Abstract
The development of targeted therapies and the resurgence of immunotherapy offer enormous potential to dramatically improve the outlook for patients with invasive urothelial carcinoma (InvUC). Optimization of these therapies, however, is crucial as only a minority of patients achieve dramatic remission, and toxicities are common. With the complexities of the therapies, and the growing list of possible drug combinations to test, highly relevant animal models are needed to assess and select the most promising approaches to carry forward into human trials. The animal model(s) should possess key features that dictate success or failure of cancer drugs in humans including tumor heterogeneity, genetic-epigenetic crosstalk, immune cell responsiveness, invasive and metastatic behavior, and molecular subtypes (e.g., luminal, basal). While it may not be possible to create these collective features in experimental models, these features are present in naturally-occurring InvUC in pet dogs. Naturally occurring canine InvUC closely mimics muscle-invasive bladder cancer in humans in regards to cellular and molecular features, molecular subtypes, biological behavior (sites and frequency of metastasis), and response to therapy. Clinical treatment trials in pet dogs with InvUC are considered a win-win scenario; the individual dog benefits from effective treatment, the results are expected to help other dogs, and the findings are expected to translate to better treatment outcomes in humans. This review will provide an overview of canine InvUC, the similarities to the human condition, and the potential for dogs with InvUC to serve as a model to predict the outcomes of targeted therapy and immunotherapy in humans.
Keywords: Urinary bladder cancer, transitional cell carcinoma, urothelial carcinoma, animal models, dog, immunotherapy, targeted therapy
THE EXPANDING NEED FOR ANIMAL MODELS TO ADVANCE InvUC THERAPY
Muscle-invasive urothelial carcinoma (InvUC) negatively impacts quality of life and is lethal in 50% of patients [1, 2]. In the last two decades, only modest improvement has occurred in the outcome of patients with InvUC [1, 2]. Recently, there has been impressive progress in drugs aimed at molecular, epigenetic, and immune targets [3]. Combinations of these new drugs, along with the finding of differential treatment responses based on molecular InvUC subtype (luminal, basal, etc.), could drive dramatic improvements in InvUC therapy [4–9]. There are, however, not enough patients to test all of the new drugs and various combinations of drugs in order to optimize therapy, especially patients with metastatic disease who are still eligible for trials having typically failed multiple therapies. This puts more pressure on pre-clinical animal studies to select therapies to move forward into humans. Current experimental animal models do not accurately recapitulate the complexities (e.g., tumor heterogeneity, genetic and epigenetic crosstalk, immune cell responsiveness) that drive drug response in human InvUC, especially in regards to invasive and metastatic cancer [10, 11]. A consequence of this is that current models fail to predict success, or more importantly to predict failure, of new cancer therapies in humans, thus leading to a high failure rate in human trials [11]. Clearly, more predictive models are essential. In this review, the case will be presented for dogs with naturally-occurring InvUC to serve as a highly relevant animal model to complement traditional models, in order to much more accurately predict treatment success and failure in humans, thereby improving the success rate in human trials.
NATURALLY-OCCURRING CANINE BLADDER CANCER
The general characteristics of naturally-occurring InvUC in dogs have recently been reviewed, and are summarized briefly herein [12–14]. Each year, approximately 6 million of the 65 million pet dogs in the United States will develop cancer [15]. With bladder cancer comprising 1-2% of all canine cancer, more than 50,000 dogs are expected to develop bladder cancer each year [12].
The most common naturally occurring bladder tumor in dogs is urothelial carcinoma, with the majority being high-grade papillary infiltrative tumors [12]. Invasion into the muscle layers of the bladder is common. Non-muscle invasive bladder cancer is uncommon in dogs. Multiple factors contribute to the development of InvUC in dogs including heritable traits and environmental exposures [12]. Known risk factors include exposure to older type flea control products and lawn chemicals as well as obesity, breed, and female sex [12, 16]. Scottish Terriers have a 21-fold increased risk while Eskimo Dogs, Shetland Sheepdogs, West Highland White Terriers, Keeshonds, Samoyeds, and Beagles have a 3–6.5-fold increased risk of developing InvUC [12]. This makes dogs an invaluable model to study heritable risk and gene-environment interactions, and to evaluate early detection and intervention strategies.
The clinical features of InvUC in dogs mimic those in humans. The most common clinical signs in dogs with InvUC are hematuria, stranguria, and pollakiuria [13]. Diagnosis is made via cystoscopic or surgically obtained biopsies for histopathology. Canine and human InvUC have similar pathology including cellular features, tumor heterogeneity, and local invasion [12, 14, 17]. Distant metastases are present in 15–20% of dogs at diagnosis, and in 50% or more of dogs at death [12]. Distant metastases occur in abdominal organs, lung, bone, and other locations [12, 18, 19].
InvUC is considered a very treatable, but rarely curable, disease in dogs. Treatment can include surgery, radiation therapy, and chemotherapy, with the latter being the mainstay of treatment. The majority of InvUC is located within the trigone region of the bladder making partial cystectomy only possible in a small subset of dogs [13]. Complete cystectomy is not typically performed in pet dogs because of the frequent extension of the tumor down the urethra, presence of metastasis at the time of diagnosis in some dogs, the morbidity associated with the surgical procedure, and the expense involved [13, 20]. The mainstay of treatment includes cyclooxygenase (COX) inhibitors, chemotherapy, and combinations of these [12–14]. COX inhibitors have antitumor effects in canine InvUC. Single agent COX inhibitor treatment leads to remission rates of 18–20% and stable disease rates of approximately 55% in dogs [12, 14]. Chemotherapy agents with known activity in dogs with InvUC include cisplatin, carboplatin, gemcitabine, vinblastine, mitoxantrone, and chlorambucil with response rates similar to those in humans [12, 14, 21–24]. COX inhibitors can enhance the effects of chemotherapy (cisplatin, carboplatin, vinblastine) when used in combination [12–14, 21]. For example, in a randomized trial in dogs with InvUC, the remission rate was significantly higher in dogs receiving vinblastine combined with piroxicam (58% ) than in dogs receiving vinblastine alone (23% ) [21]. Interestingly, dogs across all breeds appear to have similar responses to medical therapies for InvUC [12, 14, 21–24]. The placement of urethral and ureteral stents can be performed when needed to relieve urinary obstruction [13]. Overall, the cancer can be effectively managed in 75–80% of dogs with median survival times extending over a year, especially when multiple sequential therapies are used [13].
Pet owners are becoming increasingly motivated to treat their pets with InvUC and to allow their pets to participate in clinical trials. Clinical trials involving pets with naturally occurring cancer are considered a win-win scenario that benefits dogs and generates knowledge to help humans given the vast similarities seen between species (summarized in Table 1). Inclusion criteria in canine trials typically include histopathologic confirmation of InvUC, measureable disease, and expected survival of at least six weeks. Since a specific standard of care treatment has not been defined or regimented for dogs with InvUC, enrollment of dogs with treatment-naïve cancer into trials is well accepted. Depending on the goals of the trial, inclusion criteria can be set to include dogs with treatment-naïve cancer, cancer resistant to prior therapies, or both; and dogs with organ confined-disease, metastatic disease, or both.
