Abstract.
We conducted a population-based study of tuberculosis (TB) from 2009 to 2015 in an indigenous community of Brazil, the largest in the country, to investigate risk factors associated with recent TB transmission. The clinical isolates of Mycobacterium tuberculosis were genotyped by IS6110-RFLP (restriction fragment length polymorphism) and spoligotyping analysis. Among 67 isolates typed by RFLP, 69% fell into fifteen clusters, and 91% of TB cases with shared IS6110-RFLP pattern were diagnosed within 2 years of another case in the cluster. Individual risk factors associated with genetic clustering were domestic overcrowding (odds ratio [OR]: 6.10; 95% confidence interval [CI]: 1.50–24.88) and low social class (OR: 3.72; 95% CI: 1.00–13.98). Most reported contacts (76%) were identified within the household of the index TB case, but most of the genetic clustering of M. tuberculosis occurred outside of household (79%). Expanded contacts investigation and prophylaxis outside of household should be considered as a priority for TB control programs in this population.
INTRODUCTION
Despite an effective national program for the diagnosis and treatment of tuberculosis (TB) in Brazil, TB remains a leading cause of morbidity and mortality in indigenous populations.1,2 Recent studies have reported a high prevalence of latent TB2,3 and an incidence of active TB that is several fold higher in indigenous populations in Brazil compared with the national average.4 Similar disparities have been reported among indigenous populations throughout the world.
Despite these consistent findings of increased TB risk, there is limited understanding of transmission patterns within indigenous communities. In particular, the epidemiological drivers of TB, including individual and spatial risk within indigenous communities, have not been well characterized. Recent studies from high-burden, non-indigenous communities have suggested that most of the transmission occurs outside of households, but the geographic scale of transmission is poorly understood. This may have important implications for contact investigations. To address this knowledge gap, we conducted a population-based study, incorporating data from contact investigations and genotypic data, to investigate recent TB transmission in the largest indigenous community of Brazil.
MATERIALS AND METHODS
We identified and recruited all indigenous TB patients residing in the Jaguapiru and Bororó reservations who were reported to the National Notifiable Diseases Information System (SINAN) for a TB diagnosis from September 2009 to August 2015. Brazil mandates reporting of all diagnosed TB cases, and reporting rates are believed to be high.
We administered a questionnaire to all patients concerning their demographics, clinical history, occupation, and various social mixing behaviors. An index of domestic overcrowding was determined by dividing the number of individuals who were living in the household by the number of rooms, and domestic overcrowding was considering when there were more than two individuals per room. Social class was categorized according to the classifications set forth by the Brazilian Association of Research Institutes, which adopted the Brazilian Economic Classification Criterion. The study population was subsequently grouped according to the number of 10-point intervals (class D or E) that fell below the median.5 We asked participants to identify their close contacts, defined as persons who had shared indoor airspace with a person with pulmonary TB for at least 15 hours per week for one or more weeks, during a high-risk infectious period defined as the date of cough onset until 2 weeks after the initiation of appropriate anti-TB therapy.6 Data on contacts data, tuberculin skin test (TST) results, and chemoprophylaxis were collected from the clinical records using the forms used by the Indigenous Health Secretary according to the organization’s instructions.
We obtained Mycobacterium tuberculosis clinical isolates from cultures and genotyped them by using IS6110-RFLP (restriction fragment length polymorphism) and spoligotyping analysis.7,8 RFLP patterns were analyzed using an IS6110-RFLP database (RIVM–Bionumerics; Applied Maths, Sint-Martens-Latem, Belgium). A genotypic cluster was defined as a group of two or more isolates from different patients whose RFLP patterns were identical with respect to the number and size of bands. The “Spoligo-International-Type” identified were classified into spoligotype families and subfamilies with the help of the SpolDB4 database.8 We then performed bivariable logistic regression to identify significant risk factors for clustering, including demographic and clinical characteristics, as well as known TB contacts.
