Abstract.
Infection with the Rat Lungworm Angiostrongylus cantonensis is the leading cause of human eosinophilic meningoencephalitis worldwide. From its origins in southeastern Asia, the parasite was spread extensively throughout the twentieth century and is now established in many of the world’s warmer regions. Its clinical effects range from mild and transient symptoms, usually headache with peripheral nerve dysfunction, to severe and permanent central nervous system (CNS) damage, occasionally fatal. The severity and prognosis of disease are determined by the larval dose, acquired by ingesting infected intermediate hosts (slugs and snails) or, less often, paratenic hosts, such as crabs, shrimps, frogs, and monitor lizards. Early diagnosis is critical for treatment and depends on clinical suspicion, for laboratory confirmation from blood and cerebrospinal fluid can be delayed and unreliable. Treatment is fraught with difficulty, compounded by conflicting published results. Corticosteroids play a useful role in suppressing early CNS inflammation, but their duration for maintenance becomes problematic in severe infections. Because most of the pathogenesis results from host immuno-inflammatory responses to migrating and dead larvae in the CNS, anthelminthic therapy remains controversial: if effective, it kills viable larvae, arresting them in the CNS and so exacerbating the pathology. In human infections, it is now clear that many larvae do leave the CNS and reach the pulmonary arteries, sometimes with clinical consequences. Pioneering life-cycle studies in rats demonstrated a “subarachnoid phase” in larval development and migration; recent autopsy findings, outlined here, show it also occurs in humans and has some bearing on treatment. One new and four previously reported cases of human infection are analyzed here, with findings indicating that anthelminthic treatment is effective only when given early and should not be commenced beyond 3 weeks after exposure to infection. In endemic areas, treatment should start as soon as this infection is suspected, even without a clear history of exposure, given the unacceptable risks of waiting for diagnostic laboratory confirmation.
BACKGROUND
The leading cause worldwide of human eosinophilic meningoencephalitis (EME) is neuroangiostrongyliasis (NA), infection with the Rat Lungworm Angiostrongylus cantonensis which has been the subject of recent comprehensive reviews.1,2 From its presumed original focus in southeastern Asia, the parasite spread rapidly throughout the twentieth century, so that it is now endemic throughout the warmer parts of the Pacific basin, southwestern Asia, eastern Africa, South America, and around the Caribbean, including the southeastern United States. The management of human NA remains problematic, particularly regarding the use of anthelminthic agents, despite large numbers of published anecdotal case reports, which tend to be optimistic, as well as substantial clinical series studies. Our reappraisal of early experimental studies and recent human autopsy findings offer potentially useful guidelines. It seems that many clinicians compare EME with viral and bacterial infections, unaware that its pathogenesis is directly related to the number of third-stage larvae (L3) ingested, and that these worms do not multiply, but migrate, grow, and die in the central nervous system (CNS); some even proceed to the lungs. Most of the tissue damage results from host immuno-inflammatory reactions to degenerating parasites.
The life cycle.
The definitive hosts of A. cantonensis are various species of rats, mainly Rattus rattus and R. norvegicus, both originating in Asia and spread around the world by humans.3 Adult worms inhabit the right ventricle and pulmonary arteries; eggs released by females embolize into lung capillaries, where they embryonate. Hatched first-stage larvae (L1s) penetrate alveoli to be swept up the airways and swallowed, so that most are eliminated fecally. Intermediate hosts are molluscs, with many species of terrestrial and aquatic slugs and snails proving suitable in most endemic areas. They become infected by ingesting rat’s feces, although L1s might also invade through skin or gills, as probably happens with aquatic snails. Two molts lead to the L3, infective to rats that might eat the slug or snail, although paratenic hosts can intervene and include various crustaceans, amphibians, and even reptiles. Although all these can be sources of human infection, most result from ingesting undercooked slugs or snails. Contaminated snail mucus on raw leafy vegetables and L3s in drinking water are unlikely to cause heavy infection.
In rats, L3s (about 0.3 mm long) escape from ingested molluscan tissues and invade the gastrointestinal mucosa, to spread passively through all systems via the circulation. They can develop further only in the CNS, which they invade either directly, or via the peripheral nerves. Although this “neurotropism” probably reflects an ability to identify specific receptors in neural blood capillaries, it is not known how larvae travel along nerves. The life cycle was originally elucidated by Mackerras and Sandars in Brisbane, Australia, more than 60 years ago,4 before the parasite was generally recognized to be a human pathogen. (It was subsequently discovered that they had worked with the closely related Angiostrongylus mackerrasae,3 unknown at the time, and still not incriminated as a human pathogen, but the details are practically identical.) In rats, L3s start arriving in the CNS as early as 4 hours post infection (p.i.), and by 4 days, all surviving L3s are there. By 7 days p.i., these L3s have grown to almost 1 mm in length, when they molt to L4s, which continue “exploring the CNS landscape,” feeding and growing for another 5–6 days, after which they undergo their final molt. L5s (“immature adults”) develop further for a few more days before crawling out onto the surface of the brain or spinal cord, where they seek out large veins, such as one of the cerebral venous sinuses or veins leaving the spinal canal alongside spinal nerve roots.
