital and zero at the district hospital). Overall, 73% (54/74) were on ART at the time of VL diagnosis, and additional nine (12%) started ART after VL diagnosis but there was no documented ART initiation for the remaining 10 (13%) patients. Overall ART initiation (before or after VL diagnosis) was high (87% at the referral hospital and 82% at the district hospital). CPT initiation was high at both sites, with 98% documented CPT initiation at the referral hospital and 91% at the district hospital (Figure 1).
Table 2.
Characteristics of VL–HIV co-infected patients diagnosed at the University of Gondar Leishmania center, northwest Ethiopia, 2010–2015 (N = 112)
| Variable | VL after HIV diagnosis (N = 63) median (IQR) or N (%) | Concurrent VL/HIV diagnosis (N = 49) median (IQR) or N (%) |
|---|---|---|
| Time from HIV to VL diagnosis (months); N = 37 | 10 (4–19) | NA |
| CD4 count at HIV diagnosis; cells/µL | 110 (48–177) | 84 (41–133) |
| Missing | 32 | 10 |
| CD4 count at VL diagnosis; cells/µL | 45 (30–95) | 56 (32–102) |
| Missing | 6 | 2 |
| Tuberculosis at HIV diagnosis | 3 (5%) | 3 (3%) |
| ART initiation | ||
| Never | 8 (13%) | 19 (39%) |
| Before VL | 48 (76%) | NA |
| Time between VL and ART (months) | 10 (5–24) | NA |
| CD4 count at HIV diagnosis; cells/µL; N = 23 | 100 (44–208) | NA |
| CD4 count at ART initiation; cells/µL; N = 31 | 45 (33–115) | NA |
| CD4 count at VL diagnosis; cells/µL; N = 42 | 44 (21–90) | NA |
| After VL* | 7 (11%) | 30 (60%) |
| Time between first VL and ART (days); | 84 (7–153) | 31 (19–80) |
| CD4 count at ART initiation; cells/µL | 111 (64–115) | 90 (49–125) |
| Missing | 2 | 1 |
| Outcome of first VL episode | ||
| Improved and discharged | 44 (70%) | 37 (75%) |
| Died | 10 (16%) | 10 (20%) |
| Failed | 4 (6%) | 1 (1%) |
| Referred | 1 (%) | 0 (0%) |
| Absconded | 3 (0%) | 1 (2%) |
| Unknown | 1 (%) | 0 (0%) |
| Relapse after first episode | 12 (19%) | 9 (18%) |
ART = antiretroviral treatment; IQR = interquartile range; VL = visceral leishmaniasis.
Table 3.
HIV-related characteristics of VL–HIV co-infected patients diagnosed at the Metema hospital, northwest Ethiopia, 2010–2014 (N = 58)
| Variable | VL after HIV diagnosis (N = 11) | Concurrent VL/HIV diagnosis (N = 47) (%) |
|---|---|---|
| Median (IQR) or N (%) | Median (IQR) or N | |
| Time from HIV to VL diagnosis (months) | 18 (6–32) | NA |
| CD4 count at HIV diagnosis; cells/µL | 346 (137–402) | 143 (59–334) |
| Missing | 1 | 16 |
| CD4 count at VL diagnosis; cells/µL | 248 (160–340) | 143 (59–333) |
| Missing | 3 | 16 |
| Tuberculosis at HIV diagnosis | 3 (27%) | 4 (9%) |
| ART initiation | ||
| Never | 2 (18%) | 34 (74%) |
| Before VL | 7 (64%) | NA |
| Time between VL and ART | 17 (3–37) | NA |
| CD4 count at HIV diagnosis; cells/µL | – | – |
| CD4 count at ART initiation; cells/µL (N = 3) | 239 (133–585) | – |
| CD4 count at VL diagnosis; cells/µL (N = 6) | – | – |
| After VL* | 2 (18%) | 13 (28%) |
| Time between first VL and ART (months); | 1.7; 31.8 | 2.0 (1.3–3.8) |
| CD4 count at ART initiation; cells/µL | 52; 183 | 102 (40–172) |
| Missing | 0 | 5 |
| Outcome of first VL episode | ||
| Improved and discharged | 5 (45%) | 25 (53%) |
| Died | 0 | 10 (21%) |
| Failed | 0 | 0 |
| Referred | 4 (36%) | 10 (21%) |
| Absconded | 0 | 1 (2%) |
| Unknown | 2 (18%) | 4 (8%) |
| Relapse after first episode | 1 (9%) | 4 (8%) |
ART = antiretroviral treatment; IQR = interquartile range; VL = visceral leishmaniasis.
If N ≤ 2, the values are given (i.e., no median is calculated).
