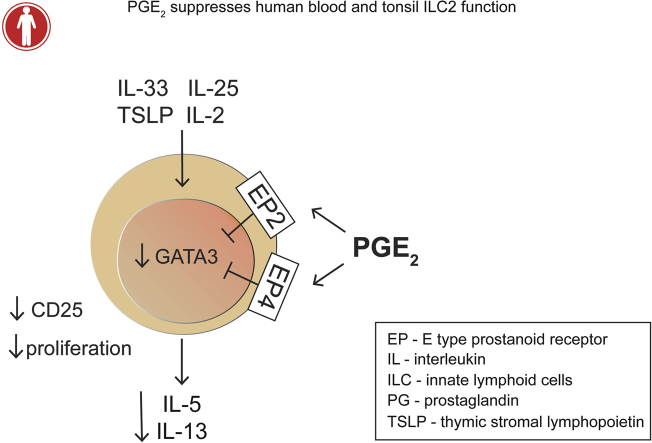
Abstract
Background
Group 2 innate lymphoid cells (ILC2s) are involved in the initial phase of type 2 inflammation and can amplify allergic immune responses by orchestrating other type 2 immune cells. Prostaglandin (PG) E2 is a bioactive lipid that plays protective roles in the lung, particularly during allergic inflammation.
Objective
We set out to investigate how PGE2 regulates human ILC2 function.
Methods
The effects of PGE2 on human ILC2 proliferation and intracellular cytokine and transcription factor expression were assessed by means of flow cytometry. Cytokine production was measured by using ELISA, and real-time quantitative PCR was performed to detect PGE2 receptor expression.
Results
PGE2 inhibited GATA-3 expression, as well as production of the type 2 cytokines IL-5 and IL-13, from human tonsillar and blood ILC2s in response to stimulation with a combination of IL-25, IL-33, thymic stromal lymphopoietin, and IL-2. Furthermore, PGE2 downregulated the expression of IL-2 receptor α (CD25). In line with this observation, PGE2 decreased ILC2 proliferation. These effects were mediated by the combined action of E-type prostanoid receptor (EP) 2 and EP4 receptors, which were specifically expressed on ILC2s.
Conclusion
Our findings reveal that PGE2 limits ILC2 activation and propose that selective EP2 and EP4 receptor agonists might serve as a promising therapeutic approach in treating allergic diseases by suppressing ILC2 function.
Key words: ILC2, allergy, prostaglandin E2, E-type prostanoid receptor 2, E-type prostanoid receptor 4
Abbreviations used: cAMP, Cyclic AMP; CRTH2, Chemoattractant receptor-homologous molecule expressed on TH2 cells; EP, E-type prostanoid receptor; ILC, Innate lymphoid cell; ILC2, Group 2 innate lymphoid cell; IMDM, Iscove modified Eagle medium; NHS, Normal human serum; NK, Natural killer; PE, Phycoerythrin; PG, Prostaglandin; RNA-seq, RNA sequencing; RT-qPCR, Quantitative RT-PCR; TSLP, Thymic stromal lymphopoietin
Graphical abstract
Group 2 innate lymphoid cells (ILC2s) were first described in mice as lymphocytes lacking expression of B- and T-cell markers and being a rich source of type 2 cytokines.1, 2 ILC2s are crucial in driving type 2 immune responses, even in the absence of adaptive T-cell activation.3, 4, 5, 6 Human ILC2s have been identified in the lung, gut, nasal polyps, thymus, tonsils, and skin and also in peripheral blood.7, 8, 9 In the mouse ILC2 development is dependent on retinoic acid receptor–related orphan receptor α and GATA-3.10 Additionally, GATA-3 plays a key role in both human and mouse ILC2 maintenance and function.10 IL-2 and IL-7 are also necessary for ILC2 maintenance and can contribute to ILC2 activation.11 On allergen stimulation, activated airway epithelial cells and alveolar macrophages produce the innate cytokines IL-33, IL-25, and thymic stromal lymphopoietin (TSLP),12 which activate nuclear factor κB and signal transducer and activator of transcription 5 signaling pathways in ILC2s.11 These events lead to GATA-3 upregulation, and GATA-3 activation triggers production of the type 2 cytokines IL-5 and IL-13.13, 14 Consequently, eosinophils, mast cells, TH2 cells, and dendritic cells are activated, and these further amplify the type 2 immune response characteristic for allergy and asthma.15, 16
Pharmacologic targeting of ILC2s is desirable because of the crucial role that ILC2s play in the pathogenesis of allergy. Although several activators of ILC2s have been described, we know relatively little about factors that restrict ILC2 responses. Lipid mediators are key molecules in controlling allergic immune responses,17 and there is clear evidence for an immunoregulatory role of prostaglandin (PG) D218 and other lipid mediators in ILC2 activation.19
