Abstract.
Repeated oral azithromycin distribution targeted only to children has proven effective in reducing the ocular Chlamydia that causes trachoma. Here, we assess whether an enhanced coverage target of at least 90% of children is superior to the World Health Organization recommendation of at least 80%. Twenty-four trachoma-endemic communities in Matamèye, Niger, were randomized to a single day of azithromycin distribution aiming for at least 80% coverage or up to 4 days of treatment and > 90% coverage of children under age 12. All distributions were biannual. Children < 5 years of age and adults > 15 years were monitored for ocular Chlamydia infection by polymerase chain reaction every 6 months for 36 months in children and at baseline and 36 months in adults. Ocular Chlamydia prevalence in children decreased from 24.9% (95% confidence interval [CI] 15.9–33.8%) to 4.4% (95% CI 0.6–8.2%, P < 0.001) at 36 months in the standard coverage arm and from 15.6% (95% CI 10.0–21.2%) to 3.3% (95% CI 1.0–5.5%; P < 0.001) in the enhanced coverage arm. Enhanced coverage reduced ocular Chlamydia prevalence in children more quickly over time compared with standard (P = 0.04). There was no difference between arms at 36 months in children (2.4% lower with enhanced coverage, 95% CI 7.7–12.5%; P = 0.60). No infection was detected in adults at 36 months. Increasing antibiotic coverage among children from 80% to 90% may yield only short term improvements for trachoma control programs. Targeting treatment to children alone may be sufficient for trachoma control in this setting.
INTRODUCTION
Annual mass azithromycin distribution is a core component of the World Health Organization (WHO)’s trachoma control strategy.1,2 When clinical trachoma prevalence is more than 10% among children less than 10 years, the WHO standard is to treat all individuals in a community. As part of this strategy, the WHO recommends mass azithromycin coverage of at least 80% in the community.3 High coverage is recommended to prevent reemergence of infection in communities.4,5 However, ocular Chlamydia infection, which causes trachoma, is overwhelmingly found in young children.6–8 Furthermore, the duration of infection is longer at younger ages,9 and children have a higher bacterial load.10 Targeting antibiotics to a core group of children may prevent infection in untreated individuals through indirect, herd-like protection.5,11 Strategies that do not involve distribution to the entire community may have advantages by conserving resources and reducing antibiotic consumption and resistance.
Mathematical models suggest that strategies involving targeting antibiotics to children require distribution of antibiotics more frequently than mass distribution to the entire community, with little margin for incomplete coverage.5 Empirically, biannual targeting of azithromycin to children has been shown to be noninferior to annual distribution to all individuals in a community for trachoma control.12 Furthermore, treatment of children leads to reduced ocular Chlamydia prevalence among untreated adults.11,12 Alternative antibiotic strategies for trachoma control, beyond annual mass azithromycin distribution, thus include strategies that target antibiotics to core groups of children.
Here, we assess two different coverage targets for biannual azithromycin distribution targeted to children aged 6 months to 12 years. We compare distribution on a single day aimed to approximate the 80% recommended by the WHO (standard coverage target) versus at least 90% (enhanced coverage target) to determine if higher antibiotic coverage leads to greater decreases in ocular Chlamydia prevalence.
METHODS
Study setting and design.
The Partnership for the Rapid Elimination of Trachoma (PRET) study was a collection of separate cluster-randomized trials designed to evaluate mass antibiotic distribution strategies for trachoma control in the Gambia, Tanzania, and Niger (NCT00792922).4,12–14 The Niger trial was the only one of the three trials to evaluate azithromycin distribution targeted specifically to children. The Niger trial enrolled participants in the Matamèye District, Zinder Region, Niger, between May 2010 and August 2013 into one of four arms designed to assess the effects of standard (> 80%) versus enhanced (> 90%) azithromycin coverage and to compare annual treatment of all individuals versus biannual treatment of children aged 12 years and younger. Here, we report a prespecified analysis of outcomes comparing standard versus enhanced biannual treatment to children aged 12 years and younger. Twenty-four grappes (smallest government health unit; henceforth, “community”) from six Centers de Santé Intégrées (CSI) were included in this analysis. Complete methods for the trial have been previously reported.12 Ethical approval for the trial was obtained from the Committee for Human Research at the University of California, San Francisco, and the Comité d’Ethique du Niger (Ethical Committee of Niger). The study was registered at ClinicalTrials.gov (NCT00792922) and implemented in accordance with the Declaration of Helsinki. Verbal informed consent was obtained from each local community chief before randomization and each individual or guardian before examination. Given low reported literacy rates in the area, both institutional review boards approved the verbal informed consent procedure.