Table 1.
Similarities between naturally-occurring canine invasive urothelial carcinoma and human invasive urothelial carcinoma
| Similarities in muscle-invasive bladder cancer between dogs and humans |
| Physiological age of onset |
| Clinical signs/symptoms |
| Cellular and pathological features including high grade, tumor heterogeneity, and local invasion |
| Molecular subtypes (e.g. luminal, basal) |
| Biological behavior (sites and frequency of metastasis) |
| Response to chemotherapy (e.g. cisplatin, carboplatin, vinblastine) |
| Shared molecular targets (e.g. EGFR, CDKN2B, PIK3CA, BRCA2, NFkB, ARHGEF4, XPA, RB1CC1, RPS6, MITF, and WT1) |
POTENTIAL ROLE FOR CANINE InvUC TO MODEL TARGETED THERAPIES
In addition to strong evidence that dogs with naturally-occurring InvUC can model traditional chemotherapy responses, there is more excitement for the potential for dogs to model and accurately predict the outcome of targeted therapies, immunotherapies, and new combinations of drugs. Although the molecular characterization of canine InvUC is in the early stages, there is already compelling evidence for the presence of druggable molecular targets shared between dogs and humans.
Shared molecular features between canine and human InvUC
The 2014 Cancer Genome Atlas Research Network comprehensive molecular characterization of urothelial bladder carcinoma provided insight into the molecular pathogenesis of human InvUC and identified potential targets for therapy [25]. Genomic alternations involving PI3K/AKT/mTOR, CDKN2A/CDK4/CCND1, and RTK/RAS pathways were identified making receptor tyrosine kinases such as EGFR, ERBB2 (Her-2), ERBB3, and FGFR3 potential targets for therapy [25].
Overexpression of epidermal growth factor receptor (EGFR) has been detected in 73% of canine InvUC, which is comparable to what has been reported in humans [26–29]. HER-2 (EGFR2/ERBB2/NEU) was also found to be significantly overexpressed in canine InvUC samples when compared to non-neoplastic urothelium [30]. Inhibitors of EGFR family proteins have been evaluated in multiple trials in humans with bladder cancer with varying success [31–33]. In a 2015 review of the literature, it was concluded that EGFR inhibitors appear to be useful in a subset of patients including patients that are chemotherapy naïve and that have cancer overexpressing EGFR or ERBB2 [34]. Prior treatment with chemotherapy can result in resistance to EGFR inhibitors, although the mechanisms of this are not clear. Canine studies could be conducted to help better understand these mechanisms of resistance and better identify subsets of patients who could benefit from EGFR inhibitor therapy. A trial of an EGFR-targeted therapy in dogs has been initiated at Purdue University by the Comparative Oncology Program.
The p53 tumor suppressor gene product is thought to play a role in the differentiation of the epithelium in the urinary bladder [35]. Loss of p53 expression has been observed in human invasive InvUC and has been associated with lymph node metastasis, advanced tumor-node-metastasis stage, and decreased survival times [35, 36]. Comparable to reports in humans, p63 expression, a homologue of p53, has been reported to be significantly lower in dogs with InvUC, compared to dogs with polypoid cystitis and normal urothelium [37]. Expression of p53, the p53 inducible gene 14-3-3σ protein, and vimentin have been documented in a portion of canine InvUC in vivo and in vitro. In humans, expression of vimentin is associated with epithelial-mesenchymal transition, cancer progression, and metastasis [40]. 14-3-3σ protein has been previously linked to tumorigenesis, and it’s expression has been evaluated in human urothelial carcinoma [41, 42].
One interesting difference between human and canine InvUC is the majority (67–85% ) of canine InvUC harbors a BRAFV595E mutation, which is homologous to the BRAFV600E mutation seen in humans with melanoma and other malignancies [43, 44] leading to constitutive activation of the MAPK pathway. While BRAF mutations are rare in human InvUC, other mutations within the MAPK pathway occur in approximately 30% of cases [25]. A study of a BRAF inhibitor in dogs with InvUC is currently underway at Purdue University with the intent to determine efficacy, safety, and resistance mechanisms that are likely to be important in humans.
Mutations in several other genes implicated in the development and progression of InvUC and other cancers in humans have been identified in canine InvUC [43, 45]. These include EGFR, CDKN2B, PIK3CA, BRCA2, NFkB, ARHGEF4, XPA, NCOA4, MDC1, UBR5, RB1CC1, RPS6, CIITA, MITF, and WT1 [25, 43, 45–57]. Additionally, other shared molecular targets will assuredly be found. In microarray analysis, there were >450 genes that were differentially expressed (between InvUC and normal bladder) and shared between dogs and humans (P < 0.05; 2FC) [27, 58]. Similarly, in RNA-seq analysis, there were 1589 genes that were differentially expressed between normal bladder and bladder cancer in dogs and in humans (selected from 2911 human/canine orthologs pairs whose respective genes were above background in both canine and human datasets) [45].
Examples of targeted therapy studies in dogs
There are published examples of studies of targeted therapies in dogs with InvUC and other cancers, including those of translational value. A canine clinical trial evaluating folate receptor expression and the safety and efficacy of folate-targeted therapy in pet dogs with InvUC was conducted [59]. Folate receptor expression was detected in 78% of canine InvUC, and folate uptake in vivo was confirmed using scintigraphy. An escalating dose of folate-targeted vinblastine (EC0905) was administered to pet dogs with biopsy-confirmed folate receptor positive InvUC. The maximum tolerated dose was determined, with neutropenia and gastrointestinal upset being dose limiting toxicities. Antitumor effects were observed with 5 dogs having partial remission and 4 dogs having stable disease out of 10 dogs treated [59]. Folate receptor expression was identified in human InvUC [59], and further work is ongoing to define the percentage of cases with folate receptor expression, and to evaluate additional folate-drug conjugates in dogs. Positive results could provide the justification for a follow up trial of folate-targeted therapy in humans.
Toceranib phosphate (SU11654, Palladia®) is a multikinase small molecule inhibitor that targets several receptor tyrosine kinases including VEGFR, PDGFR, and KIT [60]. In a phase I trial of toceranib in dogs with various spontaneous tumors, there was an objective response rate of 28% with the responses most commonly seen in dogs with cutaneous mast cell tumors that harbored KIT activating mutations [60]. Additional work was done to establish the pharmacokinetics, pharmacodynamics, and toxicity profile of toceranib in dogs [60–62]. The results of these canine studies helped lay the foundation for subsequent evaluation and ultimately FDA approval of a very closely related small molecule inhibitor, sunitinib (SU11248), in people for treatment of renal cell carcinoma and gastrointestinal stromal tumors [63, 64].
