Abstract.
Cerebral malaria (CM) is a significant cause of mortality and morbidity in sub-Saharan Africa, particularly among young children. Malarial retinopathy is the most specific clinical finding in CM, and fundus examination could help clinicians distinguish CM from other causes of encephalopathy in resource-poor areas. To assess clinician knowledge, practice patterns, and barriers to the use of funduscopy in the diagnosis of CM, we designed a descriptive multinational survey of clinicians in malaria endemic areas. Results of this survey showed that 19% of respondents were not aware of the utility of eye examinations for malarial retinopathy, and almost half (49%) never or almost never examine the eyes in cases of suspected CM. Educating clinicians about malarial retinopathy could be important in improving diagnostic specificity for CM.
INTRODUCTION
Cerebral malaria (CM) is a presentation of Plasmodium falciparum infection and a significant cause of pediatric mortality and long-term disability. Distinguishing coma caused by CM versus other causes of encephalopathy is a diagnostic challenge in resource-poor areas, and malaria overdiagnosis is a well-documented phenomenon.1–3 One prospective autopsy study by Taylor et al.,4 in Malawi, found that 23% of children who died with the diagnosis of CM actually did not have histopathological evidence of CM, but had other causes of death. There is a great need for a diagnostic test that is specific to CM and that is practical to implement in resource-poor areas.
In the research setting, funduscopic examination, by either direct or indirect retinoscopy, is a well-established diagnostic test with high specificity for pediatric CM.5,6 The characteristic retinal findings of retinal whitening, blood vessel color changes, and retinal hemorrhages are signs of parasitized red blood cells causing hypoxia by occlusion of capillaries and venules. In the autopsy study by Taylor et al.,4 retinopathy was the only laboratory or clinical finding that distinguished patients with autopsy-confirmed CM from those who had died of other causes.
In practice, CM diagnosis is made by one of a range of clinicians, many of whom have few resources at hand. There are no studies that document practice patterns of CM diagnosis among this diverse group.
Our multinational survey addresses two questions:
-
1.
Do healthcare providers in malaria-endemic areas know about, understand, and use funduscopy when diagnosing CM?
-
2.
What are the barriers to the understanding and use of funduscopy for CM diagnosis?
METHODS
This was a Duke University Institutional Review Board-exempt study. Informed consent was implied by the submission of any part of the voluntary survey.
A 26-item survey was modeled on published and validated surveys that assess clinical knowledge, perceptions, practice patterns, and barriers regarding contraception in the primary care setting in the United States.7 Assessment of practice patterns and perceptions was written as five-point Likert-type items. Special attention was paid to the wording of the title, introduction, and questions to avoid a selection bias for those who already are aware of funduscopy. The survey was offered in English and in French.
The survey was distributed via an e-mail introduction and link to the survey, which was designed to be mobile friendly. Professional contacts of the authors in various academic, governmental, and nongovernmental organizations were used. As such, the number of recipients is unknown, and a response rate could not be calculated.
Results were analyzed for frequencies of responses (descriptive statistics). Cronbach’s alpha for internal reliability was calculated using data from the following questions: “How often do you (or another clinician) examine the retinas of your patients suspected of cerebral malaria?” “Imagine that you suspect one of your patients to have cerebral malaria. How likely are you to perform or order a funduscopic exam of the eyes?” Location data were mapped, with permission, using Map Tool by Darrin Ward (http://www.darrinward.com/lat-long/).