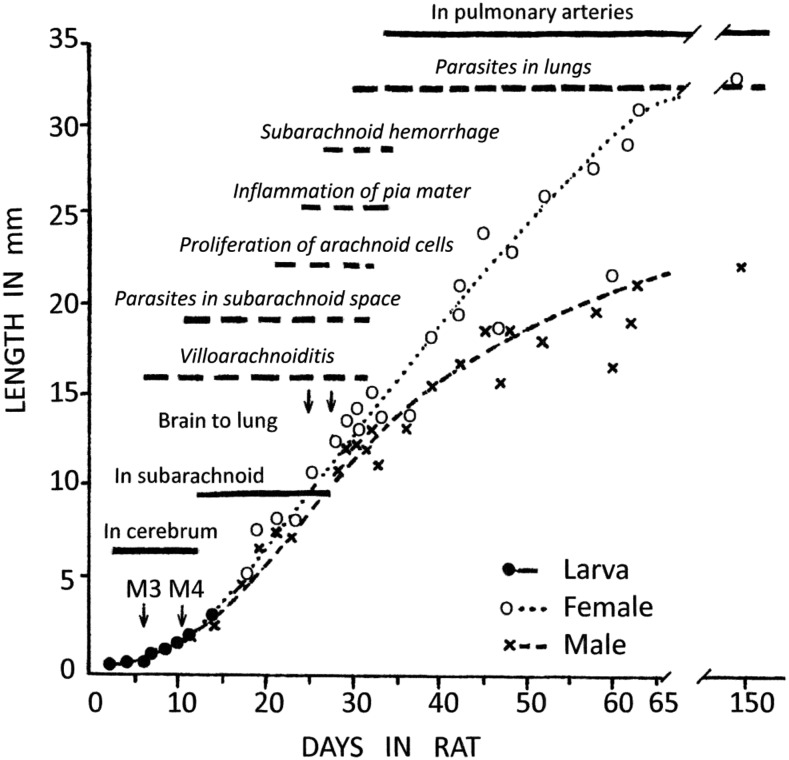
Mackerras and Sandars4 labeled this final CNS migration the “subarachnoid phase,” but critically, did not explain how their experimental rats were killed and processed. The growth rate of worms in the CNS was shown in their Figure 13 (reproduced here in Figure 1), where the “in subarachnoid” phase extends from day 12 to day 28 p.i., essentially the period beyond the 4th molt, followed immediately by “in the pulmonary arteries” (although the text indicates progressive transfer “for 4 days, the 28th to the 31st, the infection may be divided between brain and lungs”). Over this “subarachnoid” period, the worms grow from ∼3 mm length × 0.1 mm diameter to ∼15 × 0.2 mm, that is, a mass increase of 20-fold. Such remarkable growth requires copious nutrients, simply unattainable from cerebrospinal fluid (CSF), not even inflammatory CSF.
Figure 1.
Composite incorporating Figure 13 of Mackerras and Sandars4 (solid bars, normal text), depicting growth of Angiostrongylus cantonensis larvae in rat brain, with superimposed pathological findings from Figure 1 in Jindrak7 (broken bars with italicized text). M3 and M4 indicate the two larval molts in the central nervous system, beyond which the sexes are readily distinguishable; adult (L5) females grow faster and longer than males. Note the closely similar timing of both subarachnoid and pulmonary stages in both sources, given that the first study actually used A. mackerrasae.
This “subarachnoid” phase is an experimental artifact, for parasitic worms can migrate significant distances after host death.5 Tissue anoxia and acidosis ensue within minutes, stimulating viable nematode larvae to crawl actively, although even adult worms in guts can emerge from openings or invade surrounding structures (such as peritoneal cavity, liver, and kidneys). This has been well-known for the anisakids, indeed an essential feature of their life cycle,6 but rarely recognized for other species. An A. cantonensis L4 or L5 might traverse many mms in an hour under such conditions, and these worms can survive hours–days after host death. Such delays are practically unavoidable in parasitological studies, especially when large numbers of experimental animals are processed by one technician. The exponential growth of worms through the entire “subarachnoid phase” indicates that they had emerged post mortem, from a more nutritious, deeper substrate. These worms are exquisitely attuned to directions, and so could penetrate the venous system within hours of entering the subarachnoid space intra vitam. Furthermore, there is a significant pressure gradient from CSF to the central veins (even more so with inflammation), giving possibly critical hydrostatic assistance to migrating L5s that is absent in a dead host.
Jindrak’s7 detailed rat study, unequivocally using A. cantonensis, found larval development almost identical to that of Mackerras and Sandars4 study. He too showed, in his Figure 1 (with details incorporated into Figure 1 here), “parasites in the subarachnoid space” from days 10 to 32 p.i., although subarachnoid hemorrhage, which might be expected when L5s emerge from deeper tissues to seek out those big veins, did not appear until day 27 p.i., when the first worms were also found in the lungs. Occasional worms probably emerge into CSF sporadically, to return into parenchyma to continue their growth; hemorrhage would be noticeable only when large numbers do this in later development. Villoarachnoiditis was detected over days 6–28, probably in response to migrating larvae, whereas “proliferation of arachnoid cells” and “inflammation of the pia mater” did not begin until after day 21. Again, this is all consistent with premature, post-mortem larval migration into the subarachnoid space.
Human autopsy findings.
Because death from NA is rare, autopsy reports have been few, and usually not described in detail, often overlooking organs outside the CNS; A. cantonensis reaches the human lungs far more frequently than is generally recognized.3 Three documented cases from Australia are described, followed by two non-fatal cases for comparison.
Case 1.