Ibrutinib (PCI-32765: Imbruvica®), a small molecule inhibitor of Bruton’s tyrosine kinase (BTK) was evaluated in dogs with B-cell lymphoma, and was found to have good biological activity and an acceptable toxicity profile [65]. These findings helped lead to further evaluation of ibrutinib in humans and eventual FDA approval for treatment of B cell chronic lymphoid leukemia and mantle celllymphoma [66, 67].
Signaling of the mTOR pathway can contribute to the growth and progression of several cancers including osteosarcoma, and mTOR inhibitors are being evaluated for cancer therapy [68]. The mTOR inhibitor, rapamycin, has been evaluated in dogs with osteosarcoma, and the pharmacokinetics/pharmacodynamics and anticancer effects reported [69–70]. A clinical trial conducted by the National Cancer Institute (NCI) Comparative Oncology Trials Consortium is currently ongoing to evaluate oral rapamycin treatment in pet dogs with osteosarcoma [71]. Rapamycin (sirolimus) and its derivatives such as everolimus (RAD001), are currently under investigation in pre-clinical and clinical trials for people with osteosarcoma [72–75].
MODELING MOLECULAR SUBYTPES IN CANINE InvUC
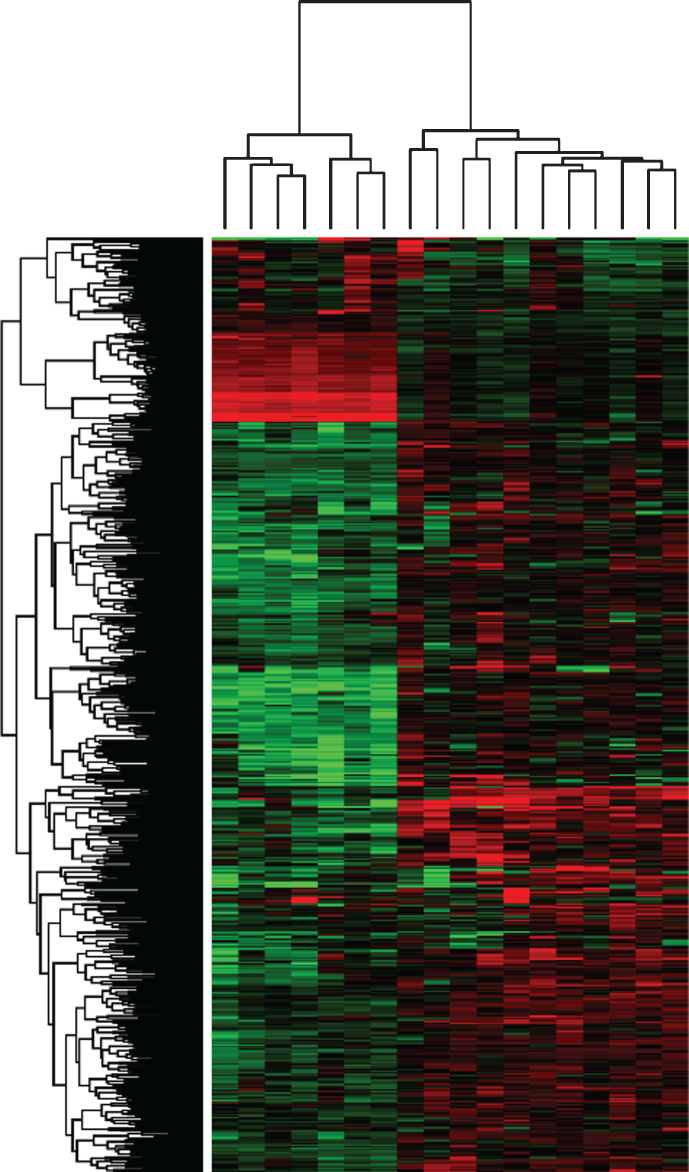
One of the compelling recent advances in InvUC is the identification of gene expression patterns that segregate human InvUC into molecular subtypes including basal, luminal, and others initially described in human breast cancer [4–7, 25, 76]. This is important because there is strong evidence that the cancer behavior and response to therapy differ between subtypes, and thus subtypes should be taken into account when evaluating new (and old) therapies [4–8]. Briefly, basal InvUCs are more prevalent in women than men; are inherently more aggressive; are associated with squamous features, more advanced stage and metastatic disease at diagnosis (although they can be more responsive to chemotherapy and immunotherapy); and are enriched for STAT3, TP63, KRT5/6A, CD44, and NFkB, c-Myc, and HIF signaling [4–8]. Some basal InvUC also express epithelial-to-mesenchymal transition markers of claudin-low breast cancer [77]. Luminal InvUC is associated with papillary histologic features and better clinical outcomes, and is enriched for ER, TRIM24, FOXA1, GATA3, PPARG, and activating FGFR3 mutations (with good response to FGFR inhibitors) [6–8, 78]. It is clear that modeling drug effects across molecular subtypes is critical, and work by our group provides strong evidence that molecular subtypes can be modeled in canine InvUC. Analysis of a “discovery” gene profiling dataset of canine InvUC and normal canine urothelium, revealed two distinct InvUC clusters [27], and a recent re-analysis of the data comparing findings to a list of >600 genes that segregate human InvUC into luminal and basal subtypes, confirmed that these two clusters align closely with luminal and basal expression patterns (Fig. 1).
Fig.1.
Clustering of differentially expressed basal and luminal genes. Gene expression profiling was performed (Canine Genome Array 2.0 Affymetrix, Santa Clara, CA). Microarray data were analyzed for canine normal bladder tissues (n = 4) and compared to canine InvUC (n = 18) using GeneSpring GX 13.1.1 (Agilent Technologies, Santa Clara, CA) and recently updated annotations by Affymetrix. Differentially expressed genes (t-test, p corr 0.05, 2FC) were selected and clustered according to basal and luminal patterns reported in human InvUC [6]. Genes clustered in two distinct groups with seven tumor samples segregating as basal (left cluster), and 11 tumor samples as luminal (right cluster).
DOGS WITH InvUC FOR MODELING IMMUNOTHERAPIES
The promise of emerging immunotherapies and challenges to be met
The unprecedented resurgence in immunotherapy has further heightened the demands for relevant animal models of cancer that can predict the outcomes (efficacy, toxicity) of immunotherapies when given as monotherapy or when combined with other therapies [79]. There is especially widespread interest in immune checkpoint inhibitors. Immune checkpoints, such PD-L1, PD-1, CTLA-4 and others, are key regulatory components of the immune system that are critical for maintaining self-tolerance [80, 81]. Immune checkpoints also modulate the duration and amplitude of physiological immune responses in peripheral tissues in order to minimize collateral tissue damage. Many cancers however, exploit immune checkpoints to avoid immune attack, particularly in evading attack by T cells that are specific for tumor antigens [80–83]. Cancer cells upregulate PD-L1 (and other immune checkpoints) in response to oncogenic signals or endogenous antitumor immune responses, and the binding of PD-L1 to PD-1 on activated T cells causes cell anergy or death [81, 83]. Antigen presenting cells, natural killer cells, and T cells also express PD-L1.
