Abstract
The cytokinin content in the primary leaves of bean (Phaseolus vulgaris) was monitored for 10 d after inoculation with white clover mosaic potexvirus. The cytokinins were isolated, purified, separated by high-performance liquid chromatography, and quantified by radioimmunoassay. The cytokinins detected at the time of inoculation (d 0) were: (a) the free bases, zeatin (Z), dihydrozeatin (DZ), and isopentenyladenine; (b) the riboside, DZ riboside (DZR); (c) the O-glucosides of DZ, DZR, and Z riboside; (d) the nucleotides, Z riboside-5′-monophosphate and isopentenyladenosine-5′-monophosphate; and (e) trace amounts of Z-9-glucoside and DZ-9-glucoside. During the 10 d after inoculation with white clover mosaic potexvirus, marked quantitative changes in this cytokinin profile were observed. The concentration of the free bases and DZR decreased, accompanied by an increase in the 9-glucosides and the nucleotides. Virus titer increased rapidly 3 d after inoculation, attaining a maximum level at d 5. This increase coincided with the increases in the 9-glucosides and the nucleotides. We propose that the decline in the cytokinin free bases and riboside may allow the increase of virus titer in bean and lead to the senescence of infected leaves.
Symptoms of viral infection in plants, such as leaf chlorosis and abscission, are similar to those observed in naturally senescing leaves (Fraser and Whenham, 1982). Because the cytokinins are implicated in the delay of senescence (Thimann, 1987; Clarke et al., 1994), we were interested in monitoring endogenous cytokinin levels after virus infection. We have recently shown that the exogenous application of the cytokinins DZ and DZR to bean (Phaseolus vulgaris) led to an inhibition of virus replication (Clarke et al., 1998). Furthermore, Sano et al. (1994, 1996) and Sano and Ohashi (1995) have implicated the cytokinins as components of the plant defense signal transduction pathway.
Changes in cytokinin-like activity after virus replication in tobacco have been reported by several groups. Kuriger and Agrios (1977) and Tavantzis et al. (1979) reported that virus infection led to a reduction in cytokinin-like activity. Furthermore, Sziraki et al. (1980) reported that the development of systemic acquired resistance coincided with an increase in the Z and ZR content of resistant leaves. They suggested that increased cytokinin concentration was essential for the development of virus resistance. However, in all of these studies low-resolution chromatographic techniques were used to separate the cytokinins, and cytokinin activity was determined using bioassay. The shortcomings of these techniques are well known (Whenham, 1989).
To combat this, Whenham (1989) used capillary GC with N2-specific detection to quantify the cytokinins found in tobacco infected with tobacco mosaic virus. He reported a decline in the concentration of Z and an increase in the storage forms Z-O-glucoside and ZROG. Dermatsia et al. (1995) observed increased 9-glucosylation in the roots of potato plants infected with PVY. Subsequently, Dermatsia and Ravnikar (1996) quantified a wide range of cytokinins in tissue-cultured potato plants 8 weeks after PVY infection and reported an increase in the total cytokinin content of infected plants. This was attributed to an increase in iP9G and ZR. There was no significant alteration in the other cytokinins quantified, but no attempt was made to quantify either the O-glucosides or the nucleotides. Furthermore, no attempt was made to inhibit the breakdown of the nucleotides, which may have resulted in an increase in the corresponding riboside.
Because the information on cytokinin metabolism in response to virus infection is incomplete and often conflicting, the aim of this study was to accurately determine the changes in endogenous cytokinin profile after virus infection. Infection of bean with WClMV was chosen as a model system because the metabolism of the cytokinins in bean is well characterized (Palmer et al., 1981; Palmer and Wong, 1985; Griggs et al., 1988, 1989; Hammerton et al., 1996), as is the response of bean to WClMV (Fry, 1959; Gibbs et al., 1966; Miki and Knight, 1967). The cytokinin extracts from these plants were purified with ion-exchange and reverse-phase chromatography. After this, the individual cytokinins were separated and quantified via HPLC and RIA. Subsequent identification of some of the cytokinin forms was achieved using electrospray MS/MS.