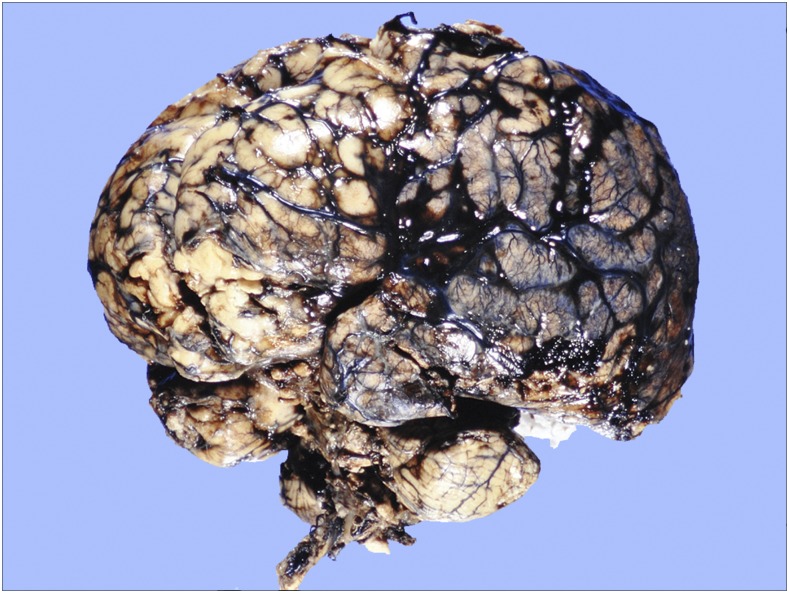
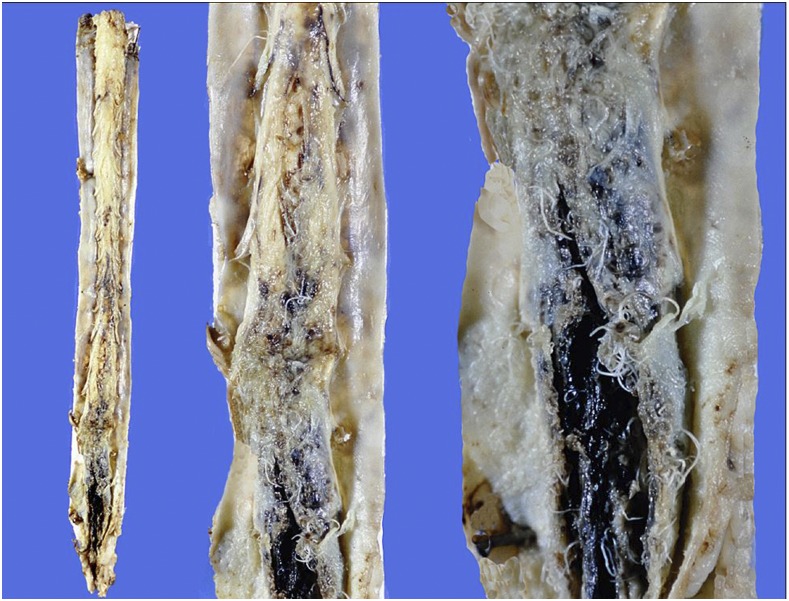
This was mentioned in an editorial,8 but not formally published, so more details are provided here. In January 1998, an 11-month-old boy was admitted to a Brisbane teaching hospital after 3 days of lethargy, malaise, rash, irritability, and lower-limb petechiae, with a provisional diagnosis of meningitis, although he was afebrile. Because his raised blood pressure (140/95 mm Hg) did not respond to routine treatment over 4 days, he was transferred to intensive care for a nitroprusside infusion. He became progressively obtunded, with reduced trunk and limb movements. A CT head scan, 9 days after admission, showed mild enlargement of the subarachnoid spaces over the frontal lobes, and CSF examination showed white cells 268 × 106/L (neutrophils 5%, eosinophils 35%, monocytes 60%); red cells 6 × 106/L; protein 1,410 mg/L (reference range, RR: 150–600 mg/L); and glucose 3.2 mmol/L (RR: 2.5–5.0 mmol/L). The peripheral blood eosinophil count was 2.7 × 109/L, up from 0.23 × 109/L at admission (RR: 0–0.7 × 109/L). Ten days after admission, he was alert but lethargic without spontaneous movements, generally hypotonic, areflexic, and with a poor gag reflex. A motile worm was seen in the vitreous of his left eye and removed at 18 days after admission: it was a 6.7 mm long, male L5 A. cantonensis (spicules 1.09 mm long). Serum enzyme immunoassay based on A. cantonensis antigens at this stage tested negative. The child’s clinical condition deteriorated, with urinary retention, priapism, diabetes insipidus, and respiratory failure requiring tracheal intubation and ventilation. Brain death was evident 23 days after admission, when a CT head scan showed generalized low density in the cerebral hemispheres, basal ganglia, and pons. Treatment was withdrawn the following day, and he died 30 minutes after extubation. Autopsy, performed 24 hours post mortem, revealed macroscopic abnormalities only in the central nervous and respiratory systems. In the cranium, the dura appeared normal, but basal meninges were thickened and discolored cream/gray. Patchy subarachnoid hemorrhages and vascular congestion were noted over the cerebral convexities, with generalized broadening of gyri and narrowing of sulci (Figure 2). Territories supplied by the middle and posterior cerebral arteries bilaterally appeared infarcted. The cerebellum also showed subarachnoid hemorrhage, with tonsillar herniation and necrosis. A few non-motile, white, threadlike worms, up to 13 mm long, were seen within the subarachnoid space of the convexities and of the brainstem. In the spinal canal, the meninges were adherent to the dura, with hundreds of worms seen in subdural and subarachnoid spaces predominantly of the lumbar and cauda equina regions (Figure 3). The caudal end of the thoracic spinal cord was expanded to approximately double its normal diameter, whereas the lumbar cord was necrotic. The internal vertebral venous plexus of lumbar and lower thoracic regions was congested.