There is compelling evidence that immune checkpoint inhibitors can drive new success in the treatment of InvUC and other cancers through the finding of durable complete remissions in heavily pre-treated patients in multiple studies [80, 82, 84–88]. There is much work to be done, however, before immune checkpoint inhibitors reach their potential in saving cancer patients. Although the remissions in patients with advanced cancer are impressive, only a minority of patients (∼20% ) have this level of benefit [84–87]. Additionally immune checkpoint inhibitors can unleash a plethora of autoimmune processes, and the monitoring and treatment of these “toxicities” requires special diligence [89]. Analyses of biomarkers to predict immune checkpoint inhibitor activity have produced conflicting results, indicating a continued need for study [80, 81, 84, 90–93].
The lack of remissions in the majority of patients and the absence of clear biomarkers of response are not surprising as the immune system’s response to cancer can fail at multiple points [81, 94–97]. In addition to immune checkpoints, causes of immune failure include low antigenicity (e.g., lack of antigens, MHC downregulation), deficient adjuvanticity (e.g., lack of damage-associated molecular patterns) to signal the immune system, ineffective T cell trafficking, immunosuppressive cells and cytokines, exhausted T cells, plus deficient numbers or function of immune effector cells in general [83, 98]. It is expected that combining drugs that positively affect different parts of the immune system will substantially increase the success rate of immune checkpoint inhibitors [81, 94–98]. This again highlights the need for relevant animal modes to help select the most promising approaches to take into human trials.
The potential for dogs with InvUC to contribute to better immunotherapy
Pet dogs with naturally-occurring cancer could serve as an invaluable model to bridge the gap between mouse models and human clinical immunotherapy trials given the development of “spontaneous cancer” in the presence of an intact immune system in dogs and the similarities between InvUC in dogs and humans described above. It is recognized that the “tool kit” that is needed to study and monitor the immune system in dogs has lagged behind that in humans and rodents. Fortunately, work has been launched to address these limitations. Along with the rapidly expanding interest in translational research in dogs, funding opportunities for the work are growing. A U01 grant program was announced through the NCI in 2017 (Canine Immunotherapy Trials and Correlative Studies, RFA-CA-17-001) [99]. This came on the heels of funding through an Administrative Supplement for P30 Cancer Center Support Grants to support research in canine immunotherapy via collaboration of NCI-designated cancer centers and veterinary medical colleges, with an emphasis put on defining neoantigens in naturally-occurring canine cancer.
In addition to assembling the tool kit to monitor immunotherapy, and gaining a deeper understanding of canine tumor immunology, another key development for translational research will be developing immune checkpoint inhibitors for use in dogs. These drugs are currently not available for dogs. Human monoclonal antibodies that target immune checkpoints have not yet been shown to bind and functionally disrupt canine checkpoints. In addition, neutralizing antibodies would form in dogs in response to administration of human antibodies. The opportunity to evaluate canine specific immune checkpoint inhibitors, however, is eagerly awaited. Studies to evaluate the antitumor effects, determine mechanisms of response and resistance, test potential combination therapies, and develop strategies to minimize adverse events are all of high interest. It is likely that dogs will develop adverse events similar to the autoimmune-related adverse events in humans. With dogs developing naturally-occurring autoimmune diseases, such as hemolytic anemia and thrombocytopenia, myasthenia gravis, hypoadrenocorticism, hypothyroidism, polyarthritis, inflammatory bowel disease, and lupus erythematosus, it is expected that immune mediated adverse events will be observed with immune checkpoint inhibitor treatment in dogs [100–102]. In fact, autoimmunity is reported to be more frequent in dogs which have undergone ovariohysterectomy or orchiectomy, compared to intact dogs, and many pet dogs, especially in the United States, have had these procedures performed [102]. It is important to point out that pet owners will not tolerate adverse events that negatively interfere with their dog’s quality of life, and strategies to minimize side effects and effectively manage them will be crucial. This is, however, certainly a worthy goal for human cancer patients as well and another opportunity for translational research aimed at improving quality of life across both species. While this work is evolving, there are already multiple examples of immunotherapy studies in dogs of translational value, including those of vaccines, muramyl tripeptides, and CAR T cells. A xenogenic (human) tyrosinase DNA vaccine was developed and approved by the U.S. Department of Agriculture for the treatment of melanoma dogs in 2007 [103]. The initial studies in dogs contributed to the development and evaluation of a similar xenogenic (mouse) tyrosinase DNA vaccine for use in humans [104]. A phase I clinical trial demonstrated that the mouse tyrosinase DNA vaccine could be safely administered to humans with melanoma [105], and additional studies are currently ongoing.
Liposomal encapsulated muramyl tripeptide phosphatidyl ethanolamine (L-MTP-PE, mifamurtide, Mepact®) is an immunomodulating drug with antitumor effects that appear to be mediated by activating monocytes and macrophages to kill tumor cells [106–108]. It was initial clinical trials in dogs with osteosarcoma that played a pivotal role in justifying further evaluation of the drug in children with osteosarcoma [106–108]. L-MTP-PE is currently approved for use in children with osteosarcoma in Europe and remains an investigational drug in the United States.
HER2/neu is a tyrosine kinase receptor that belongs to the family of EGFRs, and it is expressed in 40% of pediatric and canine osteosarcomas, as well as other cancers. Additionally, HER2 expression is associated with a decreased response to chemotherapy, increased metastasis, and decreased survival [109, 110]. ADXS31-164 is a highly attenuated, recombinant Listeria monocytogenes expressing a chimeric human HER2/neu construct [111, 112]. In a phase I canine clinical trial, ADXS31-164 was well tolerated and was highly effective at preventing pulmonary metastasis when administered to 18 pet dogs with HER2/neu+ appendicular osteosarcoma [113]. Given the promising results and translational relevance of canine studies, a phase 1b open-label dose escalation study of ADXS31-164 in humans with HER2 expressing solid tumors is currentlyunderway [114].
Next to immune checkpoint inhibitors, CAR T cells (engineered T cell or chimeric antigen receptor) are gaining the most attention and perhaps showing more promise than other current immunotherapy strategies [115]. CAR T cell therapy has been successfully delivered to tumor-bearing dogs [116]. Briefly, autologous RNA-transfected CAR T cells were generated, expanded, and administered to pet dogs with relapsed B cell lymphoma. The treatment was well tolerated and resulted in a reduction of CD20+ B cells in target lymph nodes. The results from this proof-of-concept study validate further evaluation of CAR T cell therapy in dogs, filling the gap between mouse models and translation into humans [116].