Community eligibility and randomization.
Eligible communities had a population between 250 and 600 at the most recent government census. Communities with less than 10% prevalence of trachomatous inflammation-follicular/trachomatous inflammation-intense (TF/TI) were excluded. To measure baseline TF/TI prevalence, before the first mass antibiotic treatment, a door-to-door census was conducted in eight communities per CSI. A random sample of 100 children aged 0–60 months were assessed for active trachoma according to the WHO simplified grading system.8,15 If a given community had less than 10% TF/TI prevalence, another community in the CSI was censused, and trachoma prevalence was assessed, until each CSI had eight enrolled communities.
After inclusion, communities were randomized by stratified block randomization within each CSI by high or low TF/TI prevalence in children. Communities above the median trachoma prevalence in a given CSI were considered to be “high” prevalence, and those below the median were considered to be “low” prevalence. The random allocation sequence was generated by TCP using the statistical package R (version 2.12; R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org).
Study intervention.
All communities in the biannual arms of PRET-Niger received six rounds of mass azithromycin distribution. Azithromycin was distributed via a door-to-door program. Study participants aged 6 months to 12 years were offered treatment. Children under 6 months of age and those known to be allergic to macrolides were offered topical tetracycline ointment (1%) to be applied to both eyes twice a day for 6 weeks. In the standard arm, antibiotic distributions were performed on a single day, aiming for an antibiotic coverage target (defined as the proportion of the targeted population that was treated) of 80% or greater. In the enhanced arm coverage, up to three follow-up visits were performed in an attempt to achieve coverage of 90% or greater. Individuals more than 12 years of age were not treated. Communities had received some education on face washing and the importance of hygiene, but no specific interventions (e.g., latrinization or program-related well construction) were given as part of the study. At the time of the study, annual mass azithromycin distribution according to WHO guidelines was the standard for trachoma control, distributed via a distribution point supplemented by door-to-door mop-up.
Outcome assessments.
A census was conducted in each community annually. For each 6-month data collection time point, 100 children aged 0–5 years were randomly selected (or all children if there were fewer than 100 in a given community) from the most recent census before the data collection point. Children were examined biannually at baseline and months 6, 12, 18, 24, 30, and 36. A random sample of 40 individuals over the age of 15 per community was swabbed at baseline and 36 months. Clinical examination was performed in children according to the WHO simplified grading system as previously described.8,15 Examiners were not made aware of the community’s treatment allocation and were separate personnel from treatment teams. The right and left upper tarsal conjunctiva was everted, and the right conjunctiva was swabbed. Swabs were collected without media, transported to the University of California, San Francisco and stored at −80°C. Swabs were pooled within a community and age groups into pools of five plus a remainder pool and then processed for Chlamydia with Amplicor polymerase chain reaction testing. The prevalence of ocular Chlamydia was estimated from the pooled results as previously described.16 In communities where more than 80% of pools were positive, all samples were processed individually to more accurately estimate community prevalence. All laboratory personnel were masked to the intervention arm.
Sample size.
In the parent trial, we estimated a sample size of 48 communities (12 per arm) would yield greater than 80% power to detect a 6% absolute difference in ocular Chlamydia infection in children. The current report includes only the communities randomized to the two coverage targets for biannual treatment of children.
Statistical methods.
As per our prespecified analysis plan, all analyses used a square root transformation of ocular Chlamydia infection prevalence and included adjustment for baseline prevalence. Reduction in ocular Chlamydia prevalence between baseline and at 36 months was analyzed using a paired t test. For the primary analysis, we used a linear regression model to compare the 36-month square root transformed prevalence of ocular Chlamydia in children aged 0–5 years between communities randomized to enhanced coverage compared with standard coverage, adjusting for baseline prevalence. As a prespecified secondary analysis to assess the rate of change in ocular Chlamydia prevalence over time, we used a mixed effects model with square root transformed ocular Chlamydia prevalence at each time point (months 0, 6, 12, 18, 24, 30, and 36), with treatment arm and a time point by arm interaction term as fixed effects and a random effect for community. A similar analytic strategy was used for determining the effect of treatment coverage on the prevalence of TF. All P values were calculated using an exact permutation test. All analyses were conducted in R (version 3.3.1, The R Foundation for Statistical Computing, Vienna, Austria) at the community level.