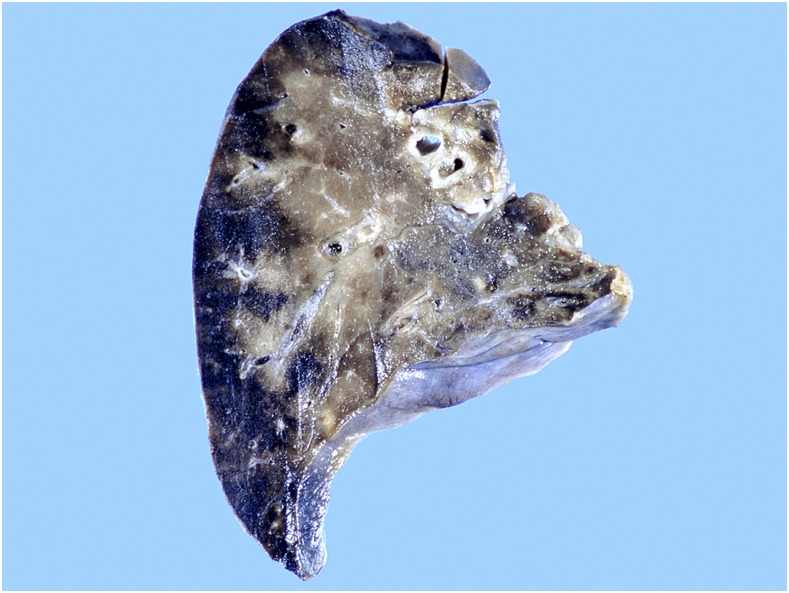
Figure 2.
Case 1: Intact brain, cerebellum, and brainstem after formalin fixation. Note vascular congestion and patchy subarachnoid hemorrhages over cerebral convexities, with generalized broadening of gyri and narrowing of sulci. Occasional worms were present on surfaces, but not evident at this magnification. This figure appears in color at www.ajtmh.org.
Figure 3.
Case 1: Triptych showing entire spinal cord (left) and increasing magnifications of cauda equina region (center and right). Note dense subarachnoid hemorrhage, with worms enmeshed among nerve roots and protruding into subdural space. This figure appears in color at www.ajtmh.org.
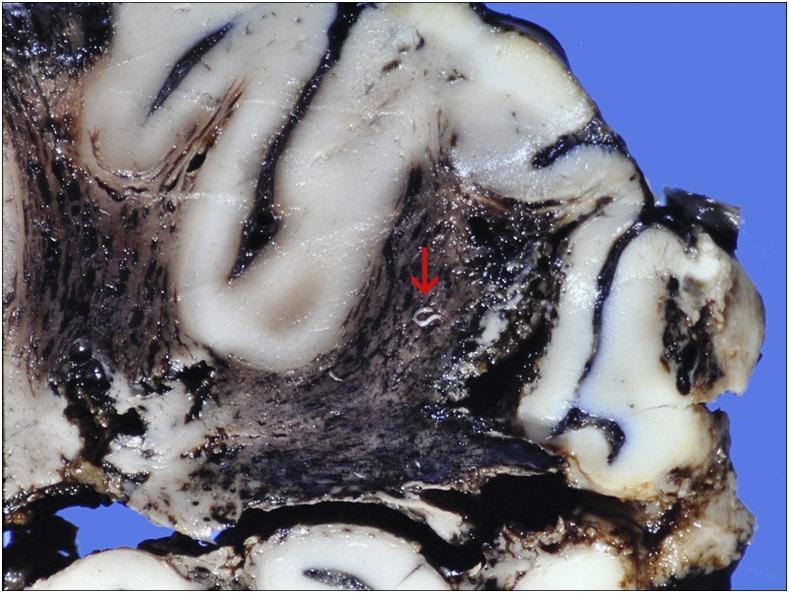
The brain weighed 1,022 g following fixation. Its cut surface showed hemorrhagic softening and expansion of deep white matter with sparing of the cortical ribbon in the temporal, parietal and occipital regions, and relative sparing of the frontal lobes. Focal linear hemorrhagic streaks extended into the cortex. Several worms protruded from the white matter (Figure 4). Microscopically, the meninges were diffusely infiltrated by lymphocytes, monocytes, and eosinophils, with occasional foreign-body–type giant cells. Virchow-Robin spaces were expanded by a similar infiltrate. Pial vessels were congested and occasionally contained degenerate worms with an associated vasculitis. Diffuse necrosis, with numerous intraparenchymal nematodes, was seen in the white matter supplied by the middle and posterior cerebral arteries. Vascular thrombosis was not seen. Most, but not all, of the worms found in the parenchyma and meninges were surrounded by mixed inflammatory cell infiltrates or occasional granulomatous foreign-body–type reactions. A degenerate worm was found in the posterior pituitary. Worms were densely packed in sections of the lumbar spinal cord and cauda equina (Figure 5). Twelve female worms extracted intact measured 9.7–14.7 mm in length and four males 7.6–12.6 mm.
Figure 4.
Case 1: Section of temporo-parietal region, showing hemorrhagic infarction of white matter and worms protruding from the cut surface (arrow at largest specimen). Cortex autolyzed. This figure appears in color at www.ajtmh.org.