MATERIALS AND METHODS
Plant Material and Experimental Design
Bean (Phaseolus vulgaris L. cv Top Crop) seedlings were grown as described previously by Clarke et al. (1998). When the primary leaves had fully expanded (14–16 d after germination), they were inoculated with either WClMV or distilled water (Clarke et al., 1998). Duplicate inoculated leaves (5 g fresh weight) were collected randomly from both virus-inoculated and water-inoculated plants on the day of inoculation (d 0) and 1, 3, 5, and 10 d after inoculation. Virus titer was determined via ELISA (Clarke et al., 1998).
Measurement of the Endogenous Cytokinins
Harvested tissue was immediately placed in modified Bieleski's solution (Jameson et al., 1987) at −20°C for 48 h. The tissue was homogenized and placed at 4°C for 48 h. The homogenate was then centrifuged, the supernatant was removed, and the pellet was resuspended in modified Bieleski's solution for an additional 48 h. The tissue was centrifuged again, and the second supernatant was pooled with the first. The internal standards [3H]ZR-trialcohol, [3H]iPA-trialcohol (50,000 cpm each), and [14C]AMP (30,000 cpm, Amersham) were added before purification.
The sample was purified by passage through a cellulose phosphate column (P1 floc cation exchanger, NH4+ form, Whatman; Badenoch-Jones et al., 1984) and washed with 0.05 m acetic acid. The cytokinin nucleotides were eluted in the wash, followed by the free bases, ribosides, and glucosides, which were eluted in 0.5 m NH4OH. The nucleotides were converted to their riboside and/or free base forms (Lewis et al., 1996) and separated using the above cellulose phosphate chromatographic conditions. The basic fraction (containing the hydrolyzed nucleotides) and the free base/riboside/glucoside fraction were passed through linked columns of DEAE-cellulose (DE-52, Whatman) and octadecyl silica (Bondesil, Analytichem International, Boston, MA; Jameson and Morris, 1989). The cytokinins were eluted from the octadecyl silica column with methanol.
Bulk separation of the free base/riboside fraction from the glucosides was achieved using normal-phase HPLC on an Alphasil 5NH2 column (250 × 4.6 mm, HPLC Technology, Cheshire, UK; Lewis et al., 1996). The O-glucosides were then converted to their respective free bases and ribosides, and the individual cytokinins were separated using reverse-phase HPLC on an octadecyl silica column (5 μm, 250 × 4.6 mm, Beckman; Lewis et al., 1996).
Two antibody clones were used for RIA: clone 16, which had a good affinity for hydroxylated cytokinins such as Z, DZ, ZR, DZR, Z9G, and DZ9G, and clone 12, which cross-reacted with iP, iPA, and iP9G (Trione et al., 1985; Lewis et al., 1996). The RIA was carried out as described previously in Jameson and Morris (1989). The antibodies were diluted in RIA buffer so that 50 μL bound 50% of the [3H]trialcohol in the absence of competitive antigen. Nonspecific binding was low for all of the assays and the minimum detection limit was between 0.6 and 1.0 pmol of ZR or iPA. Aliquots from each HPLC fraction were evaporated to dryness, and 5000 cpm [3H]ZR-trialcohol or [3H]iPA-trialcohol was added with the RIA buffer. Standard curves of ZR (clone 16) and iPA (clone 12) were conducted in triplicate with every RIA. The basis for identification was a combination of HPLC retention time and immunocross-reactivity. For quantification, values were adjusted for losses during purification and for differential cross-reactivity with the cytokinin antibodies (Lewis et al., 1996). The 7-glucosides were not cross-reactive with the antibodies used in this study and no attempt was made to detect them.