EARLY DETECTION AND INTERVENTION STUDIES IN DOGS WITH InvUC
In addition to the contributions of targeted therapies and immunotherapy, another important strategy in improving cancer management is the development of the means to detect and treat cancer in an earlier stage. The strong breed-associated risk for InvUC in dogs could allow dogs to make particularly impactful contributions to this area of research. The strong breed associated risk, such as the 21 fold higher risk in Scottish Terriers, allows for screening of a cohort of dogs that are highly likely to develop InvUC. A study is ongoing at Purdue University to screen Scottish Terriers who are at least six years old and have no evidence of urinary tract disease, with dogs being screened every six months for three years in order to detect bladder cancer early. Preliminary results indicate that cancer can be detected via screening in more than 25% of participating dogs, and that treatment is more effective in the early disease setting (unpublished work, D Knapp, 2018). This type of study offers unparalleled opportunities to evaluate various screening tests, to determine risk factors that translate into cancer development and progression, and to study early intervention in a naturally-occurring disease setting.
CONCLUSIONS
In conclusion, there is strong evidence that dogs with naturally-occurring InvUC can represent a relevant predictive model for cancer therapy in humans to complement other models. Important next steps should include parallel human and canine InvUC trials to provide further proof-of-concept for dog studies having predictive value in regards to the subsequent outcome in humans. With this proof of concept, dogs can be integrated more widely into the drug development process, and likely fill a much needed niche in predictive modeling between experimental animal work and human trials. Ultimately, dogs with InvUC could transform the success rate in human trials.
REFERENCES
- [1]. Czerniak B, Dinney C, McConkey D. Origins of bladder cancer. Annu Rev Pathol. 2016;11:149–74. [DOI] [PubMed] [Google Scholar]
- [2]. Chou R, Selph SS, Buckley DI, Gustafson KS, Griffin JC, Grusing SE, et al. Treatment of muscle-invasive bladder cancer: A systematic review. Cancer. 2016;122(6):842–51. [DOI] [PubMed] [Google Scholar]
- [3]. Rouanne M, Loriot Y, Lebret T, Soria JC. Novel therapeutic targets in advanced urothelial carcinoma. Crit Rev Oncol Hematol. 2016;98:106–15. [DOI] [PubMed] [Google Scholar]
- [4]. Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Sjödahl G, Lauss M, Lövgren K, Chebil G, Gudjonsson S, Veerla S, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012;18(12):3377–86. [DOI] [PubMed] [Google Scholar]
- [6]. Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Damrauer JS, Hoadley KA, Chism DD, Fan C, Tiganelli CJ, Wobker SE, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci USA. 2014;111(8):3110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Rebouissou S, Bernard-Pierrot I, de Reyniès A, Lepage ML, Krucker C, Chapeaublanc E, et al. EGFR as a potential therapeutic target for subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci Transl Med. 2014;6(244):244ra91. [DOI] [PubMed] [Google Scholar]
- [9]. Li QQ, Hao JJ, Zhang Z, Hsu I, Liu Y, Tao Z, et al. Histone deacetylase inhibitor-induced cell death in bladder cancer is associated with chromatin modification and modifying protein expression: A proteomic approach. Int J Oncol. 2016;48(6):2591–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Kobayashi T, Owczarek TB, McKiernan JM, Abate-Shen C. Modeling bladder cancer in mice: Opportunities and challenges. Nat Rev Cancer. 2015;15(1):42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Mak IW, Evaniew N, Ghert M. Lost in translation: Animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6(2):114–8. [PMC free article] [PubMed] [Google Scholar]
- [12]. Knapp DW, Ramos-Vara JA, Moore GE, Dhawan D, Bonney PL, Young KE. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014;55:100–18. [DOI] [PubMed] [Google Scholar]
- [13]. Fulkerson CM, Knapp DW. Management of transitional cell carcinoma of the urinary bladder in dogs: A review. Vet J. 2015;205:217–25. [DOI] [PubMed] [Google Scholar]
- [14]. Fulkerson CM, Dhawan D, Ratliff TL, Hahn NM, Knapp DW. Naturally occurring canine invasive urinary bladder cancer: A complementary animal model to improve the success rate in human clinical trials of new cancer drugs. Int J Genomics. 2017;2017:6589529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Center for Cancer Research. COP – Pet owners – Disease information [Internet]. 2017. [updated 2017 Jul 21; cited 2017 Sep 29]. Available from:https://ccr.cancer.gov/comparative-oncology-program/pet-owners/disease-info
- [16]. Bryan JN, Keeler MR, Henry CJ, Bryan ME, Hahn AW, Caldwell CW. A population study of neutering status as a risk factor for canine prostate cancer. Prostate. 2007;67:1174–81. [DOI] [PubMed] [Google Scholar]
- [17]. Shapiro SG, Raghunath S, Williams C, Motsinger-Reif AA, Cullen JM, Liu T, et al. Canine urothelial carcinoma: Genomically aberrant and comparatively relevant. Chromosome Res. 2015;23(2):311–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Meuten DJ, Meuten TLK. Tumors of the urinary system In: Meuten DJ, editor. Tumors in domestic animals. 5th ed Hoboken: Wiley; 2014. p. 632. [Google Scholar]
- [19]. Charney VA, Miller MA, Heng HG, Weng HY, Knapp DW. Skeletal metastasis of canine urothelial carcinoma: Pathologic and computed tomographic features. Vet Pathol. 2017;54(3):380–6. [DOI] [PubMed] [Google Scholar]
- [20]. Vilar FO, de Araújo LA, Lima SV. Total bladder replacement with de-epithelialized ileum. Experimental study in dogs. Int Braz J Urol. 2004;30:237–44. [DOI] [PubMed] [Google Scholar]
- [21]. Knapp DW, Ruple-Czerniak A, Ramos-Vara JA, Naughton JF, Fulkerson CF, Honkisz SI. A nonselective cyclooxygenase inhibitor enhances the activity of vinblastine in a naturally-occurring canine model of invasive urothelial carcinoma. Bl Cancer. 2016;2(2):241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Knapp DW, Henry CJ, Widmer WR, Tan KM, Moore GE, Ramos-Vara JA, et al. Randomized trial of cisplatin versus firocoxib versus cisplatin/firocoxib in dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 2013;27(1):126–33. [DOI] [PubMed] [Google Scholar]
- [23]. Robat C, Burton J, Thamm D, Vail D. Retrospective evaluation of doxorubicin-piroxicam combination for the treatment of transitional cell carcinoma in dogs. J Small Anim Pract. 2013;54(2):67–74. [DOI] [PubMed] [Google Scholar]
- [24]. Marconato L, Zini E, Lindner D, Suslak-Brown L, Nelson V, Jeglum AK. Toxic effects and antitumor response of gemcitabine in combination with piroxicam treatment in dogs with transitional cell carcinoma of the urinary bladder. J Am Vet Med Assoc. 2011;238(8):1004–10. [DOI] [PubMed] [Google Scholar]