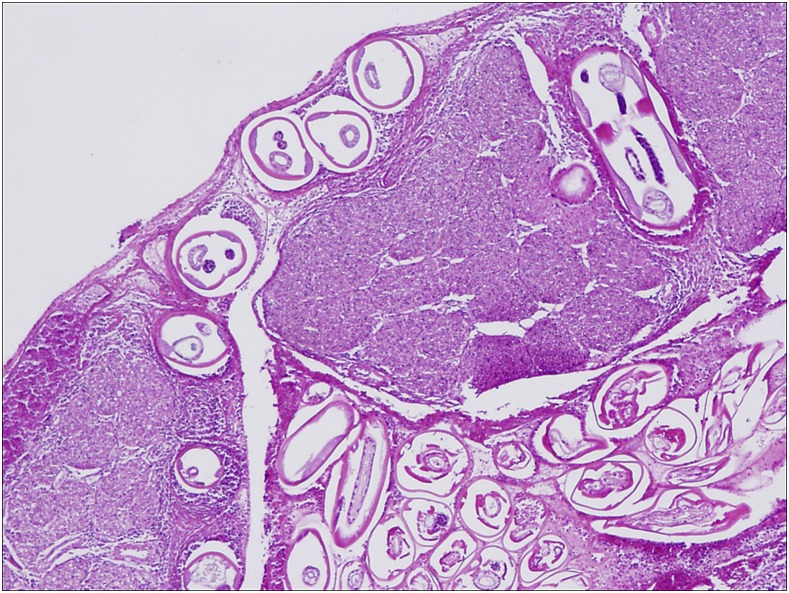
Figure 5.
Case 1: Cauda equina transverse section, high power (H&E stained) showing numerous worms in subarachnoid space and within nerve roots, with surrounding inflammatory response. This figure appears in color at www.ajtmh.org.
In the thorax, pleuritis involved the inferior and posterior aspects of both left and right lower lobes, without pleural effusions. The cut lung surfaces showed variable congestion, with a wedge-shaped hemorrhagic infarction in the right lower lobe (Figure 6). Numerous thrombi were seen within blood vessels in all lobes; microscopically, those in pulmonary arteries were found to contain large numbers of nematodes (Figure 7). Both vessel walls and thrombi were heavily infiltrated with mixed inflammatory cells, including numerous eosinophils and occasional Langhans cells. Secondary changes of pulmonary infarction, intra-alveolar hemorrhage and edema were present.
Figure 6.
Case 1: Formalin-fixed lobe of lung, exhibiting numerous thrombi, extensive congestion and peripheral hemorrhagic infarction. This figure appears in color at www.ajtmh.org.
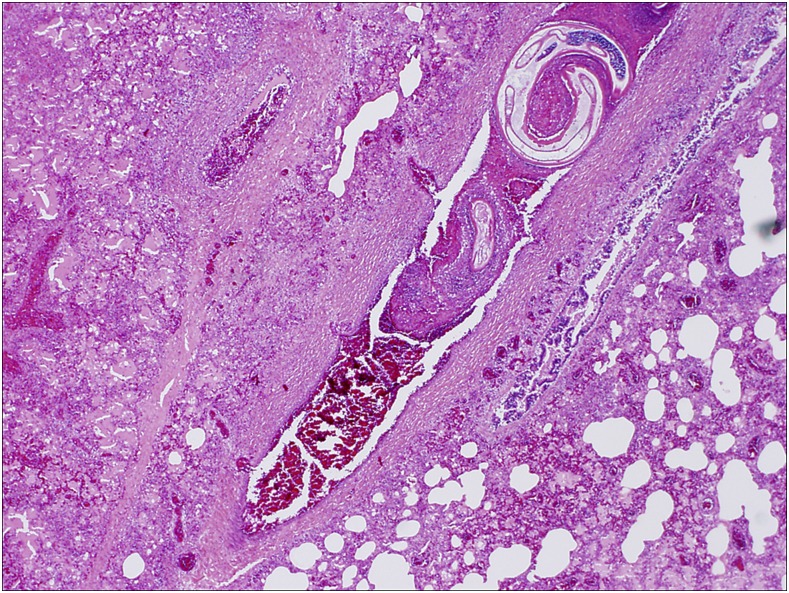
Figure 7.
Case 1: High power microscopic view of pulmonary section (H&E stained) showing worm within thrombosed pulmonary artery, surrounded by extensive congestion and inflammation. This figure appears in color at www.ajtmh.org.
The nematodes, some degenerate and others apparently viable, had all the morphological features of adult A. cantonensis. Many males contained developing spermatozoa in their testes, whereas females had oöcytes in their uteri; some even appeared to have copulated, with spermatozoa present. One female extracted from a pulmonary artery was 18 mm long (maximal diameter 0.30 mm), and one male was 12 mm long, with 1.33 mm spicules.
With hundreds of worms in his CNS, this child had died in the late “subarachnoid phase”; those on the surface would have migrated from deeper tissues post mortem, reaching even into the subdural space as a result of the 24-hour delay before autopsy. The anatomy of the cauda equina might present a bottleneck, with unsuitable veins for escape, perhaps explaining the intense clustering of worms in that region; this need not be a post-mortem artifact. At least as many were in the lungs, where some were alive and sexually active. Their size allowed an estimate of the infection’s duration. In experimental monkeys, 30 days p.i., L5s from the CNS are 4.8–9.8 mm long,9 whereas in rats, worms reach that size by day 20, growing to 12–18 mm by day 32.4 Assuming a comparable 10-day delay in humans, the timing of exposure for this child was narrowed to the last week of 1997. His parents recalled that on Boxing Day, they had found their son grubbing around in a large poolside plant pot, intently sucking his dirty fingers; they removed him and covered the soil with a damp towel. The towel was still in place after his death, covering clear imprints of his fingertips across the soil, as well as several small garden-snail shells (Helicarion species), almost certainly the source of his infection; this, indicated that his illness started about 10 days p.i., with demise on day 38.