Identification of Z9G via Electrospray MS
The identity of Z9G was confirmed using electrospray MS/MS. The virus inoculation experiment was repeated as described above using cv Top Crop grown from seed purchased from Aylett Nurseries (Radlett, Hertfordshire, UK) and a freshly isolated strain of WClMV. Leaf samples were removed from the inoculated plants at 0 and 10 d after virus inoculation (10.6 and 11.6 g fresh weight, respectively). The cytokinins were extracted as outlined above, although only the glucoside fraction was investigated. The glucosides were separated from the free bases and ribosides via normal-phase HPLC using the Alphasil 5NH2 column. They were treated with β-glucosidase and further separated using the Alphasil 5NH2 column. One fraction was collected at the retention time of Z9G/DZ9G, whereas the rest of the sample was collected as a second fraction.
The two fractions were evaporated to dryness and dissolved in methanol for analysis via LC/MS/MS (API 300, Perkin-Elmer). The samples were injected by a fused silica capillary tube (i.d. 1–2 μm) attached to a syringe pump (model 2400-001, Harvard Apparatus, South Natick, MA) at 5 μL min−1, and a potential between 4.5 and 5.0 kV was applied to the sample to induce electrospray. A coaxial air spray was also applied at 0.7 L min−1. The conditions were optimized for the standards Z9G and DZ9G at an ion-spray voltage of 5.2 kV, an orifice voltage of 90 V, and a focus ring of 300 V for Z9G and 320 V for DZ9G. Scans were carried out in the first quadrupole with a range between 50 and 500 m/z, in steps of 0.1 atomic mass unit with a dwell time of 1.0 ms. Further structural information was produced by fragmentation of the molecular ion with collision-induced dispersion, and the ion-spectrum product was measured using the third quadrupole.
RESULTS
Cytokinin Content of the Virus and Water-Inoculated Leaves
Free Bases and Ribosides
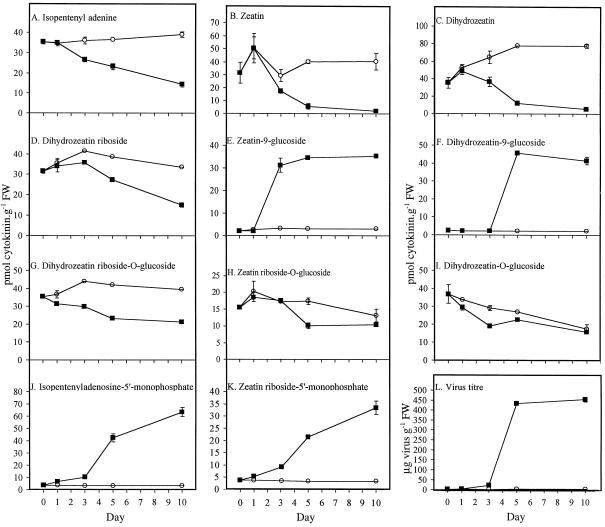
Leaves inoculated with WClMV showed a marked decline in the free bases, beginning just 1 d after virus inoculation (Fig. 1, A–C). Within 10 d the concentration of iP had decreased to 15 pmol g−1 fresh weight, one-half of the level detected in the control leaves (Fig. 1A). The concentrations of both Z and DZ dropped from approximately 30 pmol g−1 fresh weight to about 10 pmol g−1 fresh weight at d 5 and continued to decline over the next 5 d to less than 5 pmol g−1 fresh weight (Fig. 1, B and C). The concentration of Z in the control leaves remained close to that detected at the beginning of the experiment (Fig. 1B). In contrast, the concentration of DZ in the control leaves increased to 80 pmol g−1 fresh weight over the first 5 d, after which it remained constant (Fig. 1C).
Figure 1.
Changes in endogenous cytokinins in primary leaves of bean during the 10 d after inoculation with either water (○) or WClMV (▪). A through K, Cytokinins detected are noted at the top of each box; and L, virus titer measured over the same period. Where se bars are smaller than the data point, they are not displayed. FW, Fresh weight.