RESULTS
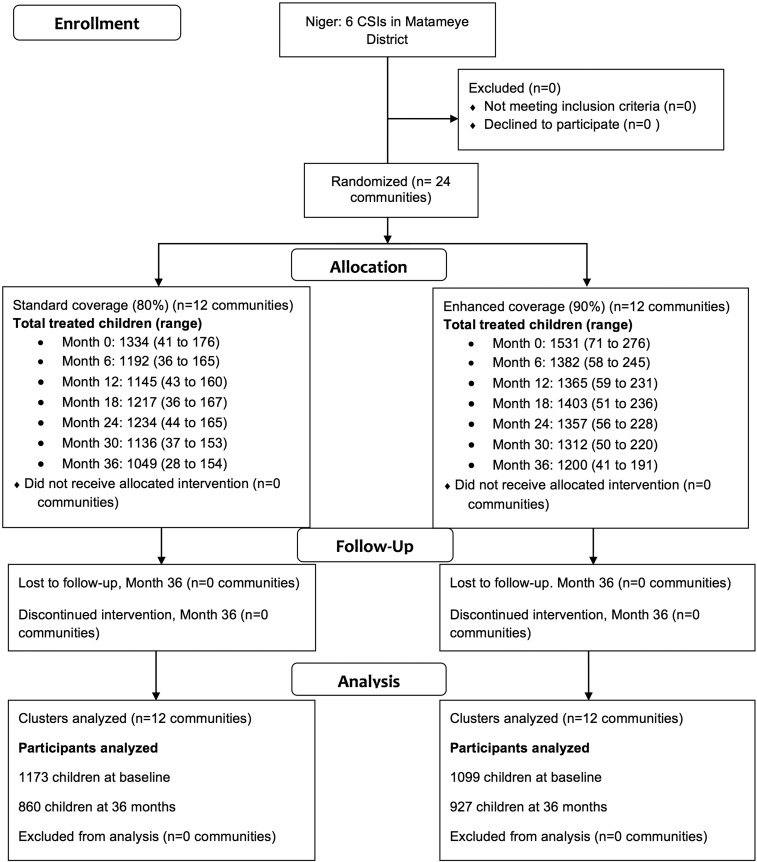
At baseline, the 12 communities randomized to standard coverage had a mean of 111 (range 41–176) children aged 0 to 5 years treated, and the 12 communities randomized to enhanced coverage had a mean of 126 (range 71–276) treated (Figure 1). Baseline characteristics, including age and gender and prevalence of TF and infection, were well balanced at baseline (Table 1). Antibiotic coverage among children aged 0–5 ranged from 75.8% (95% confidence interval [CI] 71.6–80.1%) to 84.3% (95% CI 80.1– 88.6%) in the standard coverage arm and from 91.1% (95% CI 88.1–94.1%) to 94.2% (95% CI 92.1–96.4%) in the enhanced coverage arm (Table 2).
Figure 1.
Flow diagram of study communities.
Table 1.
Baseline characteristics of study communities
| Characteristic | Standard coverage | Enhanced coverage |
|---|---|---|
| Number of communities | 12 | 12 |
| Total number of individuals per community (range) | 497 (325–670) | 458 (310–569) |
| Mean number of children aged 0–5 years per community (range) | 143 (60–236) | 144 (77–290) |
| Proportion of female participants (95% CI) | 51.6% (50.1–53.1%) | 49.9% (48.1–51.6%) |
| Prevalence of ocular Chlamydia in children aged 0–5 years (95% CI) | 24.9 (15.9–33.8) | 15.6 (10.0–21.1) |
| Prevalence of TF in children aged 0–5 years (95% CI) | 24.7 (16.4–32.9) | 23.9 (16.3–31.5) |
CI = confidence interval; TF = trachomatous inflammation–follicular.
Table 2.