Case 2.
Around the same time as in Case 1, another 11-month-old boy died in a Melbourne teaching hospital of NA acquired in Fiji.10 His initial presentation was with extensive spinal cord disease, undiagnosed but clinically responding to early IV methylprednisolone treatment. When this was stopped after 2 weeks, his condition deteriorated rapidly, with bulbar signs and respiratory compromise indicating brainstem involvement. Recommencement of steroids proved futile, the child dying 41 days after admission, that is, 47 days after the onset of illness (the source of infection was never identified), so at least 50 days after exposure. Autopsy showed extensive damage in the brain and spinal cord; microscopically, only dead worms were found in parts of his brainstem and cord, among zones of extensive inflammation; none was seen on the surface. The lungs were affected severely by arterial thrombosis, patchy infarction, and bronchopneumonia, and live and sexually mature adult worms were found throughout the pulmonary arteries. They were confirmed as A. cantonensis, with evidence of copulation but not egg release; sizes were not reported. The authors wrote “Had this infection been suspected, anthelmintic treatment would have been commenced in addition to steroids, even though there is a known risk of a reaction to dying parasites.”10
Case 3.
This involved a 10-month-old girl, admitted to a Sydney teaching hospital with suspected Guillain–Barré syndrome.11 The illness had started with lethargy and irritability 6 days pre-admission, then progressed gradually. High-dose corticosteroids were commenced on day 20 then albendazole was added (around 27 days after the onset of illness) after a brain biopsy showed eosinophilic vasculitis. Severe clinical deterioration prompted cessation of all treatment, and the girl died 27 days after admission (33 days after illness onset; the infection source was not identified). “At autopsy, numerous 10- to 12-mm threadlike organisms were visible macroscopically in the lumbar subdural space and over the cerebral surface and brainstem.”11 Microscopically, dead and live worms were found in the subarachnoid space and CNS parenchyma, with patchy, hemorrhagic menigitis in the brain and spinal cord. The published photograph of the cauda equina entangled in a mass of worms in the subdural space closely resembled the findings in Case 1 aforementioned. Worms “identified as A. cantonensis” were also found in the pulmonary arteries, with inflammation and infarction, but no more details were reported. The worms were similar in size to those in Case 1, suggesting this girl died about 37–40 days after exposure to infection, with about a week of incubation before her illness commenced.
Non-fatal human infections.
Case 4.
This was the first human infection to be reported from Sydney.12 A young man presented to a teaching hospital with a 3-day history of headache, nausea, vomiting, neck stiffness, and photophobia, 3 weeks after an acute gastrointestinal illness (nausea, abdominal cramps, diarrhea, myalgia, and fever) that had lasted a week. Eosinophilia was detected in his blood (1.6 × 109/L) but not CSF, and he was treated for suspected herpes simplex virus infection, although blood tests subsequently came back negative, as did serology for Strongyloides and Angiostrongylus. He was well enough to leave after 12 days, but then returned 5 days later with increasing headache and drowsiness. On admission, he was irritable, with gross bilateral papilledema. Blood eosinophils had increased to 3.1 × 109/L, and CSF taken by cisternal puncture was under high pressure and cloudy owing to numerous polymorphs (1,008 × 106/L), of which 90% were shown to be eosinophils by special staining. Magnetic resonance imaging (MRI) brain scan showed multiple lesions consistent with NA in deep white matter of both cerebral hemispheres and corpus callosum. It was only at this point that he was questioned specifically about ingesting slugs or snails, revealing that he had swallowed two live garden slugs, for a dare, 5 weeks previously, that is, just before his initial gastrointestinal disturbance. Repeat Angiostrongylus serology now tested positive. He was treated with repeated CSF drainage (once via cisternal puncture, then twice via lumbar puncture), acetazolamide, and dexamethasone, initially IV then orally, the latter continued at reducing doses for 4 weeks after discharge, with a good recovery. Anthelminthics were not used.
Respiratory involvement was not a feature in this case, suggesting the number of invasive larvae was not catastrophic and consistent with his satisfactory recovery.
Case 5.
This involved another young man from Sydney, but with devastating consequences.13 He had been admitted to hospital with a 3-day history of insomnia and lower-limb paresthesia, and provisionally diagnosed with Guillain–Barré syndrome. Blood on admission showed mild eosinophilia (0.5 × 109/L; normal < 0.4 × 109/L), but CSF was clear. His illness extended to autonomic involvement, and by the second week he was hallucinating, with fluctuating levels of consciousness. Repeat lumbar puncture showed CSF under high pressure (31 cm; normal < 20), with a raised protein level and 30 × 109/L “polymorphs,” although differential staining was not done. He was given empirical antibiotic and antiviral treatment, as well as IV hydrocortisone (100 mg four times daily). His condition deteriorated, “with a declining level of consciousness, progressive quadriparesis, and respiratory failure necessitating endotracheal intubation and mechanical ventilation on day 12 after admission.” His peripheral blood eosinophilia peaked at 1.9 × 109/L by day 24, and a third CSF sample, stained this time, showed eosinophils to comprise 37% of 504 × 109/L white cells. The fact that this man had eaten a large garden slug seems to have been considered only at this late stage, even though he had mentioned it on admission, to various attendants. CSF tested positive by enzyme immunoassay for anti-Angiostrongylus IgG, and a progress MRI on day 26 showed multiple focal lesions in his cerebral hemispheres, cerebellum, brain stem, and spinal cord, all consistent with NA. Given his dire condition, albendazole 400 mg twice daily was commenced, with continuing high dose steroid cover, and continued for 1 month, that is, from about 33 days after infection, until 60 days. This man survived, but in an almost vegetative state, albeit with very gradual improvement. He required a ventriculo-peritoneal shunt, suffered recurrent convulsions, and remained on a ventilator in intensive care for almost 15 months, before being transferred into rehabilitative care 22 months after admission, “where there has been ongoing gradual improvement.”13 Despite his respiratory failure and prolonged ventilation, pulmonary complications and chest radiology findings were not mentioned in the report.