RESULTS
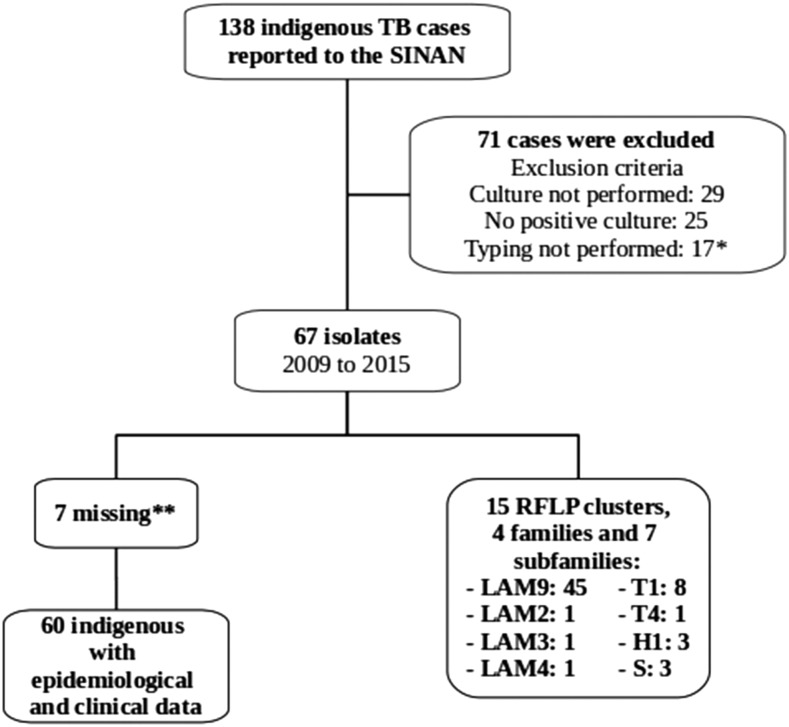
A total of 138 indigenous TB cases were reported to SINAN between September 2009 and August 2015 (Figure 1). Of these, 84% completed treatment, 2% failed, 12% died, and 2% transferred to other municipalities. During the study period, the annual incidence of TB in this population was 144 cases per 100,000 (95% confidence interval [CI]: 121–170 cases per 100,000 persons). Among the reported cases, isolates were available for genotyping by RFLP from 67 (49%). Demographic and clinical characteristics of the patients whose isolates were available for genotyping did not differ from those whose isolates were not available, except that those included were less likely to have extrapulmonary disease (10% versus 25%, P = 0.0003). Among the 67 included patients, 473 contacts were identified, all indigenous, of whom 360 (76%) resided in the same house as the TB case index. Among the identified contacts, TST data were available for 432 (91%), of which 117 (94%) had a TST > 5 mm and 8 (6%) had a TST < 5 mm. There was no difference in TST positivity between household contacts identified outside of the household (P = 0.68). Among all contacts, 125 (26%) received chemoprophylaxis.
Figure 1.
Flowchart for the recruitment of indigenous patients with tuberculosis. * Five strains were not re-culturable after freezing; five strains had no restriction fragment length polymorphism (RFLP) signal; four strains had contamination and could not be re-isolated; and three strains had < 5 bands of IS6110. SINAN = Sistema de Informação de Agravos de Notificação (National Notifiable Diseases Information System). ** One indigenous participant died before the interview and six without epidemiological clinical data.
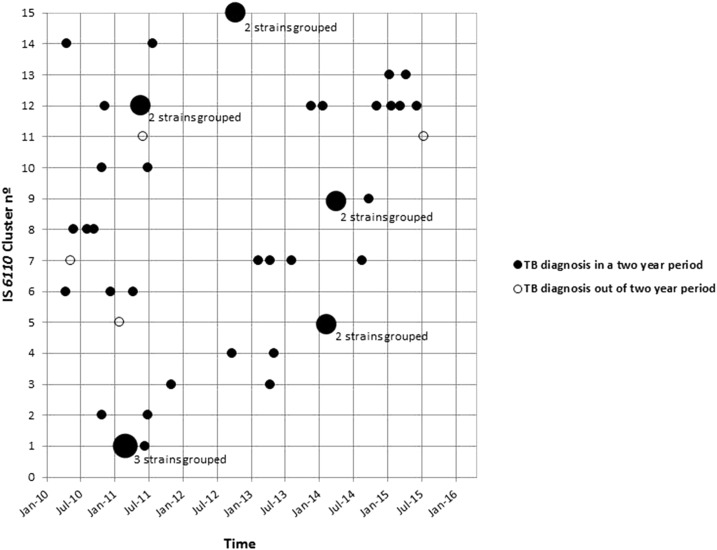
Regarding the RFLP profile, 46 (69%) isolates fell into 15 clusters, whereas 21 (31%) isolates had unique RFLP patterns. The median size of the clusters was two (interquartile range [IQR] 2–3). To assess whether there were larger RFLP groups present, we relaxed the definition of clusters to allow for one band difference, and this did not significantly affect cluster assignment and size. The temporal distribution of these 46 M. tuberculosis isolates revealed that 91% of TB patients with shared IS6110-RFLP patterns were diagnosed within 2 years of another case within their respective cluster (Figure 2). Among RFLP-clustered cases, 21% (8/38) lived in the same house as another case (Table 1); none of these individuals received isoniazid preventive therapy. In bivariate analysis, risk factors associated with RFLP-clustering included domestic overcrowding (odds ratio [OR]: 4.83; 95% CI: 1.44–16.26), the presence of a Bacillus Calmette–Guérin scar (OR: 0.32; 95% CI: 0.08–1.28), history of contact with TB (OR: 4.87; 