The concentration of DZR followed a pattern similar to the free bases, remaining fairly constant at about 35 pmol g−1 fresh weight in the control leaves, but declining to about one-half of that level in the virus-inoculated leaves (Fig. 1D).
Glucosides
One day after WClMV inoculation a rapid increase in Z9G concentration occurred. This stabilized after d 5 at approximately 35 pmol g−1 fresh weight (Fig. 1E). The concentration of DZ9G also began to increase sharply, this time 3 d after infection, and again stabilized after d 5 at about 45 pmol g−1 fresh weight (Fig. 1F). Only trace levels of Z9G and DZ9G were detected in the control leaves. Because Z9G and DZ9G had not previously been detected in bean tissue, the experiment was repeated and a sample at the retention time of Z9G/DZ9G was taken for analysis via electrospray MS/MS. This confirmed the identity of Z9G (Table I). However, in this second experiment DZ9G could not be detected in either the d-10 or the d-0 samples. However, the presence of the other glucosyl dihydro-derivatives, DZOG and DZROG, was confirmed (Table I).
Table I.
Identification of cytokinin standards and putative cytokinin forms from the primary leaves of bean 10 d after inoculation with WCIMV, as determined by electrospray MS/MS
| Compound | Parent Ion (Relative Intensity) | Constituent Ions (Relative Intensity) |
|---|---|---|
| Z9G standard | 382 (100) | 382 (8), 220 (59), 202 (10), 148 (4), 136 (19) |
| DZ9G standard | 384 (100) | 384 (10), 222 (82), 204 (0.1), 148 (1), 136 (6) |
| Putative Z9G | 382 (100) | 382 (5), 220 (58), 202 (7), 148 (4), 136 (26) |
| Putative DZa | 222 (100) | –b |
| Putative DZRa | 354 (100) | 354 (7), 222 (68) |
Aglycone of the O-glucoside following β-glucosidase treatment.
–, Compound not subject to MS fragmentation.
In contrast to the 9-glucosides, O-glucoside levels tended to decrease after WClMV inoculation (Fig. 1, G–I). The concentration of DZR-O-glucoside declined to one-half of that observed in the control leaves over the 10 d (Fig. 1G). The concentration of ZROG increased in both the control and infected leaves immediately after inoculation. However, this was followed by a decline, which was somewhat sharper in the virus-inoculated leaves than in the controls. By d 10, the concentration of ZROG in both the control and infected leaves had decreased to a similar level (Fig. 1H). A decrease in the DZOG concentration began in the leaves immediately after inoculation, and again this was more rapid in the virus-inoculated leaves than in the control leaves. By d 10, the concentration of DZOG had declined to a similar level (approximately 20 pmol g−1 fresh weight) in both leaf types (Fig. 1I).
Nucleotides
In the virus-infected leaves the concentration of iPA-5′-monophosphate began to increase from low levels only 1 d after inoculation; by d 10 it had increased to 70 pmol g−1 fresh weight (Fig. 1J). A similar trend was observed in ZR-5′-monophosphate levels, where its concentration rose to 35 pmol g−1 fresh weight in infected leaves by d 10 (Fig. 1K). In both cases the concentration of nucleotide observed in the control leaves was less than 5 pmol g−1 fresh weight and remained at this level throughout the experiment.
Influence of Viral Infection on the Total Cytokinin Pool
The total cytokinin content of the primary leaves was calculated for each of the cytokinin groups (free bases and riboside, O-glucosides, N-glucosides, and nucleotides) in both control and virus-infected leaves over the 10 d of the experiment (Table II). At the end of the experiment, the total cytokinin pool was the same in the virus-inoculated as in the control leaves. However, the profile of the individual cytokinin groups in the virus-infected leaf tissue had changed markedly. The free bases and riboside were reduced by nearly 80% and the O-glucosides were reduced by 33%. In contrast, there was a marked increase in both the 9-glucosides and the nucleotides, with little detection of these compounds in the control leaves (Table II).
Table II.