IMPLICATIONS FOR TREATMENT
All three children (Cases 1–3), and the adult in case 5, suffered heavy infection, in which manifestations of CNS involvement often appear about a week after exposure. Could they have been saved by timely anthelminthic treatment? If most of the damage arises from tissue reactions to disintegrating worms, then killing the parasites very early should prove beneficial to the host, especially given that a large proportion of larvae are destined to die in the CNS anyway, after growing considerably in size. The pathology, comprising elements of eosinophilic radiculo-myelo-meningo-encephalitis, results from host inflammatory responses mediated by the immune system superimposed upon vascular and other trauma inflicted by migrating worms. Although some question the contributory role of eosinophils,14 their tissue-destructive capacity in other parasitic infections is well-recognized, such as in human eosinophilic enteritis caused by hookworm infections.15 Histologically, the finding of viable larvae in normal tissues indicates either that they have moved since the sample was taken (or the host died), or that intra vitam inflammation follows as a “condensation trail.” (The intense cluster found in the cauda equina in Cases 1 and 3 might have been in situ before the patients’ death, possibly reflecting an anatomical bottleneck.) However, dead larvae invariably are seen enmeshed within dense cellular infiltrates. A dead larva in the CNS will remain there until disposal by inflammatory-repair mechanisms, over months to years, depending on its size and extent of lesions induced, often irreversible. The longer anthelminthic treatment is delayed, the less its benefits, and the greater the potential detriment; growing numbers of naturally dead worms will be extending inflammation, whereas the dwindling numbers of viable larvae should be left alone to leave the CNS.
The “subarachnoid phase” ranges 10–32 p.i. in rats.7 It is considerably delayed in monkeys, but they seem even less suitable hosts for A. cantonensis than humans, for worms fail to reach their lungs.9 In us, the final molt occurs around 14 days p.i., then the 2–3 mm L5s double in length by day 20, and can persist in the CNS until day 40 p.i., as indicated by Cases 1–3.
An early study of anthelminthic treatment in experimentally infected mice (50 A. cantonensis L3s per mouse)16 found that 2–3 week courses of albendazole, commenced within the first 2 weeks of infection, could kill most or all invading larvae, whereas beyond 3 weeks p.i., treatment had little effect. There was no mention of adverse effects, even though some mice were treated for 3 weeks from day 20 p.i., and examined at 44 days p.i., when dead, large worms would have been in their CNS. If this is a valid model for human infections, it not only emphasizes the importance of treating as early as possible, but also dampens concerns about the negative effects of killing worms in the later subarachnoid stages. In Case 5, albendazole was not commenced until 33 days after exposure, when most worms would have already perished, whereas any survivors would have been on the verge of leaving the CNS (or perhaps already in the pulmonary arteries).Had these been killed by the late treatment, they might have added to the already extensive CNS damage. Chest X-ray findings were not reported, but would have included significant nonspecific abnormalities consistent with pulmonary invasion by adult worms.
All this indicates that anthelminthic therapy in human NA commencing beyond about 3 weeks p.i. will be futile at best or risks exacerbating the disease at worst. Corticosteroids effectively suppress eosinophilic inflammation, and so will alleviate some clinical manifestations, but in heavy infections will also delay resolution of inflammation and “disposal” of the worm carcases, whose continued presence will provoke ongoing damage; the steroids have to be withdrawn at some point.
The presupposition here, of course, is that the exposure event is known, which is rarely the case, and often requires meticulous fieldwork that can still prove futile. Sadly, in both adults (Cases 4 and 5), an opportunity was lost by not eliciting and/or following up on the clear history of garden-slug ingestion. An added difficulty is that early infection is impossible to diagnose confidently, given its nonspecific manifestations and inconclusive laboratory findings: blood and CSF eosinophilia can be erratic and/or delayed, whereas serology is unreliable and often slow to convert. By the time all test results are confirmatory, it is far too late to commence anthelminthic therapy. Given the dire prognosis of heavy infection, albendazole with steroid cover should be given to any patient in whom the list of provisional diagnoses includes NA. In Cases 1–3, had treatment been commenced on admission, before any laboratory evidence of NA could be available, there is a good chance all three children would be alive now, although possibly with varying degrees of neurological impairment. Cases 3 and 5 demonstrate a common scenario, with a provisional diagnosis of Guillain–Barré syndrome directing therapy. Urgent treatment of this condition is not critical, whereas in NA it might make a huge difference; perhaps all children (and adults?) in A. cantonensis-endemic areas presenting with suspected Guillain–Barré syndrome should be given albendazole routinely. In Case 3, albendazole was commenced only after the positive brain biopsy, probably around 27 days following infection, far too late for any beneficial effects, and possibly exacerbating neurological damage by killing worms on the verge of leaving the CNS. In endemic areas, any child seen to ingest a slug or snail should be offered albendazole prophylaxis immediately—in such a case, even a short course might prove highly effective. In another condition caused by nematode L3s in tissues, cutaneous larva migrans, a single dose of albendazole is often effective, with a 5-day course guaranteeing 100% success.17 The risk of mild side effects would be more than outweighed by the dire consequences of delayed treatment in NA.