95% CI: 1.49–15.95), homeowner (OR: 5.60; 95% CI: 0.65–48.19), social class ≤ 10 from the median (D or E) (OR: 3.69; 95% CI: 1.14–11.93), and work in a sugarcane factory (OR: 0.21; 95% CI: 0.05–0.80). In multivariable analysis, variables associated with genetic clustering included domestic overcrowding (OR: 6.10; 95% CI: 1.50–24.88) and social class ≤ 10 from the median (D or E) (OR: 3.72; 95% CI: 1.00–13.98) (Table 1).
Figure 2.
Temporal distribution of Mycobacterium tuberculosis strains isolated from indigenous population in Dourados, Brazil, clustered by IS6110 restriction fragment length polymorphism analysis, and stratified by month and year of isolation and number of the identified cluster. Strains isolated within 2 months in the same IS6110 cluster are depicted with a single, larger circle.
Table 1.
Bivariable and multivariable analyses for IS6110 restrict fragment length polymorphism (RFLP) clustering among indigenous patients with TB
| RFLP clustered | Unique profile | ||||
|---|---|---|---|---|---|
| Variables | (number/percentage) | P value | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Sex, male | 23/38 (60) | 15/22 (68) | 0.553 | – | – |
| Domestic overcrowding* | 29/35 (83) | 11/21 (52) | 0.008 | 4.83 (1.44–16.26) | 6.10 (1.50–24.88)§ |
| BCG vaccine scar | 24/36 (67) | 19/22 (86) | 0.096 | 0.32 (0.08–1.28) | – |
| Alcohol use | 9/38 (24) | 7/22 (32) | 0.492 | – | – |
| Smoking | 6/38 (16) | 1/21 (5) | 0.210 | – | – |
| Illicit drug use over the last year | 3/38 (8) | 1/22 (5) | 0.616 | – | – |
| History of contact with TB case | 31/38 (82) | 10/21 (48) | 0.007 | 4.87 (1.49–15.95) | – |
| Household contact | 8/38 (21) | 0/22 (0) | 0.021 | † | – |
| HIV/AIDS | 1/34 (3) | 0/20 (0) | 0.439 | † | – |
| Previous TB | 4/37 (11) | 5/22 (23) | 0.218 | – | – |
| Homeowner | 30/38 (79) | 21/22 (95) | 0.084 | 5.60 (0.65–48.19) | – |
| Individual income < US$100 | 23/35 (66) | 11/21 (52) | 0.323 | – | – |
| Social class ≤ 10 from the median (D or E)‡ | 31/38 (82) | 11/21 (52) | 0.018 | 3.69 (1.14–11.93) | 3.72 (1.00–13.98)§ |
| Less than 4 year of schooling | 23/38 (60) | 9/21 (43) | 0.192 | – | – |
| Living with partner | 23/38 (60) | 12/22 (54) | 0.651 | – | – |
| Sugarcane worker | 4/38 (10) | 8/22 (36) | 0.016 | 0.21 (0.05–0.80) | – |
AIDS = acquired immune deficiency syndrome; BCG = Bacillus Calmette–Guérin; CI = confidence interval; HIV = human immunodeficiency virus; OR = odds ratio; TB = tuberculosis.
More than two individuals per room.
The logit estimators use a correction of 0.5 in every cell of those tables that contain a zero.
Social class was categorized according to the classifications set forth by the Brazilian Association of Research Institutes and grouped according to the number of 10-point intervals (class D or E) that fell below the median.