Mean treatment coverage among children 0–5 years during mass antibiotic distributions by study visit
| Time point | Standard coverage | Enhanced coverage |
|---|---|---|
| 0 months (95% CI) | 80.8% (76.4–85.1%) | 94.2% (92.1–96.4%) |
| 6 months (95% CI) | 78.6% (74.6–82.6%) | 92.1% (89.1–95.2%) |
| 12 months (95% CI) | 81.1% (78.3–84.0%) | 93.6% (91.7–95.5%) |
| 18 months (95% CI) | 82.2% (77.2–87.1%) | 92.9% (88.4–97.3%) |
| 24 months (95% CI) | 84.3% (80.1–88.6%) | 91.4% (89.7–93.1%) |
| 30 months (95% CI) | 78.2% (74.0–82.4%) | 91.2% (87.9–94.4%) |
| 36 months (95% CI) | 75.8% (71.6–80.1%) | 91.1% (88.1–94.1%) |
CI = confidence interval.
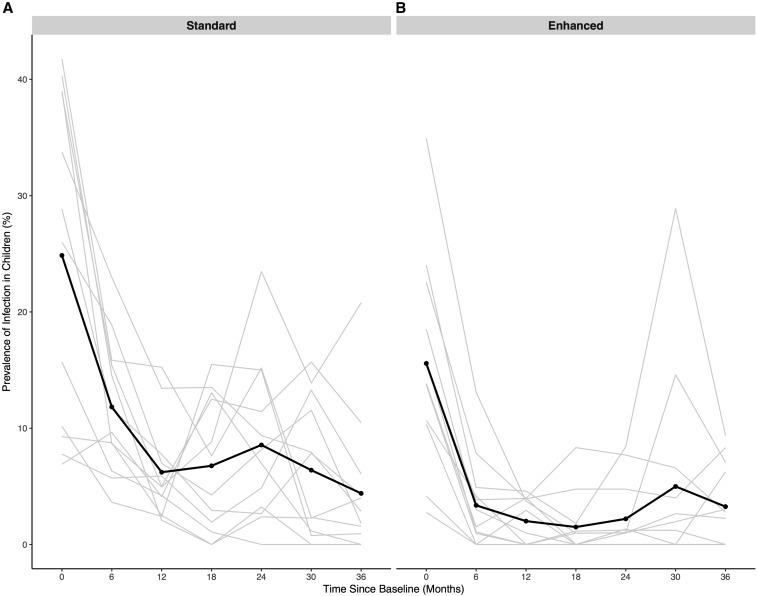
Ocular Chlamydia prevalence was reduced from 24.9% (95% CI 15.9–33.8%) to 4.4% (95% CI 0.6–8.2%, P < 0.001; Figure 2) in the standard coverage arm and from 15.6% (95% CI 10.0–21.2%) at baseline to 3.3% (95% CI 1.0–5.5%; P < 0.001) at 36 months in the enhanced coverage arm. In a model adjusting for ocular Chlamydia prevalence at baseline, there was no difference in ocular Chlamydia prevalence at 36 months in communities randomized to the standard versus enhanced coverage arm (mean difference 2.4% in the enhanced versus standard arm at 36 months, 95% CI 7.7–12.5%; P = 0.60). In a repeated measures model, ocular Chlamydia prevalence reduced more quickly over time in the enhanced arm compared with that in the standard arm (P = 0.04; Table 3).
Figure 2.
Prevalence of ocular Chlamydia infection in children aged 0–5 years in communities randomized to standard (A) or enhanced (B) coverage. All communities received biannual mass azithromycin treatment in children less than 12 years of age.
Table 3.