Distribution of cytokinins in the control and WCIMV-infected primary leaves of bean during the 10 d after inoculation
| d | Virus-Free Control
|
WCIMV Infected
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FB/R | O-G | N-G | NT | Total | FB/R | O-G | N-G | NT | Total | |
| pmol g−1 fresh wt | ||||||||||
| 0 | 134.1 | 87.6 | 5.2 | 5.1 | 232.0 | 133.5 | 85.3 | 5.4 | 5.3 | 229.5 |
| 1 | 174.1 | 90.8 | 5.1 | 4.9 | 274.9 | 168.0 | 79.1 | 5.3 | 10.2 | 262.6 |
| 3 | 172.0 | 90.2 | 5.3 | 5.1 | 272.6 | 116.5 | 66.5 | 33.4 | 19.4 | 235.8 |
| 5 | 193.6 | 86.1 | 5.1 | 5.4 | 290.2 | 73.2 | 56.1 | 80.2 | 66.6 | 276.1 |
| 10 | 191.1 | 70.2 | 5.2 | 5.1 | 272.6 | 36.8 | 47.4 | 86.7 | 103.2 | 274.1 |
FB, Free bases; R, ribosides; O-G, O-glucosides; N-G, N-glucosides; and NT, nucleotides.
The changes in the endogenous cytokinin pool of the infected plants coincided with an increase in virus titer (Fig. 1L). Virus titer did not increase until after d 3 when a rapid increase was observed; after d 5 it stabilized at approximately 450 μg virus g−1 fresh weight. The decline in the total free bases, riboside, and O-glucosides and the increase in the N-glucosides and nucleotides were associated with this increase in virus titer.
DISCUSSION
A detailed picture of the changes in cytokinin profile in response to virus infection is presented. Metabolically, it appears that after infection of bean with WClMV, the free base, riboside, and O-glucoside forms are diverted into the production of nucleotides and inactive 9-glucosides (Table II). How these changes in metabolism are controlled is uncertain. However, the increase in nucleotides may represent a block in the cytokinin biosynthetic pathway, the inhibition of nucleotide turnover to active forms, or the increased activity of enzymes that convert the free bases and ribosides to nucleotide forms (Jameson, 1994). The increase in the 9-glucosides may represent increased activity of N-glucosyltransferases (Mok and Martin, 1994), with a decrease in side chain removal (Chatfield and Armstrong, 1986) and/or compartmentation of the 9-glucosides away from degrading enzymes (Jameson, 1994).
In this work the appearance of the 9-glucosides was one of the major consequences of viral infection. Z9G and DZ9G have not previously been detected in bean (Palmer et al., 1981; Palmer and Wong, 1985; Griggs et al., 1988, 1989; Hammerton et al., 1996). However, in those studies only healthy, unstressed tissue was examined for cytokinin content. In the present study, trace amounts of the 9-glucosides were detected in healthy leaves, whereas viral infection led to the accumulation of high concentrations of the 9-glucosides (Fig. 1, E and F; Table II).
Because this is the first time, to our knowledge, that 9-glucosides have been detected in bean, the experiment was repeated to allow confirmation of their identity via electrospray MS/MS. Although the identity of Z9G was unequivocally confirmed, DZ9G was not detected in the 10-d-old virus-inoculated tissue. This was unexpected, especially considering the presence of the other glucosyl-dihydroderivative forms in this tissue. It is possible, however, that some differences in cytokinin metabolism may have occurred because of the use of an altered seed source for the cv Top Crop plants or the newly isolated virus. Further analysis will be required to confirm these possibilities.
The 9-glucosides are believed to be products of cytokinin deactivation (Jameson, 1994). Therefore, it appears that in the first 5 d after virus infection removal of the active cytokinins is occurring. We propose that the production of the 9-glucosides is a direct response to WClMV infection in bean.