RESULTS
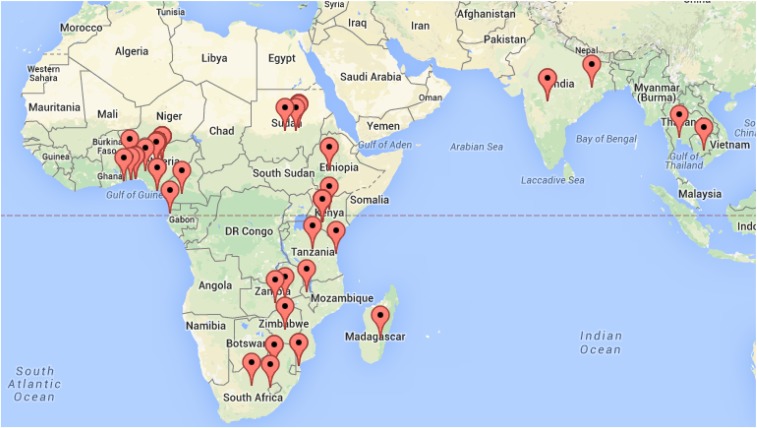
Seventy-four responses were submitted from sub-Saharan Africa and Asia, representing 21 different countries. All answered questions were included in the analyses (52–55 responses per item). The distribution of completed surveys corresponds to the most recent map of malaria endemicity (Figure 1),8 a measure that is closely related to incidence of CM.9 Ninety-eight percent (52/53) indicated that they had internet access at least once a week. See Table 1 for the demographic characteristics of the respondents.
Figure 1.
Geographic distribution of survey responses. Locations were mapped primarily using user-reported city and country information, and geographic coordinates of Internet Protocol addresses in cases where location was not provided by the user. Google Maps image used here in accordance with Google’s Permissions Policy; map data from Google Maps and National Institute of Statistics and Geography, Mexico (INEGI). Locations plotted using Map Tool by Darrin Ward, with permission. This figure appears in color at www.ajtmh.org.
Table 1.
Survey respondent characteristics
| Specialty | No. | %, N = 53 |
|---|---|---|
| Pediatrics | 34 | 64 |
| Other | 17 | 32 |
| Critical care | 8 | 15 |
| Medicine | 7 | 13 |
| Patient age group | No. | %, N = 54 |
| 0–15 years old | 42 | 78 |
| > 15 years old | 23 | 43 |
| Practice setting | No. | %, N = 54 |
| Teaching hospital | 33 | 61 |
| Urban | 26 | 48 |
| Clinic | 10 | 19 |
| Rural | 8 | 15 |
| District hospital | 7 | 13 |
| Mission hospital | 5 | 9 |
Demographic questions were multiple-selection “Check all that apply” format.
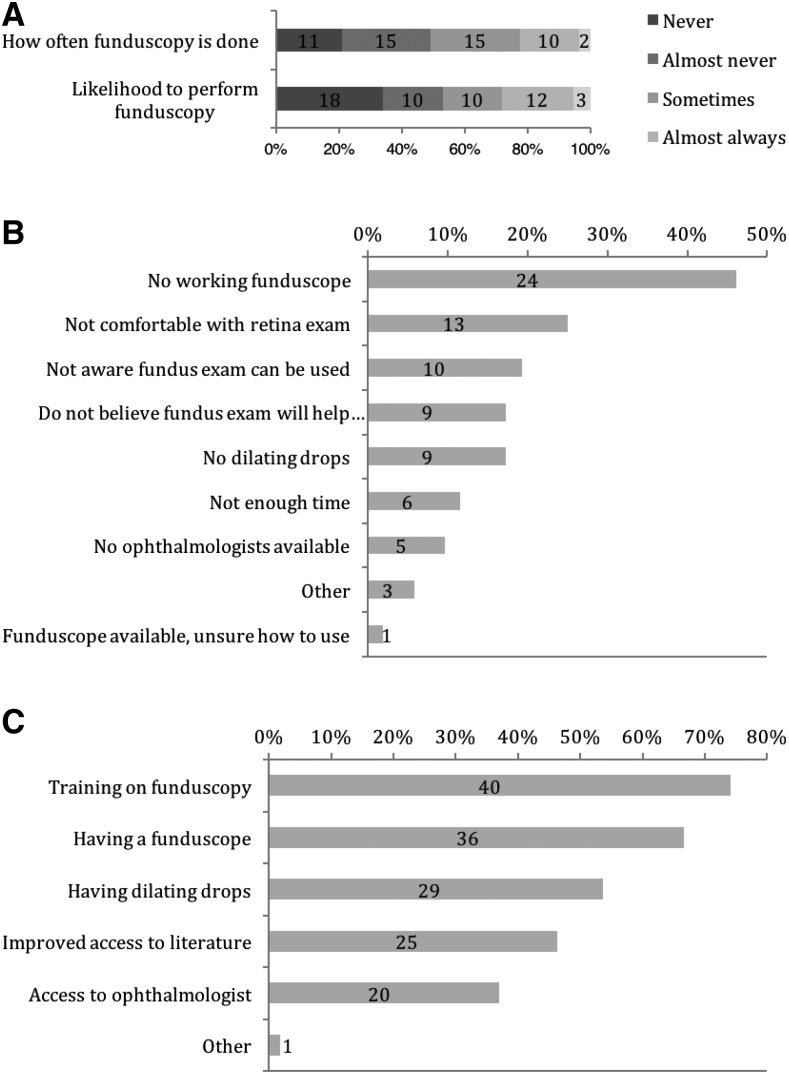
Sixty percent (32/53) reported having diagnosed CM in the last year. More than one-third of respondents (34%, 18/53) are not at all likely to perform funduscopy in a case of suspected CM (Figure 2A). Cronbach’s alpha for internal reliability for these two questions assessing this clinical behavior was 0.96, indicating that responses were highly consistent between the two items. The most common barriers to funduscopy (Figure 2B) were lack of a working funduscope (46% of 53 respondents), discomfort with retina examination (25%), lack of awareness of the use of funduscopy in CM (19%), a belief that funduscopy would not help with CM diagnosis (17%), and lack of dilating drops (17%). Thirty-six percent (19/53) reported having no working funduscope available. When asked what would help the respondent perform funduscopy, training on funduscopy (74% of 53 respondents) and having a working funduscope (67%) were the most frequent answers (Figure 2C).
Figure 2.
Funduscopic practice patterns and barriers. (A) Respondents were asked about current behavior, “How often do you (or another clinician) examine the retinas of your patients suspected of cerebral malaria?” (top) and about likelihood to perform funduscopy in a hypothetical case, “Imagine that you suspect one of your patients to have cerebral malaria. How likely are you to perform or order a funduscopic exam of the eyes?” (bottom). N = 53, Cronbach’s alpha=0.96. (B) Frequency of answers to the question, “What are the barriers to performing funduscopy on patients suspected of having cerebral malaria?” N = 52. (C). “Which of the following would help you perform ocular fundus examination of patients with suspected cerebral malaria?” N = 54.