- [25]. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Hanazono K, Fukumoto S, Kawamura Y, Endo Y, Kadosawa T, Iwano H, et al. Epidermal growth factor receptor expression in canine transitional cell carcinoma. J Vet Med Sci. 2014;77(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Dhawan D, Paoloni, Shukradas S, Choudhury DR, Craig BA, Ramos-Vara JA, et al. Comparative gene expression analyses identify luminaland basal subtypes of canine invasive urothelial carcinoma thatmimic patterns in human invasive bladder cancer. PLoS One. 2015;10(9):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Chaux A, Cohen JS, Schultz L, Albadine R, Jadallah S, Murphy KM, et al. High epidermal growth factor receptor immunohistochemicalexpression in urothelial carcinoma of the bladder is notassociated with EGFR mutations in exons 19 and A study usingformalin-fixed, paraffin-embedded archival tissues. Hum Pathol. 2012;43(1):1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Chow NH, Chan SH, Tzai TS, Ho CL, Liu HS. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res. 2001;7(7):1957–62. [PubMed] [Google Scholar]
- [30]. Millanta F, Impellizeri J, McSherry L, Rocchigiana G, Aurisicchio L, Lubas G. Overexpression of HER-2 via immunohistochemistry in canine urinary bladder transitional cell carcinoma – a marker of malignancy and possible therapeutic target. Vet Comp Oncol. 2017. [cited 2017 Sep 23]. doi: 0.1111/vco.12345. [Epub ahead of print] [DOI] [PubMed]
- [31]. Pruthi RS, Nielsen M, Heathcote S, Wallen EM, Rathmell WK, Godley P, et al. A phase II trial of neoadjuvant ertlotinib in patients with muscle-invasive bladder cancer undergoing radical cystectomy: Clinical and pathological results. BJU Int. 2010;106(3):349–54. [DOI] [PubMed] [Google Scholar]
- [32]. Petrylak DP, Tangen CM, Van Veldhuizen PJ Jr, Goodwin JW, Twardowski PW, Atkins JN, et al. Results of the Southwest Oncology Group phase II evaluation (study S0031) of ZD1839 for advanced transitional cell carcinoma of the urothelium. BJU Int. 2010;105(3):317–21. [DOI] [PubMed] [Google Scholar]
- [33]. Wülfing C, Machiels JP, Richel DJ, Grimm MO, Treiber U, DeGroot MR, et al. A single-arm, multicenter, open-label phase 2study of lapatinib as the second-line treatment of patients withlocally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115(13):2881–90. [DOI] [PubMed] [Google Scholar]
- [34]. Mooso BA, Vinall RL, Mudryj M, Yap SA, deVere White RW, Ghosh PM. The role of EGFR family inhibitors in muscle invasive bladder cancer: A review of clinical data and molecular evidence. J Urol. 2015;193(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Watanabe R, Tomita Y, Nishiyama T, Tanikawa R, Sato S. Correlation of p53 protein expression in human urothelial transitional cell cancers with malignant potential and patient survival. Int J Urol. 1994;1(1):43–8. [DOI] [PubMed] [Google Scholar]
- [36]. Chatterjee SJ, Datar R, Youssefzadeh D, George B, Goebell PJ, Stein JP, et al. Combined effects of p53, p21, and pRB expression in the progression of bladder transitional cell carcinoma. J Clin Oncol. 2004;22(6):1007–13. [DOI] [PubMed] [Google Scholar]
- [37]. Hanazono K, Nishimori T, Fukumoto S, Kawamura Y, Endo Y, Kadosawa, et al. Immunohistochemical expression of p63, Ki67 and β-catenin in canine transitional cell carcinoma and polypoid cystitis of the urinary bladder. Vet Comp Oncol. 2016;14(3):263–9. [DOI] [PubMed] [Google Scholar]
- [38]. Suárez-Bonnet A, Herráex P, Aguirre M, Suárez-Bonnet E, Andrada M, Rodríguez F. Expression of cell cycleregulators, 14-3-3σ and p53 proteins, and vimentin incanine transitional cell carcinoma of the urinary bladder. Urol Onco. 2015;33(7):332.e1–332.e7. [DOI] [PubMed] [Google Scholar]
- [39]. Dhawan D, Ramos-Vara JA, Stewart JC, Zheng R, Knapp DW. Canine invasive transitional cell carcinoma cell lines: In vitro tools to complement a relevant animal model of invasive urinary bladder cancer. Urol Oncol. 2009;27(3):284–92. [DOI] [PubMed] [Google Scholar]
- [40]. Baumgart E, Cohen MS, Silva Neto B, Jacobs MA, Wotkowicz C, Rieger-Christ KM, et al. Identification and prognostic significance of an epithelial-mesenchymal transition expression profile in human bladder tumors. Clin Cancer Res. 2007;13(6):1685–94. [DOI] [PubMed] [Google Scholar]
- [41]. Kunze E, Wendt M, Schlott T. Promoter hypermethylation of the 14-3-3 σ, SYK and CAGE-1 genes is related to the various phenotypes of urinary bladder carcinomas and associated withprogression of transitional cell carcinomas. Int J Mol Med. 2006;18(4):547–57. [PubMed] [Google Scholar]
- [42]. Mhawech P, Benz A, Cerato C, Greloz V, Assaly M, Desmond JC, et al. Downregulation of 14-3-3sigma in ovary, prostate and endometrial carcinomas is associated with CpG island methylation. Mod Pathol. 2005;18(3):340–8. [DOI] [PubMed] [Google Scholar]
- [43]. Decker B, Parker HG, Dhawan D, Kwon EM, Karlins E, Davis BW, et al. Homologous mutation to human BRAF V600E is common in naturally occurring canine bladder cancer – evidence for a relevant model system and urine-based diagnostic test. Mol Cancer Res. 2015;13(6):993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Mochizuki H, Shapiro SG, Breen M. Detection of BRAF mutation in urine DNA as a molecular diagnostic for canine urothelial and prostatic carcinoma. PLoS One. 2015;10(12):e0144170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Ramsey Sa, Xu T, Goodall C, Rhodes AC, Kashyap A, He J, et al. Cross-species analysis of the canine and human bladder cancer transcriptome and exome. Genes Chromosomes Cancer. 2017;56(4):328–343. [DOI] [PubMed] [Google Scholar]
- [46]. Ross JS, Wang K, Al-Rohil RN, Nazeer T, Sheehan CE, Otto GA, et al. Advanced urothelial carcinoma: Next-generation sequencing reveals diverse genomic alterations and targets of therapy. Mod Pathol. 2014;27(2):271–80. [DOI] [PubMed] [Google Scholar]
- [47]. Bilgrami SM, Qureshi SA, Pervez S, Abbas F. Promoter hypermethylation of tumor suppressor genes correlates with tumor grade and invasiveness in patients with urothelial bladder cancer. Springerplus. 2014;3(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Yap KL, Kiyotani K, Tamura K, Antic T, Jang M, Montoya M, et al. Whole-exome sequencing of muscle-invasive bladder canceridentifies recurrent mutations of UNC5C and prognostics importanceof DNA repair gene mutations on survival. Clin Cancer Res. 2014;20(24):6605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Wang X, Ji P, Zhang Y, LaComb JF, Tian X, Li E, et al. Aberrant DNA methylation: Implications in racial health disparity. PLoS One. 2016;11(4):e0153125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Zhi Y, Ji H, Pan J, He P, Zhou X, Zhang H, et al. Downregulated XPA promotes carcinogenesis of bladder cancer via impairment of DNA repair. Tumour Biol. 2017;39(2):1010428317691679. [DOI] [PubMed] [Google Scholar]