Variables with P < 0.05.
Of the 67 isolates that were spoligotyped, 63 (94%) contained spoligotypes described in the international database (SpolDB4), belonging to four families (Latin American Mediterranean-LAM, S family, Haarlem-H and T family) and seven subfamilies (LAM2, LAM3, LAM4, LAM9, T1, T4, and H1). LAM was the main spoligotype family (48/67; 72%), with 45 (67%) isolates belonging to the subfamily LAM9, followed by LAM 2, 3, and 4, with 1 (1.5%) isolates in each one. T1 and T4 were the subfamilies of the 8 (12%) and 1 (1.5%) isolates, respectively, whereas 3 (4.5%) isolates belonged to H1 and 3 (4.5%) to S Family (Figure 1).
DISCUSSION
Although indigenous people comprise < 5% of the global population, evidence suggests that the burden of TB falls heavily on these populations,9 as seen among the indigenous groups of Brazil.10 However, there have been few data concerning the recent transmission of TB and its importance within indigenous families and villages, which often live in conditions of poor housing quality and overcrowding.11
During the study period of 2009–2015, the indigenous populations of Dourados were disproportionately affected by TB when compared with the nearby nonindigenous population (144 cases/100,000 persons; 95% CI: 121–170 cases/100,000 persons versus 23 cases/100,000 persons; 95% CI: 20–26 cases/100,000 persons). In this setting, we found small genetic clusters, with 79% of clustering of M. tuberculosis occurring outside of the household. These findings are consistent with several recent molecular epidemiologic studies from nonindigenous populations, which found that > 80% of transmission could not be linked to households, plots (multifamily land areas), or known contacts.12,13
The median number of household contacts identified in whom TST was performed (6; IQR: 2–10) and proportion of contacts that had received chemoprophylaxis (26%) were high compared with the other studies in local nonindigenous populations.14,15 A previous study conducted in the nearby municipalities of Dourados and Amambai showed the expansion of treatment of latent TB infection in this population had a strong protective effect (relative risk: 0.03, 95% CI: 0.01–0.27).16 These data may explain the low degree of genetic clustering in the household in this area. Most of the patients who were in genotypic clusters were diagnosed outside of the household and were not identified as a contact and within 2 years of another case in their cluster, which underscores the importance of contact investigations and prophylaxis. In addition, TST positivity did not differ between household and non-household contacts. Taken together, these findings suggest that expanding the investigation and prophylaxis to contacts beyond the household may be an effective measure to control TB in indigenous communities.
Previous studies have found sociodemographic factors to be predictors of recent TB transmission, such as younger age, minority race/ethnicity status, male sex, homelessness, incarceration, and drug use.17,18 In our study, domestic overcrowding and social class were associated with genetic clustering, indicating that poverty and socioeconomic status, which are known factors associated with the risk of developing TB,19 are also linked to recent transmission of the disease in the indigenous population.
After analysis by spoligotyping, the predominance of the LAM family was observed, responsible for grouping approximately 72% of the isolates. This percentage is much higher than that reported elsewhere in Brazil (46%).20 Of the 12 subfamilies already identified for the LAM family,8 four were observed in our study. The families T, H, and S, have also been described in Brazil, in smaller proportions, as in our study.20
This study was subject to several limitations. The window for the molecular study was only 6 years, and only half of the cases had isolates available for genotyping; both of these factors could lead to underestimation of the proportion of cases belonging to clusters. RFLP provides limited resolution for genetic differences between isolates, and future studies using whole genome sequencing may improve inference about specific transmission events between individuals.
In summary, our data demonstrate that differences in socioeconomic status contribute to the risk of TB recent transmission in indigenous communities, indicating that TB prevention should address the social factors that produce unequal health outcomes among this population. A substantial proportion of TB transmission in these two indigenous communities occurs between individuals who did not reside in the same household. These findings underscore the importance of expanding contact investigations and preventive therapy outside of households to improve TB control in these indigenous communities.
Acknowledgment:
We thank the UFGD tuberculosis study group.