Participants generally highly rated their ability to distinguish coma caused by CM from coma due to other causes. Nearly half (49%, 26/53) indicated that it is extremely important to improve his/her methods of diagnosing CM. The large majority of respondents preferred in-person training to learn more about funduscopy for CM diagnosis (81%, 43/53).
DISCUSSION
Malarial retinopathy is well established as the most specific diagnostic sign for pediatric CM,10 and funduscopy is recommended in the World Health Organization Management of Severe Malaria.11 We showed that, in this group of voluntary online survey respondents, the likelihood to perform funduscopy is low for both technical and perceptual reasons. Our study suggests that clinician awareness and acceptance are just as important as physical resources and technical skill. The respondents’ self-rated ability to distinguish malarial coma from other causes was high, but published research suggests that this perception contributes to the problem of overdiagnosis.3 In our survey, the results on educational preferences suggest that an evidence-based educational intervention such as one proposed by Chandler et al.,12 could be effective in changing clinician mindsets toward funduscopy. Several reports show promising results when non-ophthalmologists or nonphysicians are trained to detect retinopathy of prematurity,13 diabetic retinopathy,14 or cytomegalovirus retinopathy.15 With the advent of ever increasing mobile access in sub-Saharan Africa, smart-phone-based fundus imaging, and ultra-low-cost funduscopes, adoption of a new diagnostic practice pattern is more feasible.
There are several limitations and biases to our study. Those diagnosing CM are general medical clinicians and health workers in resource-poor and remote areas. This survey is likely to have reached the most privileged of that group, and there were no respondents from the highly endemic areas of central sub-Saharan Africa, e.g., the Democratic Republic of the Congo. There is no way to verify the self-reported data. However, there is good internal reliability of the questions assessing funduscopic examination practice, one of the main outcomes. A response rate cannot be calculated because it is impossible to estimate the number of people who saw the recruitment e-mail. The power cannot be calculated because this is the first survey of its kind in the field, and the size of the target audience is ill defined and spans continents. There is a selection bias for respondents who have connections to contacts of the authors, who, in turn, have close ties to developed countries. The online survey also selects for those most likely to have internet access.