- [51]. Lee JH, Park SJ, Kim SW, Hariharasudhan G, Jung SM, Jun S, et al. c-Fos-dependent miR-22 targets MDC1 and regulates DNA repair in terminally differentiated cells. Oncotarget. 2017;8(29):48204–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Wang J, Zhao X, Jin L, Wu G, Yang Y. UBR5 contributes to colorectal cancer progression by destabilizing the tumor suppressor ECRG4. Dig Dis Sci. 2017;62(10):2781–2789. [DOI] [PubMed] [Google Scholar]
- [53]. Eissa S, Matboli M, Awad N, Kotb Y. Identification and validation of a novel autophagy gene expression signature for human bladder cancer patients. Tumour Biol. 2017;39(4):1010428317698360. [DOI] [PubMed] [Google Scholar]
- [54]. Couty S, Westwood IM, Kalusa A, Cano C, Travers J, Boxall K, et al. The discovery of potent ribosomal S6 kinase inhibitors by high-throughput screening and structure-guided drug design. Oncotarget. 2013;4(10):1647–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Mottok A, Steidl C. Genomic alterations underlying immune privilege in malignant lymphomas. Curr Opin Hematol. 2015;22(4):343–54. [DOI] [PubMed] [Google Scholar]
- [56]. Leclerc J, Ballotti R, Bertolotto C. Pathways from senescence to melanoma: Focus on MITF sumoylation. Oncogene. 2017;36(48):6659–67. [DOI] [PubMed] [Google Scholar]
- [57]. Sacristan R, Gonzalez C, Fernández-Gómez JM, Fresno F, Escaf S, Sánchez-Carbayo M. Molecular classification of non-muscle-invasive bladder cancer (pTa low-grade, pT1 low-grade, and pT1 high-grade subgroups) using methylation of tumor-suppressor genes. J Mol Diagn. 2014;16(5):564–72. [DOI] [PubMed] [Google Scholar]
- [58]. Dhawan D, Ramos-Vara JA, Hahn NM, Waddell J, Olbricht GR, Zheng R, et al. DNMT An emerging target in the treatment of invasive urinary bladder cancer. Urol Oncol. 2013;31(8):1761–9. [DOI] [PubMed] [Google Scholar]
- [59]. Dhawan D, Ramos-Vara JA, Naughton JF, Cheng L, Low PS, Rothenbuhler R, et al. Targeting folate receptors to treat invasive urinary bladder cancer. Cancer Res. 2013;73(2):875–84. [DOI] [PubMed] [Google Scholar]
- [60]. London CA, Hannah AL, Zadovoskaya R, Chien MB, Kollias-Baker C, Rosenberg M, et al. Phase I dose-escalating study of SU11654, a small molecular receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9(7):2755–68. [PubMed] [Google Scholar]
- [61]. London CA, Malpas PB, Wood-Follis SL, Boucher JK, Rusk AW, Rosenberg MP, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15(11):3856–65. [DOI] [PubMed] [Google Scholar]
- [62]. Pryer NK, Lee LB, Zadovaskaya R, Yu X, Sukbuntherng J, Cherrington JM, et al. Proof of target for SU11654: Inhibition of KIT phosphorylation in canine mast cell tumors. Clin Cancer Res. 2003;9(15):5729–34. [PubMed] [Google Scholar]
- [63]. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixie O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24. [DOI] [PubMed] [Google Scholar]
- [64]. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomized controlled trial. Lancet. 2006;368(9544):1329–38. [DOI] [PubMed] [Google Scholar]
- [65]. Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmunedisease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107(29):13075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-celllymphoma. N Engl J Med. 2013;369(6):507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Egas-Bejar D, Anderson PM, Agarwal R, Corrales-Medina F, Devarajan E, Huh WW, et al. Theranostic profiling for actionable aberrations in advanced high risk osteosarcoma with aggressive biology reveals high molecular diversity: The human fingerprint hypothesis. Oncoscience. 2014;1(2):167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Larson JC, Allstadt SD, Fan TM, Khanna C, Lunghofer PJ, Hasen RJ, et al. Pharmacokinetics of orally administered low-dose rapamycin in healthy dogs. Am J Vet Res. 2016;77(1):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Paoloni MC, Mazcko C, Fox E, Fan T, Lana S, Kisseberth W, et al. Rapamycin pharmacokinetic and pharmacodynamics relationships in osteosarcoma: A comparative oncology study in dogs. PLoS One. 2010;5(6):e11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Center for Cancer Research. COP – Pet owners – Open clinical trials [Internet]. 2017 [updated 2017 Jul 21; cited 2017 Sep 29]. Available from:https://ccr.cancer.gov/Comparative-Oncology-Program/pet-owners/trials
- [72]. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02429973, Trial with gemcitabine and rapamycin in second line of metastatic osteosarcoma (GEIS-29); 2015 Apr 29 [cited 2017 Sep 29]. Available from: ccr.cancer.gov
- [73]. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT01216826, Phase II study of everolimus in children and adolescents with refractory or relapsed osteosarcoma; 2010 Oct 7 [updated 2013 Aug 7; cited 2017 Sep 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT01216826
- [74]. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02517918, Metronomic chemotherapy in patients with advanced solid tumor with bone metastasis and advanced pretreated osteosarcoma (METZOLIMOS); 2015 Aug 7 [updated 2016 Nov 9; cited 2017 Sep 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT02517918
- [75]. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT01830153, RAD001 in advanced sarcoma; 2013 Apr 12 [cited 2017 Sep 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT01830153 ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT01804374, Phase II open label, non-randomized study of sorafenib and everolimus in relapsed and non-resectable osteosarcoma (SERIO); 2013 Mar 5 [updated 2015 Jun 17; cited 2017 Sep 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT01804374
- [76]. Choi W, Ochoa A, McConkey DJ, Aine M, Höglund M, Kim WY, et al. Genetic alterations in the molecular subtypes of bladder cancer: Illustration in the cancer genome atlas dataset. Eur Urol. 2017;72(3):354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. Aine M, Sjödahl G, Eriksson P, Veerla S, Lindgren D, Ringnér M, et al. Integrative epigenomic analysis of differential DNA methylation in urothelial carcinoma. Genome Med. 2015;7(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. Tabernero J, Bahleda R, Diestmann R, Infante JR, Mita A, Italiano A, et al. Phase I dose-escalation study of JNJ-42756493, an oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2015;33(30):3401–8. [DOI] [PubMed] [Google Scholar]
- [79]. Farina MS, Lundgren KT, Bellmunt J. Immunotherapy in urothelial cancer: Recent results and future perspectives. Drugs. 2017;77(10):1077–89. [DOI] [PubMed] [Google Scholar]