REFERENCES
- 1.Castro DB, Pinto RC, Albuquerque BC, Sadahiro M, Braga JU, 2016. The socioeconomic factors and the indigenous component of tuberculosis in Amazonas. PLoS One 11: e0158574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basta PC, Coimbra CE, Jr, Welch JR, Correa Alves LC, Santos RV, Bastos Camacho LA, 2010. Tuberculosis among the Xavante Indians of the Brazilian Amazon: an epidemiological and ethnographic assessment. Ann Hum Biol 37: 643–657. [DOI] [PubMed] [Google Scholar]
- 3.Malacarne J, Rios DP, Silva CM, Braga JU, Camacho LA, Basta PC, 2016. Prevalence and factors associated with latent tuberculosis infection in an indigenous population in the Brazilian Amazon. Rev Soc Bras Med Trop 49: 456–464. [DOI] [PubMed] [Google Scholar]
- 4.Cunha EA, Ferrazoli L, Riley LW, Basta PC, Honer MR, Maia R, Costa IP, 2014. Incidence and transmission patterns of tuberculosis among indigenous populations in Brazil. Mem Inst Oswaldo Cruz 109: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ABEP , 2015. Brazilian Economic Classification Criterion [in Portuguese]. Available at: www.abep.org. Accessed June 15, 2017.
- 6.Fiske CT, Yan FX, Hirsch-Moverman Y, Sterling TR, Reichler MR, Tuberculosis Epidemiologic Studies Consortium Task Order 2 Team, 2014. Risk factors for treatment default in close contacts with latent tuberculous infection. Int J Tuberc Lung Dis 18: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Embden JD, et al. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 31: 406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demay C, Liens B, Burguière T, Hill V, Couvin D, Millet J, Mokrousov I, Sola C, Zozio T, Rastogi N, 2012. SITVITWEB–a publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infect Genet Evol 12: 755–766. [DOI] [PubMed] [Google Scholar]
- 9.United Nations Permanent Forum on Indigenous Issues , 2012. State of the World’s Indigenous Peoples. ST/ESA/328. New York, NY: United Nations. Available at: http://www.unhcr.org/refworld/docid/4b6700ed2.html. Accessed December 10, 2012.
- 10.Tollefson D, Bloss E, Fanning A, Redd JT, Barker K, McCray E, 2013. Burden of tuberculosis in indigenous peoples globally: a systematic review. Int J Tuberc Lung Dis 17: 1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Collaborating Centre for Aboriginal Health , 2010. Housing as a Social Determinant of First Nations, Inuit and Métis Health. Available at: http://www.nccah-ccnsa.ca/docs/fact%20sheets/social%20determinates/NCCAH_fs_housing_EN.pdf. Accessed October 5, 2014.
- 12.Middelkoop K, Mathema B, Myer L, Shashkina E, Whitelaw A, Kaplan G, Kreiswirth B, Wood R, Bekker LG, 2015. Transmission of tuberculosis in a South African community with a high prevalence of HIV infection. J Infect Dis 211: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glynn JR, Whiteley J, Bifani PJ, Kremer K, van Soolingen D, 2002. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg Infect Dis 8: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puma DV, Perez-Quilez O, Roure S, Martinez-Cuevas O, Bocanegra C, Feijoo-Cid M, Valerio L, 2017. Risk of active tuberculosis among index case of householders—A long-term assessment after the conventional contacts study. Public Health Nurs 34: 112–117. [DOI] [PubMed] [Google Scholar]
- 15.Fox GJ, Barry SE, Britton WJ, Marks GB, 2013. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 41: 140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuhara LS, Sacchi FP, Croda J, 2013. Impact of latent infection treatment in indigenous populations. PLoS One 8: e71201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saavedra-Campos M, Welfare W, Cleary P, Sails A, Burkitt A, Hungerford D, Okereke E, Acheson P, Petrovic M, 2016. Identifying areas and risk groups with localised Mycobacterium tuberculosis transmission in northern England from 2010 to 2012: spatiotemporal analysis incorporating highly discriminatory genotyping data. Thorax 71: 742–748. [DOI] [PubMed] [Google Scholar]
- 18.Fenner L, et al. 2012. Mycobacterium tuberculosis transmission in a country with low tuberculosis incidence: role of immigration and HIV infection. J Clin Microbiol 50: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuel B, et al. 2016. Relationship between nutritional support and tuberculosis treatment outcomes in West Bengal, India. J Tuberc Res 4: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes HM, et al. 2012. Spoligotypes of Mycobacterium tuberculosis complex isolates from patients residents of 11 states of Brazil. Infect Genet Evol 12: 649–656. [DOI] [PubMed] [Google Scholar]