Although there have been no other reports regarding the effect of viral infection on the endogenous cytokinin pool in bean, evidence in several other species supports a decline in the active cytokinin free bases and ribosides after viral infection. Kuriger and Agrios (1977), Tavantzis et al. (1979), and Whenham (1989) all reported a decline in Z- and ZR-like compounds after tobacco ringspot virus and tobacco mosaic virus infection of cowpea and tobacco. Although ZR was not detected in our work a decline in iP, Z, DZ, and DZR was observed in infected leaves (Fig. 1, A–D). However, Dermatsia et al. (1995) reported an increase in both ZR and iP9G in PVY-infected potato leaves with no detected alteration in other cytokinins. This result conflicts with the above reports. However, it appears that Dermatsia et al. (1995) did not attempt to inhibit phosphatase activity to prevent nucleotide breakdown during their initial cytokinin extraction. The nucleotides may have been a source of the increased levels of ZR in their infected material; we have shown that the nucleotides increased significantly postinfection. Furthermore, the plants were maintained in vitro for the duration of the study, which was noted to stimulate cytokinin synthesis in the same study (Dermatsia et al., 1995). Nonetheless, the report by Dermatsia et al. (1995) of an increase in iP9G is significant, particularly with respect to the increased concentration of Z9G and DZ9G observed in this study (Fig. 1, E and F; Table II).
The symptoms of viral infection such as induced leaf chlorosis and abscission are similar to those changes that occur during natural leaf senescence (Fraser and Whenham, 1982). Leaf senescence is usually associated with a decline in chlorophyll content, loss in turgidity, decline in nucleic acids and proteins, breakdown of cellular membranes, and finally, abscission of the leaf (Thimann, 1987). Both senescence (Singh et al., 1992) and viral infection (this work) resulted in a decline in the cytokinin free bases, ribosides, and O-glucosides. There are no studies on the changes of the N-glucosides during senescence. The changes that we measured in cytokinin metabolism would have been too late to act as a signal of virus infection and are more likely to be related to later facets of virus infection, such as induced senescence. Furthermore, virus-induced senescence may enhance virus movement through the plant. Increased movement of metabolites from the leaf (because of senescence) may facilitate systemic virus spread via the phloem.
Cytokinins can act as oxygen-free-radical scavengers and may thus inhibit senescence (Musgrave, 1994). It is interesting that the increase in virus replication that we observed in this study began 3 d after virus inoculation (Fig. 1L), by which time the cytokinin free bases and ribosides had already declined to 68% of that in the controls. By d 5, when the virus titer had reached its peak, the free bases and ribosides had decreased to 38% of that in the controls, before declining after 5 d to 19%. We propose that a reduction in these cytokinins, with a consequent reduction in oxygen-free-radical scavengery (either directly or through a reduction in the production of enzymes that detoxify oxygen free radicals), may account for some of the symptoms exhibited by virus-infected plants and may also play a role in virus replication.
Recently, we have shown that the application of DZ and DZR to the xylem of bean plants led to a reduction in virus replication at the point of double-stranded RNA synthesis and prevented the normal virus-induced decrease in free-radical-scavenging enzymes (Clarke, 1996; Clarke et al., 1998). The role of oxygen free radicals, the enzymes involved in their detoxification, and the influences of plant hormones and virus infection over them will be reported in a future paper.
ACKNOWLEDGMENTS
We gratefully acknowledge the gift of monoclonal antibodies from Prof. R.O. Morris (University of Missouri, Columbia). We acknowledge the use of equipment purchased by funds from the University Grants Committee and the New Zealand Lottery Grants Board for P.E.J. We thank Dr. Gill Norris and Michael Wilson (Massey University) for sample analysis via electrospray MS/MS and for compiling Figure 1, respectively, and Prof. Peter Bannister (University of Otago) for advice on statistics.