Despite these limitations, the novel strength of this study is its geographic breadth, which revealed clear themes of current practice patterns and barriers in CM diagnosis. Our work highlights the need for targeted educational efforts in addition to technological innovation of low-cost, easy-to-use ophthalmoscopy.
Acknowledgments:
We thank Scott Clifford and the Duke Social Science Research Institute for help with survey design and Qualtrics. Special thanks go to the members of the Arab-African Society of Retina Specialists and of the African Collaborative Research Network, who were invaluable in distributing the survey.
Disclaimer: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) L. S., N. A. V. B., O. O., and T. H. M. have no relationships that might have an interest in the submitted work in the previous 3 years; (2) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (3) L. S., N. A. V. B., O. O., and T. H. M. have no nonfinancial interests that may be relevant to the submitted work.
REFERENCES
- 1.Strøm GEA, Haanshuus CG, Fataki M, Langeland N, Blomberg B, 2013. Challenges in diagnosing paediatric malaria in Dar es Salaam, Tanzania. Malar J 12: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandler CIR, Mwangi R, Mbakilwa H, Olomi R, Whitty CJM, Reyburn H, 2008. Malaria overdiagnosis: is patient pressure the problem? Health Policy Plan 23: 170–178. [DOI] [PubMed] [Google Scholar]
- 3.Chandler CIR, Jones C, Boniface G, Juma K, Reyburn H, Whitty CJM, 2008. Guidelines and mindlines: why do clinical staff over-diagnose malaria in Tanzania? A qualitative study. Malar J 7: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME, 2004. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 10: 143–145. [DOI] [PubMed] [Google Scholar]
- 5.Beare NA V, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg 2006;75:790–797. [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor TE, Molyneux ME, 2015. The pathogenesis of pediatric cerebral malaria: eye exams, autopsies, and neuroimaging. Ann N Y Acad Sci 1342: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin SE, Fletcher J, Stein T, Segall-Gutierrez P, Gold M, 2011. Determinants of intrauterine contraception provision among US family physicians: a national survey of knowledge, attitudes and practice. Contraception 83: 472–478. [DOI] [PubMed] [Google Scholar]
- 8.Gething PW, Patil AP, Smith DL, Guerra CA, Elyazar IR, Johnston GL, Tatem AJ, Hay SI, 2011. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J 10: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snow RW, et al. 1997. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 349: 1650–1654. [DOI] [PubMed] [Google Scholar]
- 10.Beare NAV, Lewallen S, Taylor TE, Molyneux ME, 2011. Redefining cerebral malaria by including malaria retinopathy. Future Microbiol 6: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) , 2012. Management of Severe Malaria. Geneva, Switzerland: WHO. [Google Scholar]
- 12.Chandler CIR, Meta J, Ponzo C, Nasuwa F, Kessy J, Mbakilwa H, Haaland A, Reyburn H, 2014. The development of effective behaviour change interventions to support the use of malaria rapid diagnostic tests by Tanzanian clinicians. Implement Sci 9: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saunders RA, Donahue ML, Berland JE, Roberts EL, Von Powers B, Rust PF, 2000. Non-ophthalmologist screening for retinopathy of prematurity. Br J Ophthalmol 84: 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma L, Prakash G, Tewari HK, Gupta SK, Murthy GVS, Sharma N, 2003. Screening for diabetic retinopathy by non-ophthalmologists: an effective public health tool. Acta Ophthalmol Scand 81: 373–377. [DOI] [PubMed] [Google Scholar]
- 15.Tun N, London N, Kyaw MK, Smithuis F, Ford N, Margolis T, Drew WL, Lewallen S, Heiden D, 2011. CMV retinitis screening and treatment in a resource-poor setting: three-year experience from a primary care HIV/AIDS programme in Myanmar. J Int AIDS Soc 14: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]