Longitudinal prevalence of ocular Chlamydia among a random sample of 0- to 5-year-old children
| Prevalence of ocular Chlamydia, 0- to 5-year-old children | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Community | Month 0 | Month 6 | Month 12 | Month 18 | Month 24 | Month 30 | Month 36 | |||||||
| Standard coverage | ||||||||||||||
| 1 | 7.8% | (8/103) | 5.7% | (6/105) | 5.9% | (6/102) | 1.9% | (2/104) | 4.9% | (5/103) | 13.3% | (15/113) | 6.1% | (6/99) |
| 2 | 26.0% | (13/50) | 18.9% | (10/53) | 7.0% | (3/43) | 4.3% | (2/47) | 8.2% | (4/49) | 11.5% | (6/52) | 1.8% | (1/55) |
| 3 | 39.0% | (39/100) | 8.7% | (9/104) | 5.0% | (5/101) | 8.8% | (9/102) | 23.5% | (23/98) | 13.9% | (10/72) | 20.8% | (16/77) |
| 4 | 33.8% | (27/80) | 23.0% | (17/74) | 13.4% | (9/67) | 13.5% | (10/74) | 9.4% | (6/64) | 7.9% | (5/63) | 2.9% | (1/35) |
| 5 | 40.3% | (27/67) | 14.5% | (9/62) | 2.1% | (1/48) | 0 | (0/49) | 2.4% | (1/42) | 2.3% | (1/44) | 4.0% | (2/50) |
| 6 | 9.3% | (9/97) | 8.8% | (7/80) | 2.5% | (1/40) | 0 | (0/51) | 3.2% | (1/31) | 0 | (0/23) | 0 | (0/11) |
| 7 | 41.7% | (43/103) | 15.8% | (16/101) | 15.2% | (16/105) | 7.6% | (8/105) | 15.2% | (17/112) | 2.3% | (2/86) | 1.6% | (1/64) |
| 8 | 6.9% | (6/87) | 9.6% | (8/83) | 4.1% | (3/73) | 13.0% | (9/69) | 7.0% | (6/86) | 1.2% | (1/86) | 0 | (0/73) |
| 9 | 38.8% | (40/103) | 15.4% | (16/104) | 5.0% | (5/100) | 12.5% | (14/112) | 11.4% | (12/105) | 15.7% | (16/102) | 10.5% | (11/105) |
| 10 | 15.7% | (16/102) | 6.3% | (6/95) | 4.2% | (4/95) | 1.1% | (1/92) | 0 | (0/120) | 0 | (0/108) | 0 | (0/89) |
| 11 | 28.8% | (30/104) | 11.7% | (12/103) | 7.8% | (8/103) | 2.9% | (3/102) | 2.7% | (3/113) | 7.8% | (8/102) | 4.3% | (4/94) |
| 12 | 10.2% | (18/177) | 3.6% | (6/165) | 2.4% | (4/165) | 15.5% | (24/155) | 15.0% | (21/140) | 0.8% | (1/129) | 0.9% | (1/108) |
| Enhanced coverage | ||||||||||||||
| 13 | 22.5% | (23/102) | 7.8% | (8/102) | 3.9% | (4/102) | 4.8% | (5/105) | 4.8% | (5/105) | 4.0% | (4/100) | 8.3% | (8/96) |
| 14 | 10.3% | (6/58) | 0 | (0/76) | 2.9% | (2/68) | 0 | (0/72) | 0 | (0/62) | 0 | (0/56) | 0 | (0/61) |
| 15 | 15.8% | (15/95) | 1.1% | (1/93) | 0 | (0/93) | 1.1% | (1/87) | 1.2% | (1/81) | 1.2% | (1/82) | 0 | (0/78) |
| 16 | 18.5% | (20/108) | 3.0% | (3/100) | 1.0% | (1/101) | 0 | (0/104) | 1.0% | (1/100) | 2.7% | (3/113) | 2.2% | (2/89) |
| 17 | 4.2% | (3/72) | 0 | (0/69) | 0 | (0/53) | 0 | (0/43) | 0 | (0/55) | 0 | (0/38) | 6.3% | (2/32) |
| 18 | 13.7% | (14/102) | 1.0% | (1/105) | 0 | (0/91) | 1.0% | (1/103) | 1.0% | (1/100) | 2.0% | (2/102) | 3.0% | (3/99) |
| 19 | 10.7% | (8/75) | 4.2% | (3/72) | 0 | (0/66) | 0 | (0/78) | 1.3% | (1/75) | 0 | (0/70) | 0 | (0/65) |
| 20 | 34.9% | (37/106) | 13.1% | (14/107) | 3.7% | (4/107) | 1.0% | (1/103) | 1.0% | (1/99) | 14.6% | (14/96) | 7.1% | (6/85) |
| 21 | 13.8% | (9/65) | 1.5% | (1/66) | 4.0% | (2/50) | 0 | (0/54) | 0 | (0/68) | 0 | (0/51) | 0 | (0/50) |
| 22 | 2.8% | (3/109) | 0 | (0/98) | 0 | (0/103) | 0 | (0/107) | 0 | (0/96) | 0 | (0/107) | 0 | (0/104) |
| 23 | 15.5% | (16/103) | 3.9% | (4/104) | 4.0% | (4/101) | 8.3% | (9/108) | 7.7% | (8/104) | 6.6% | (5/76) | 2.8% | (2/72) |
| 24 | 24.0% | (25/104) | 4.9% | (5/102) | 4.6% | (5/109) | 1.8% | (2/110) | 8.4% | (9/107) | 28.9% | (26/90) | 9.4% | (9/96) |
In adults aged 15 years and older, ocular Chlamydia prevalence was 1.8% (95% CI 0.04–3.6%) in the standard arm and 0.5% (95% CI 0–1.2%) in the enhanced arm at baseline. At 36 months, there was no infection detected in adults in any community, and no difference in infection at 36 months between the two arms.