Beyond the CNS, all three children, and probably the adult in Case 5, had severe pulmonary pathology. Killing the worms early in infection would have averted this, although in later stages, anthelminthics will probably be of little benefit, given the severity of CNS (and pulmonary) involvement. The adult worms in thrombosed pulmonary arteries were bound to die there, so accelerating this event would have made no difference to the clinical outcome in any case. It would be fascinating, biologically, to determine if A. cantonensis survives long enough in human lungs to produce patent infection; survivors of NA should be followed up with microscopy for the fecal passage of L3s.
Acknowledgments:
We are grateful to Dr. Christopher Burke, formerly Pediatric Neurologist at the Royal Children’s Hospital, Brisbane, for allowing us to include his patient (Case 1) in this report and to Dr. Les Hall for compiling Figure 1 from the originals.
REFERENCES
- 1.Wang Q-P, Lai D-H, Zhu X-Q, Chen X-G, Lun Z-R, 2008. Human angiostrongylosis. Lancet Infect Dis 8: 621–630. [DOI] [PubMed] [Google Scholar]
- 2.Barratt J, Chan D, Sandaradura I, Malik R, Spielman D, Lee R, Marriott D, Harkness J, Ellis J, Stark D, 2016. Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 143: 1087–1118. [DOI] [PubMed] [Google Scholar]
- 3.Prociv P, Spratt DM, Carlisle MS, 2000. Neuro-angiostrongyliasis: unresolved issues. Int J Parasitol 30: 1295–1303. [DOI] [PubMed] [Google Scholar]
- 4.Mackerras MJ, Sandars DF, 1955. The life history of the rat lung-worm, Angiostrongylus cantonensis (Chen) (Nematoda: Metastrongylidae). Aust J Zool 3: 1–21. [Google Scholar]
- 5.Prociv P, 1989. Observations on the post-mortem migration of nematode larvae and its role in tissue digesting techniques. J Helminthol 63: 281–287. [DOI] [PubMed] [Google Scholar]
- 6.Cipriani P, Acerra V, Bellisario B, Sbaraglia GL, Cheleschi R, Nascetti G, Mattiucci S, 2016. Larval migration of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae) in European anchovy, Engraulis encrasicolus: implications to seafood safety. Food Control 59: 148–157. [Google Scholar]
- 7.Jindrak K, 1970. The pathology of intracranial angiostrongylosis in rats. J Comp Pathol 80: 287–297. [DOI] [PubMed] [Google Scholar]
- 8.Prociv P, 1999. Eosinophilic meningitis—crossing paths with the rat lungworm, Angiostrongylus cantonensis. Med J Aust 170: 517–518.10397038 [Google Scholar]
- 9.Cross JH, 1979. Experimental studies on Angiostrongylus species and strains in monkeys and laboratory animals. Studies on Angiostrongyliasis in Eastern Asia and Australia Taipei, Taiwan: US Naval Medical Research Unit No. 2, 118–137. [Google Scholar]
- 10.Cooke-Yarborough CM, Kornberg AJ, Hogg GG, Spratt DM, Forsyth JRL, 1999. A fatal case of angiostrongyliasis in an 11-month-old infant. Med J Aust 170: 541–543. [DOI] [PubMed] [Google Scholar]
- 11.Morton NJ, Britton P, Palasanthiran P, Bye A, Sugo E, Kesson A, Ardern-Holmes S, Snelling TL, 2013. Severe hemorrhagic meningoencephalitis due to Angiostrongylus cantonensis among young children in Sydney, Australia. Clin Infect Dis 57: 1158–1161. [DOI] [PubMed] [Google Scholar]
- 12.Senanayake SN, Pryor DS, Walker J, Konecny P, 2003. First report of human angiostrongyliasis acquired in Sydney. Med J Aust 179: 430–431. [DOI] [PubMed] [Google Scholar]
- 13.Blair NF, Orr CF, Delaney AP, Herkes GK, 2013. Angiostrongylus meningoencephalitis: survival from minimally conscious state to rehabilitation. Med J Aust 198: 440–442. [DOI] [PubMed] [Google Scholar]
- 14.Gosnell WL, Kramer KJ, 2013. The role of eosinophils in angiostrongyliasis: multiple roles for a versatile cell? Hawaii J Med Public Health 72 (Suppl 2): 49–51. [PMC free article] [PubMed] [Google Scholar]
- 15.Prociv P, 1997. Pathogenesis of human hookworm infection: insights from a “new” zoonosis. Freedman DO, ed. Chemical Immunology: Immunopathogenetic Aspects of Disease induced by Helminth Parasites Basel, Switzerland: Karger AG, S, 62–98. [DOI] [PubMed] [Google Scholar]
- 16.Hwang KP, Chen ER, 1988. Larvicidal effect of albendazole against Angiostrongylus cantonensis in mice. Am J Trop Med Hyg 39: 191–195. [DOI] [PubMed] [Google Scholar]
- 17.Caumes E, 2000. Treatment of cutaneous larva migrans. Clin Infect Dis 30: 811–814. [DOI] [PubMed] [Google Scholar]