Abbreviations:
- DZ
dihydrozeatin
- DZ9G
DZ-9-glucoside
- DZOG
DZ-O-glucoside
- DZR
DZ riboside
- iP
isopentenyladenine
- iP9G
iP-9-glucoside
- iPA
iP-adenosine
- PVY
potato virus Y
- RIA
radioimmunoassay
- WClMV
white clover mosaic potexvirus
- Z
zeatin
- Z9G
Z-9-glucoside
- ZR
zeatin riboside
- ZROG
ZR-O-glucoside
LITERATURE CITED
- Badenoch-Jones J, Letham DS, Parker CW, Rolfe BG. Quantitation of cytokinins in biological samples using antibodies against zeatin riboside. Plant Physiol. 1984;75:1117–1125. doi: 10.1104/pp.75.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield JM, Armstrong DJ. Regulation of cytokinin oxidase activity in callus tissues of Phaseolus vulgaris cv Great Northern. Plant Physiol. 1986;80:493–499. doi: 10.1104/pp.80.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SF (1996) Physiological and molecular interactions between plant hormones and white clover mosaic virus infection of Phaseolus vulgaris L. PhD thesis. University of Otago, Dunedin, New Zealand
- Clarke SF, Burritt DJ, Jameson PE, Guy PL. Influence of plant hormones on virus replication and pathogenesis-related proteins in Phaseolus vulgaris L. infected with white clover mosaic potexvirus. Physiol Mol Plant Pathol. 1998;53:195–207. [Google Scholar]
- Clarke SF, Jameson PE, Downs C. The influence of 6-benzylaminopurine on post-harvest senescence of floral tissues of broccoli (Brassica oleracea var. Italica) Plant Growth Regul. 1994;14:21–27. [Google Scholar]
- Dermatsia M, Ravnikar M. Physiol Mol Plant Pathol. 1996;48:65–71. [Google Scholar]
- Dermatsia M, Ravnikar M, Kovac M. Increased cytokinin-9-glucosylation in roots of susceptible Solanum tuberosum cultivar infected by potato virus YNTN. Mol Plant-Microbe Interact. 1995;8:327–330. [Google Scholar]
- Fraser RSS, Whenham RJ. Plant growth regulators and virus infection: a critical review. Plant Growth Regul. 1982;1:37–59. [Google Scholar]
- Fry PR. A clover mosaic virus in New Zealand pastures. NZ J Agric Res. 1959;2:971–981. [Google Scholar]
- Gibbs AJ, Varma A, Woods RD. Viruses occurring in white clover (Trifolium repens L.) from permanent pastures in Britain. Ann Appl Biol. 1966;58:213–240. [Google Scholar]
- Griggs P, Stuchbury T, Wang TL. Dihydrozeatin from Phaseolus sap: quantification by RIA and GC-MS. Phytochemistry. 1988;27:1583–1587. [Google Scholar]
- Griggs P, Wang TL, Stuchbury T. Cytokinin production in rooted bean (Phaseolus vulgaris L.) cuttings. J Plant Physiol. 1989;135:439–445. [Google Scholar]
- Hammerton R, Nicander B, Tillberg E. Identification of some major cytokinins in Phaseolus vulgaris and their distribution. Physiol Plant. 1996;96:77–84. [Google Scholar]
- Jameson PE. Cytokinin metabolism and compartmentation. In: Mok DWS, Mok MC, editors. Cytokinins: Chemistry, Activity, and Function. Boca Raton, FL: CRC Press; 1994. pp. 113–128. [Google Scholar]
- Jameson PE, Letham DS, Zhang R, Parker CW, Badenoch-Jones J. Cytokinin translocation and metabolism in lupin species. I. Zeatin riboside introduced into the xylem at the base of Lupinus angustifolius stems. Aust J Plant Physiol. 1987;14:695–718. [Google Scholar]
- Jameson PE, Morris RO. Zeatin-like cytokinins in yeast: detection by immunological methods. J Plant Physiol. 1989;135:385–396. [Google Scholar]
- Kuriger WE, Agrios GN. Cytokinin levels and kinetin-virus interactions in tobacco ringspot virus infected cowpea plants. Phytopathology. 1977;67:604–609. [Google Scholar]