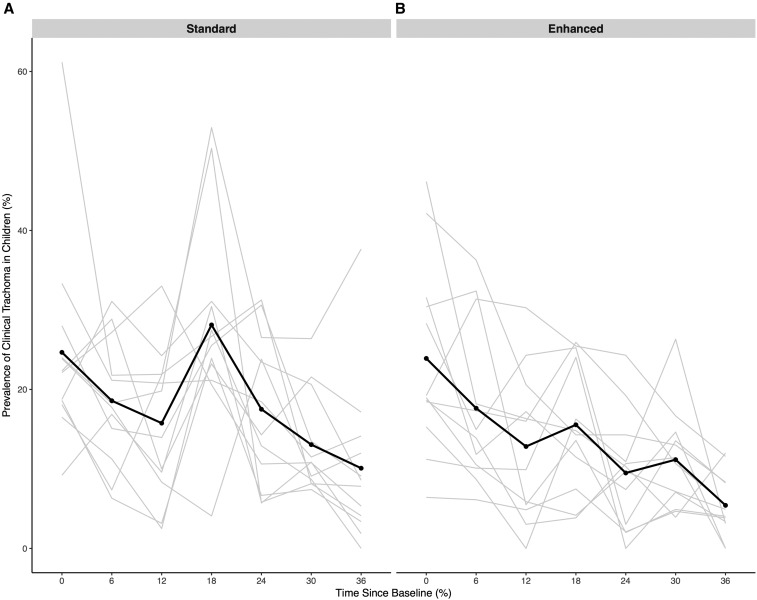
TF prevalence was reduced from 24.7% (95% CI 16.4–32.9%) to 10.1% (95% CI 3.7–16.5%; P = 0.001; Figure 3) in the standard coverage arm and from 23.9% (95% CI 16.3–31.5%) at baseline to 5.4% (95% CI 2.9–7.9%; P = 0.0001) at 36 months in the enhanced coverage arm. Adjusting for baseline, there was no difference between TF prevalence in the standard compared with the enhanced arm (mean difference −4.6% in the enhanced versus the standard arm at 36 months, 95% CI 11.1–1.9%; P = 0.20). In a repeated measures model, there was no evidence of differential change over time in TF prevalence between the enhanced and standard arms (P = 0.19).
Figure 3.
Prevalence of trachomatous inflammation in children aged 0–5 years in communities randomized to standard (A) or enhanced (B) coverage. All communities received biannual mass azithromycin treatment in children less than 12 years of age.
DISCUSSION
In this study, we demonstrate that achieving higher antibiotic treatment coverage in communities treating children biannually may have resulted in a faster decrease in ocular Chlamydia prevalence. However, after 36 months, the prevalence was not significantly different from standard coverage. In this mesoendemic setting in Niger, for biannual distribution of azithromycin to children, increasing antibiotic coverage above 80% may yield only marginal returns for trachoma control programs. Consistent with results from other mesoendemic regions, strategies targeting treatment only to children appear to be effective for reducing the prevalence of ocular Chlamydia.17
The WHO recommends annual mass azithromycin distribution for 3–5 years followed by an impact assessment in communities with clinical trachoma prevalence above 10% in children younger than 10 years of age. If the assessment shows control has not been achieved then annual distributions would continue. In the present study, biannual distribution with approximately 80% coverage did not reduce TF prevalence below 10% in children younger than 5 years of age. Biannual treatment strategies involve greater costs and are more logistically intense than annual treatment strategies. However, treatment of children is considered easier than that of adults.17 In a previous analysis, we demonstrated that biannual treatment of children alone was noninferior to annual mass azithromycin distribution.12 Although we did not compare biannual with annual distribution in the present analysis, these results may indicate that in some regions treatment may be required for a longer duration to reduce TF prevalence below 10%.