Figure 1.
Flowchart displaying the VL–HIV co-infected patients included in the study at Metema district hospital and the University of Gondar Leishmania center, northwest Ethiopia. ART = antiretroviral treatment; CTX = cotrimoxazole prophylaxis; VL = visceral leishmaniasis.
The remaining 96 (56%) patients had both infections diagnosed concurrently, with a median CD4 count at VL diagnosis of 56 cells/µL at the referral hospital and 143 cells/µL at the district hospital. A total of 19 (20%) of these patients died during admission (20% at the referral hospital, 19% at the district hospital). Although at the referral hospital ART initiation was 67% among cured patients, only 36% had documented evidence for the start of ART at the district hospital. For the entire patient group with concurrent VL/HIV diagnosis, overall ART initiation was 61% at the referral hospital and 28% at the district hospital. At the district hospital, referrals were relatively common, and ART initiation was low in this group. Among cured patients, CPT initiation was 89% at the referral hospital and 72% at the district hospital.
DISCUSSION
This is one of the first studies evaluating ART initiation among VL–HIV co-infected patients in East Africa. A relatively high proportion of patients were diagnosed with VL while in HIV follow-up. In this patient group, ART initiation was good as most were on ART at VL diagnosis or started after VL diagnosis. This patient group provides opportunities for designing additional preventative strategies to be implemented during their routine HIV consultations. By contrast, ART initiation was low in patients with concurrent diagnosis of VL and HIV, especially at the district hospital. However, national and international guidelines clearly stipulate that all co-infected patients must be started on ART without delay as VL is considered an AIDS-defining condition.6
There might be many reasons behind the apparent low ART initiation in those with an unknown HIV status at the time of VL diagnosis. At the district level, this may mainly be related to the high rate of referral and substantial mortality in co-infected patients. Some referred cases are likely to have enrolled in the ART program at the referral hospital. The low ART initiation among VL-cured patients might be due to self-referral or undocumented referral after cure to other treatment centers for ART initiation. In this respect, it is important to highlight that, particularly at the district level, the HIV-care program deals with highly mobile populations, with substantial numbers of seasonal migrants. This could have contributed to the low ART initiation as they might have moved back to their place of origin to start ART. However, it could also be that they became LTFU and never received ART or they started ART after a long delay. Clearly, operational research evaluating the linkage and referral system appears to be indicated as better understanding of the underlying reasons would allow for corrective actions. At the referral hospital level, ART initiation was better. Nevertheless, the lack of ART initiation in 33% of VL-cured patients represents opportunities for improvement. Undocumented referral of patients, poor documentation practices or patients being lost to care might be involved and need emphasis for improvement.
For TB/HIV co-infection, TB program indicators have been put in place, measuring the extent of TB/HIV collaborative activities. This includes reporting on the proportion of TB patients starting ART within 8 weeks of TB diagnosis.10 A similar indicator would be considered for the VL program as a quality measure related to the needs of co-infected patients. But to monitor program performance, information on all VL–HIV co-infected patients should be available. Similar challenges have been observed for TB/HIV co-infection, especially when TB and ART services are not on the same premises.11 Good data collection systems have been shown to be required to document ART initiation in TB patients referred from the TB clinic.12,13 It would be worthwhile to conduct a follow-up study to determine how many of the patients LTFU or transferred-out to another ART center were ultimately initiated on ART.
We also aimed to quantify the proportion of VL that occurs among patients enrolled in HIV care. This is of particular interest as VL occurring in patients in regular HIV care might be preventable. Moreover, case fatality rates of this subgroup tended to be lower compared with patients diagnosed with HIV at the time of VL diagnosis, at least at the district hospital level. This may be related to early detection of VL as these patients are in medical follow-up or the fact that they were more likely to be on ART at the time of VL diagnosis. With the role-out of provider-initiated testing at every clinical encounter (hence not related to VL), the proportion of VL cases diagnosed in patients enrolled in HIV care is expected to increase further. Preventative strategies have been shown to be effective against opportunistic infections. A good example is isoniazid prophylactic therapy given to all HIV patients to prevent TB.11,14 Although this should be confirmed by a larger study, around 43% of the VL–HIV burden in our study occurred within the HIV cohort. At the same time, clinical prediction tools should be developed relying on clinical and laboratory markers to identify those at subsequent risk of VL. If sufficiently performant, this could constitute the basis for a screen and treat approach, as used for other opportunistic infections.9 Such a strategy would entail defining those at high risk of developing VL over the subsequent months and providing prophylactic therapy to prevent the progression to VL.