- [80]. Zhou TC, Sankin AI, Porcelli SA, Perlin DS, Schoenberg MP, Zang X. A review of the PD-1/PL-L1 checkpoint in bladder cancer: From mediator of immune escape to target for treatment. Urol Oncol. 2017;35(1):14–20. [DOI] [PubMed] [Google Scholar]
- [81]. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-pro-grammeddeath-1 (MDX-1106) in refractory solid tumors: Safety, clinicalactivity, pharmacodynamics, and immunologic correlates. J ClinOncol. 2010;28(19):3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicenter, phase 2 trial. Lancet. 2017;389(10064):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: Tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4(7):e1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84]. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm multicenter, phase 2 trial. Lancet. 2016;387(10031):1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. [DOI] [PubMed] [Google Scholar]
- [86]. Inman BA, Longo TA, Ramalingam S, Harrison MR. Atezolizumab: A PD-L1-blocking antibody for bladder cancer. Clin Cancer Res. 2017;23(8):1886–90. [DOI] [PubMed] [Google Scholar]
- [87]. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution ofPD-1/PD-L1 immunotherapy for bladder cancer: The future is now. Cancer Treat Rev. 2017;54:58–67. [DOI] [PubMed] [Google Scholar]
- [89]. Hahn AW, Gill DM, Agarwal N, Maughan BL. PD-1 checkpoint inhibition: Toxicities and management. Urol Oncol. 2017;35(12):701–7. [DOI] [PubMed] [Google Scholar]
- [90]. Johnson DB, Frapton GM, Rioth MJ, Yusko E, Xu Y, Guo X, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res. 2016;4(11):959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92]. Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17(4):209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94]. Galluzzi L, Zitvogel L, Kroemer G. Immunological mechanisms underneath the efficacy of cancer therapy. Cancer Immunol Res. 2016;4(11):895–902. [DOI] [PubMed] [Google Scholar]
- [95]. Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44(2):343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Bidnur S, Savdie R, Black PC. Inhibiting immune checkpoints for the treatment of bladder cancer. Bladder Cancer. 2016;2(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98]. Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infectionand cancer. Trends Immunol. 2015;36(4):265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99]. RFA-CA-17-001: Canine Immunotherapy Trials and Correlative Studies (U01) [Internet]. National Institutes of Health. U.S. Department of Health and Human Services; [cited 2017 Sep 30]. Available from: https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-17-001.html
- [100]. Gershwin LJ. Current and newly emerging autoimmune diseases. Vet Clin North Am Small Anim Pract. 2018;48(2):323–38. [DOI] [PubMed] [Google Scholar]
- [101]. O’Kell AL, Wasserfall C, Catchpole B, Davison LJ, Hess RS, Kushner JA, et al. Comparative pathogenesis of autoimmune diabetes in humans, NOD mice, and canines: Has a valuable animal model of type 1 diabetes been overlooked. Diabetes. 2017;66(6):1443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Sundburg CR, Belanger JM, Bannasch DL, Famula TR, Oberbauer AM. Gonadectomy effects on the risk of immune disorders in the dog: A retrospective study. BMC Vet Res. 2016;12(1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].USDA licenses DNA vaccine for treatment of melanoma in dogs. J Am Vet Med Assoc. 2010;236(5):495. [DOI] [PubMed] [Google Scholar]
- [104]. Wolchok JD, Yuan J, Houghton AN, Gallardo HF, Rasalan TS, Wang J, et al. Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Mol Ther. 2007;15(11):2044–50. [DOI] [PubMed] [Google Scholar]
- [105]. Yuan J, Ku GY, Adamow M, Mu Z, Tandon S, Hannaman D, et al. Immunologic responses to xenogenic tyrosinase DNA vaccine administered by electroporation in patients with malignant melanoma. J Immunother Cancer. 2013;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. MacEwen EG, Kurzman ID, Rosenthan RC, Smith BW, Manley PA. Therapy for osteosarcoma in dogs with intravenous infection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst. 1989;8112 935–8. [DOI] [PubMed] [Google Scholar]
- [107]. Kurzman ID, MacEwen EG, Rosenthal RC, Fox LF, Keller ET. Adjuvant therapy for osteosarcoma in dogs: Results of randomized clinical trials using combined liposome-encapsulated murmayl tripeptide and cisplatin. Clin Cancer Res. 1995;1(12):1595–601. [PubMed] [Google Scholar]
- [108]. Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival – a report from the Children’s Oncology Group. J Clin Oncol. 2008;26(4):633–8. [DOI] [PubMed] [Google Scholar]
- [109]. Rainusso N, Brawley VS, Ghazi A, Hicks MJ, Gottschalk S, Rosen JM, et al. Immunotherapy targeting HER2 with genetically modified Tcells eliminates tumor-initiating cells in osteosarcoma. Cancer Gene Ther. 2012;19(3):212–7. [DOI] [PubMed] [Google Scholar]
- [110]. Flint AF, U’Ren L, Legare ME, Withrow SJ, Dernell W, Hanneman WH. Overexpression of the erbB-2 proto-oncogene in canine osteosarcoma cell lines and tumors. Vet Pathol. 2004;41(3):291–6. [DOI] [PubMed] [Google Scholar]
- [111]. Seavey MM, Pan ZK, Maciag PC, Wallecha A, Rivera S, Paterson Y, et al. A novel human Her-2/neu chimeric molecule expression by Listeria monocytogenes can elicit potent HLA-A2 restricted CD8-positive T cell responses and impact the growth and spread of Her-2/neu-positive breast tumors. Clin Cancer Res. 2009;15(3):924–32. [DOI] [PubMed] [Google Scholar]
- [112]. Shahabi V, Seavey MM, Maciag PC, Rivera S, Wallecha A. Development of a live and highly attenuated Listeria monocytogenes-based vaccine for the treatment of Her2/neu-overexpressing cancers in human. Cancer Gene Ther. 2011;18(1):53–62. [DOI] [PubMed] [Google Scholar]
- [113]. Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, et al. Immunotherapy with a HER2-targeting Listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res. 2016;22(17):4380–90. [DOI] [PubMed] [Google Scholar]
- [114]. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02386501. Dose escalation study of ADXS312-164 in subjects with HER2 expressing solid tumors; 2015 Mar 12 [updated 2017 Jan 20; cited 2017 Sep 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT02386501
- [115]. Gomes-Silva D, Ramos CA. Cancer immunotherapy using CAR-T cells: From the research bench to the assembly line. Biotechnol J. 2018;13(2):1700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116]. Panjwani MK, Smith JB, Schutsky K, Gnanandarajah J, O’Connor CM, Powell DJ Jr, et al. Feasibility and safety of RNA-transfected CD20-specific chimeric antigen receptor T cells in dogs with spontaneous B cell lymphoma. Mol Ther. 2016;24(9):1602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]