- Lewis DH, Burge GK, Schmierer DM, Jameson PE. Cytokinins and fruit development in the kiwifruit (Actinidia delicosa). I. Changes during fruit development. Physiol Plant. 1996;98:179–186. [Google Scholar]
- Miki T, Knight CA. Some chemical studies on a strain of white clover mosaic virus. Virology. 1967;31:55–63. doi: 10.1016/0042-6822(67)90007-4. [DOI] [PubMed] [Google Scholar]
- Mok DWS, Martin RC. Cytokinins and plant development: an overview. In: Mok DWS, Mok MC, editors. Cytokinins: Chemistry, Activity, and Function. Boca Raton, FL: CRC Press; 1994. pp. 155–166. [Google Scholar]
- Musgrave ME. Cytokinins and oxidative process. In: Mok DWS, Mok MC, editors. Cytokinins: Chemistry, Activity, and Function. Boca Raton, FL: CRC Press; 1994. pp. 167–178. [Google Scholar]
- Palmer MV, Scott IM, Horgan R. Cytokinin metabolism in Phaseolus vulgaris L. II Comparative metabolism of exogenous cytokinins by detached leaves. Plant Sci Lett. 1981;22:187–195. [Google Scholar]
- Palmer MV, Wong OC. Identification of cytokinins from xylem exudate of Phaseolus vulgaris L. Plant Physiol. 1985;79:296–298. doi: 10.1104/pp.79.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Ohashi Y. Involvement of small GTP-binding proteins in defense signal-transduction of higher plants. Proc Natl Acad Sci USA. 1995;92:4138–4144. doi: 10.1073/pnas.92.10.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Seo S, Koizumi N, Niki T, Iwamura H, Ohashi Y. Regulation by cytokinins of endogenous levels of jasmonic and salicylic acids in mechanically wounded tobacco plants. Plant Cell Physiol. 1996;37:762–769. [Google Scholar]
- Sano H, Seo S, Orudgev E, Youssefian S, Ishizuka K, Ohashi Y. Expression of the gene for a small GTP-binding protein in transgenic tobacco elevates endogenous cytokinin levels, abnormally induces salicylic acid in response to wounding, and increases resistance to tobacco mosaic virus infection. Proc Natl Acad Sci USA. 1994;91:10556–10560. doi: 10.1073/pnas.91.22.10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Letham DS, Palni LMS. Cytokinin biochemistry in relation to leaf senescence. VII. Endogenous cytokinin levels and exogenous application of cytokinins in relation to sequential leaf senescence of tobacco. Physiol Plant. 1992;86:388–397. [Google Scholar]
- Sziraki I, Balazs E, Kiraly Z. Role of different stresses in inducing systemic acquired resistance to TMV and increasing cytokinin levels in tobacco. Physiol Plant Pathol. 1980;16:277–284. [Google Scholar]
- Tavantzis SM, Smith SH, Witham FH. The influence of kinetin on tobacco ringspot virus infectivity and the effect of virus infection on the cytokinin activity in intact leaves of Nicotiana glutinosa L. Physiol Plant Pathol. 1979;14:227–233. [Google Scholar]
- Thimann K (1987) Plant senescence: a proposed integration of the constituent processes. In WW Thomson, EA Nothangel, RC Huffaker, eds, Plant Senescence: Its Biochemistry and Physiology. American Society of Plant Physiologists, Rockville, MD, pp 1–19
- Trione EJ, Krygier BB, Banowetz GM, Kathrein JM. The development of monoclonal antibodies against the cytokinin zeatin riboside. J Plant Growth Regul. 1985;4:101–109. [Google Scholar]
- Whenham RJ. Effect of systemic tobacco mosaic virus infection on endogenous cytokinin concentration in tobacco (Nicotiana tabacum L.) leaves: consequences for the control of resistance and symptom development. Physiol Mol Plant Pathol. 1989;35:85–95. [Google Scholar]