Many of the patients developing VL while in HIV care had low CD4 counts at the time of ART initiation and VL diagnosis, suggesting that advanced HIV disease and/or poor immune recovery on ART facilitated the progression toward VL. Obviously, early HIV diagnosis and ART initiation remain vital strategies as well.
This study was done by collecting data from two of the major VL treatment hospitals in northwest Ethiopia. Despite the intrinsic limitations of a retrospective study using routine data (e.g., missing data), important lessons can be learned from this study. First, it highlights the importance of documentation of referrals and linkage between HIV and VL programs. Second, VL, as TB, should be a disease that needs routine screening during the follow up of HIV patients in the endemic areas. Future studies should also look at ART maintenance therapy, beyond ART initiation, and investigate the causes for the low ART initiation.
In conclusion, this study identified a low rate of documented ART initiation among VL–HIV co-infected patients, especially at the district level and among patients that did not know their HIV status at VL diagnosis. Further studies are required to better understand the reasons behind this, but suboptimal documentation practices and the lack of systems to track ART initiation in referred cases are likely to contribute. In addition, we identified that a substantial proportion of VL–HIV cases occur among patients in regular follow-up for HIV care. Further studies are now indicated to assess whether accurate and reliable risk markers can be identified to target preventive strategies to those most at risk. In VL endemic areas, routine symptomatic screening for VL in HIV program merits further exploration.
REFERENCES
- 1.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP, Gradoni L, Ter Horst R, Lopez-Velez R, Moreno J, 2008. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev 21: 334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diro E, Lynen L, Ritmeijer K, Boelaert M, Hailu A, van Griensven J, 2014. Visceral Leishmaniasis and HIV coinfection in East Africa. PLoS Negl Trop Dis 8: e2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurissa Z, Gebre-Silassie S, Hailu W, Tefera T, Lalloo DG, Cuevas LE, Hailu A, 2010. Clinical characteristics and treatment outcome of patients with visceral leishmaniasis and HIV co-infection in northwest Ethiopia. Trop Med Int Health 15: 848–855. [DOI] [PubMed] [Google Scholar]
- 4.Ritmeijer K, ter Horst R, Chane S, Aderie EM, Piening T, Collin SM, Davidson RN, 2011. Limited effectiveness of high-dose liposomal amphotericin B (AmBisome) for treatment of visceral leishmaniasis in an Ethiopian population with high HIV prevalence. Clin Infect Dis 53: e152–e158. [DOI] [PubMed] [Google Scholar]
- 5.ter Horst R, Collin SM, Ritmeijer K, Bogale A, Davidson RN, 2008. Concordant HIV infection and visceral leishmaniasis in Ethiopia: the influence of antiretroviral treatment and other factors on outcome. Clin Infect Dis 46: 1702–1709. [DOI] [PubMed] [Google Scholar]
- 6.van Griensven J, Ritmeijer K, Lynen L, Diro E, 2014. Visceral leishmaniasis as an AIDS defining condition: towards consistency across WHO guidelines. PLoS Negl Trop Dis 8: e2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO , 2010. Control of the leishmaniasis. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March, 2010. WHO technical report series 949. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 8.van Griensven J, Carrillo E, Lopez-Velez R, Lynen L, Moreno J, 2014. Leishmaniasis in immunosuppressed individuals. Clin Microbiol Infect 20: 286–299. [DOI] [PubMed] [Google Scholar]
- 9.van Griensven J, Diro E, Lopez-Velez R, Ritmeijer K, Boelaert M, Zijlstra E, Hailu A, Lynen L, 2014. A screen and treat strategy targeting visceral leishmaniasis in HIV infected individuals in endemic east-African countries: the way forward? PLoS Negl Trop Dis 8: e3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO , 2015. A Guide to Monitoring and Evaluation for Collaborative TB/HIV Activities. 2015 revision.Geneva, Switzerland: World Health Organization. [Google Scholar]
- 11.WHO , 2012. WHO Policy on Collaborative TB/HIV Activities: Guidelines for National Programmes and Other Stakeholders. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 12.Tweya H, et al. 2016. Developing a point-of-care electronic medical record system for TB/HIV co-infected patients: experiences from Lighthouse Trust, Lilongwe, Malawi. BMC Res Notes 9: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard AA, Gasana M, Getahun H, Harries A, Lawn SD, Miller B, Nelson L, Sitienei J, Coggin WL, 2012. PEPFAR support for the scaling up of collaborative TB/HIV activities. J Acquir Immune Defic Syndr 60 (Suppl 3): S136–S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangaka MX, Wilkinson RJ, 2013. Isoniazid prevention of HIV-associated tuberculosis. Lancet Infect Dis 13: 825–827. [DOI] [PubMed] [Google Scholar]