With repeated antibiotic distributions, perfect coverage of all individuals in a community may not be required for elimination, as untreated children may receive indirect protection from those treated. Children form a core group for infection in mathematical models.5 However, models suggest that increasing antibiotic coverage should result in more rapid elimination.5 Previous work from the parent study of this analysis found that biannual treatment of children yielded comparable results to annual treatment of all adults in the community and that infection in adults reduced similarly in communities where only children were treated and where all individuals were treated.12 Previous evidence from a hyperendemic region of Ethiopia demonstrated a herd effect for trachoma. With quarterly treatment of children younger than 10 years of age, ocular Chlamydia prevalence was reduced by approximately half in adults, even though adults themselves had not received treatment.11 In Nepal, mass treatment of children was found to be cost effective compared with mass treatment of the entire community.18 The present results add to the evidence that treatment of a subgroup of the population may be sufficient for trachoma control among children and adults. However, achieving higher coverage of antibiotic treatment is both costlier and more logistically challenging. These results indicate that even with treatment targeted only to children, imperfect treatment coverage will not jeopardize trachoma control programs.
Treatment targeted only to children younger than the age of 12 years resulted in no infection in adults aged 15 and older by 36 months. Previous work in Ethiopia has demonstrated that treatment of children in communities leads to decreases in ocular Chlamydia prevalence in adults via herd-like protection.11,19 In the present study, infection prevalence was low at baseline. Whereas it is possible that over the 3-year period infection would have disappeared in the community in the absence of treatment of children, these results indicate that treatment of adults may not be required for trachoma control programs.
There may be additional benefits to limiting antibiotic distributions in communities. Mass azithromycin distribution selects for macrolide resistance in Streptococcus pneumoniae isolates from Ethiopian children.20 Although this selection pressure is removed and the prevalence of resistance decreases when antibiotic distributions are discontinued,21 minimizing the amount of antibiotic distributed may help minimize the extent of antibiotic resistance. Strategies that target only children and have lower coverage targets reduce antibiotic consumption, potentially resulting in less antibiotic resistance.
The results of this study must be evaluated in the context of several limitations. Outcomes, including ocular Chlamydia prevalence and TF, were measured in a random sample of children, and thus elimination in a given community cannot be definitively determined. Thus, an observed prevalence estimate of 0% does not guarantee elimination, and infection could return over time after cessation of treatment. In addition, this study took place in a mesoendemic region of Niger, and as such, these results may not generalize to regions with differing endemicity. Although clinical examiners were not made aware of the intervention arm of communities, it is possible they could have asked community members about the treatment that had occurred in a given community, which could have compromised masking of clinical examination. However, the primary outcome was ocular Chlamydia prevalence, and all laboratory personnel were fully masked.
Although azithromycin distribution programs have been extremely effective in reducing trachoma prevalence worldwide, trachoma control has remained a challenge in some severely affected districts. Alternative strategies, such as targeting core groups of children, increasing the frequency of distributions, and expanding antibiotic coverage, have been considered in these areas. The results of this study indicate that increasing antibiotic coverage above the WHO recommendation may not be necessary to effectively reduce trachoma prevalence in mesoendemic settings similar to that of this community-randomized trial.
REFERENCES
- 1.Mabey DC, Solomon AW, Foster A, 2003. Trachoma. Lancet 362: 223–229. [DOI] [PubMed] [Google Scholar]
- 2.Taylor HR, Burton MJ, Haddad D, West F, Wright H, 2014. Trachoma. Lancet 384: 2142–2152. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization , 2006. Trachoma Control: A Guide from Prgoramme Managers Geneva, Switzerland: World Health Organization, 1–53. [Google Scholar]
- 4.Harding-Esch EM, et al. Partnership for Rapid Elimination of Trachoma (PRET) study group , 2013. Mass treatment with azithromycin for trachoma: when is one round enough? Results from the PRET trial in the Gambia. PLoS Negl Trop Dis 7: e2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lietman T, Porco T, Dawson C, Blower S, 1999. Global elimination of trachoma: how frequently should we administer mass chemotherapy? Nat Med 5: 572–576. [DOI] [PubMed] [Google Scholar]
- 6.Last AR, Burr SE, Weiss HA, Harding-Esch EM, Cassama E, Nabicassa M, Mabey DC, Holland MJ, Bailey RL, 2014. Risk factors for active trachoma and ocular Chlamydia trachomatis infection in treatment-naïve trachoma-hyperendemic communities of the Bijagós Archipelago, Guinea Bissau. PLoS Negl Trop Dis 8: e2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schemann JF, Sacko D, Malvy D, Momo G, Traore L, Bore O, Coulibaly S, Banou A, 2002. Risk factors for trachoma in Mali. Int J Epidemiol 31: 194–201. [DOI] [PubMed] [Google Scholar]
- 8.Amza A, et al. PRET Partnership , 2012. Community risk factors for ocular Chlamydia infection in Niger: pre-treatment results from a cluster-randomized trachoma trial. PLoS Negl Trop Dis 6: e1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grassly NC, Ward ME, Ferris S, Mabey DC, Bailey RL, 2008. The natural history of trachoma infection and disease in a Gambian cohort with frequent follow-up. PLoS Negl Trop Dis 2: e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon AW, et al. 2004. Mass treatment with single-dose azithromycin for trachoma. N Engl J Med 351: 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.House JI, et al. 2009. Assessment of herd protection against trachoma due to repeated mass antibiotic distributions: a cluster-randomisedtrial. Lancet 373: 1111–1118. [DOI] [PubMed] [Google Scholar]
- 12.Amza A, et al. 2017. A cluster-randomized trial to assess the efficacy of targeting trachoma treatment to children. Clin Infect Dis 64: 743–750. [DOI] [PubMed] [Google Scholar]
- 13.Yohannan J, Munoz B, Mkocha H, Gaydos CA, Bailey R, Lietman TA, Quinn T, West SK, 2013. Can we stop mass drug administration prior to 3 annual rounds in communities with low prevalence of trachoma?: PRET Ziada trail results. JAMA Ophthalmol 131: 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stare D, Harding-Esch E, Munoz B, Bailey R, Mabey D, Holland M, Gaydos C, West S, 2011. Design and baseline data of a randomized trial to evaluate coverage and frequency of mass treatment with azithromycin: the partnership for rapid elimination of trachoma (PRET) in Tanzania and the Gambia. Ophthalmic Epidemiol 18: 20–29. [DOI] [PubMed] [Google Scholar]
- 15.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR, 1987. A simple system for the assessment of trachoma and its complications. Bull World Health Organ 65: 477–483. [PMC free article] [PubMed] [Google Scholar]
- 16.Ray KJ, Zhou Z, Cevallos V, Chin S, Enanoria W, Lui F, Lietman TM, Porco TC, 2014. Estimating community prevalence of ocular Chlamydia trachomatis infection using pooled polymerase chain reaction testing. Ophthalmic Epidemiol 21: 86–91. [DOI] [PubMed] [Google Scholar]
- 17.Holm SO, et al. 2001. Comparison of two azithromycin distribution strategies for controlling trachoma in Nepal. Bull World Health Organ 79: 194–200. [PMC free article] [PubMed] [Google Scholar]
- 18.Frick KD, Lietman TM, Osaki-Holm S, Jha H, Chaudary J, Bhatta RC, 2001. Cost-effectiveness of trachoma control measures: comparing targeted household treatment and mass treatment of children. Bull World Health Organ 79: 201–207. [PMC free article] [PubMed] [Google Scholar]
- 19.Chidambaram JD, et al. 2004. Mass antibiotic treatment and community protection in trachoma control programs. Clin Infect Dis 39: e95–e97. [DOI] [PubMed] [Google Scholar]
- 20.Skalet AH, et al. 2010. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med 7: e1000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haug S, et al. 2010. The decline of pneumococcal resistance after cessation of mass antibiotic distributions for trachoma. Clin Infect Dis 51: 571–574. [DOI] [PubMed] [Google Scholar]