One highly abundant lipid mediator in the lung is PGE2, which exerts its effects through 4 different G protein–coupled E-type prostanoid receptors (EPs [EP1-EP4]). PGE2 has been shown to be protective in mouse models of asthma and allergy,20, 21 as well as in the human lung, where inhaled PGE2 inhibits allergen-induced bronchoconstriction.22 Furthermore, PGE2 shows inhibitory effects on TH2 cells, macrophages, mast cells, neutrophils, and eosinophils.23, 24, 25, 26, 27
Importantly, the role of PGE2 in regulating ILC2 function has not been described yet. Here we reveal PGE2 as a potent suppressor of human ILC2 cytokine production and proliferation, most likely through downregulating the expression of GATA-3 and IL-2 receptor α (CD25). Furthermore, ILC2s express exclusively the EP2 and EP4 receptors, and PGE2 uses both these receptors to suppress ILC2 function. Our findings indicate that targeting the PGE2-EP2/EP4 pathway might be an efficient way to suppress ILC2 function as a therapeutic strategy for allergy and asthma.
Methods
A detailed description of the methods and materials used in this study is provided in the Methods section in this article's Online Repository at www.jacionline.org.
Isolation and flow cytometric sorting of tonsillar and blood ILC2s
ILC2s were obtained from human tonsils and buffy coats. Cells were isolated by means of flow cytometric sorting with antibodies against specific cell-surface markers, as previously described.28 A detailed description of antibodies used for ILC2 isolation can be found in the Methods section in this article's Online Repository.
Culture, expansion, and treatments of ILC2s
Freshly sorted ILC2s were expanded for 2 weeks in culture with a feeder cell mixture, as previously described.29 ILC2s were treated with PGE2 (30 nmol/L) for 10 minutes before adding IL-2 (10 U/mL) with or without IL-33, TSLP, and IL-25 (50 ng/mL each). The EP2 receptor antagonist PF-04418948 and the EP4 receptor antagonist ONO AE3-208 were added 20 minutes before PGE2. In some experiments ILC2s were treated with the EP2 receptor agonist butaprost and the EP4 receptor agonist L-902,688 ten minutes before the cytokine stimulation. Expanded ILC2s were incubated for 24 or 72 hours, and freshly sorted ILC2s were incubated for 5 days.
Flow cytometric staining and analysis of intracellular cytokines, transcription factors, and proliferation
Cell-surface staining of expanded tonsillar ILC2s was assessed by using specific antibodies. CellTrace violet dye was used for analyzing cell proliferation. Intracellular cytokine detection and GATA-3 expression were determined after specific fixation and permeabilization protocols. After staining, cells were acquired immediately on an LSR Fortessa flow cytometer (BD, San Jose, Calif).
ELISA
Collected cell supernatants were analyzed with the IL-5 ELISA Duo-Set Kit (R&D Systems, Minneapolis, Minn) and the IL-13 ELISA Kit (Sanquin, Amsterdam, The Netherlands).
Quantitative RT-PCR
Total RNA from expanded tonsillar ILC2s was isolated with the RNeasy Micro Kit (Qiagen, Hilden, Germany). After cDNA synthesis, quantitative PCR reactions were performed with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, Calif) and primers for human EP1 to EP4 receptors and the housekeeping gene RPS18 (all primers were from Bio-Rad Laboratories). Quantitative RT-PCR (RT-qPCR) was performed in the CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories).
RNA sequencing analysis
Single-cell RNA sequencing (RNA-seq) expression patterns were obtained as reads per kilobase gene model and million mappable reads from the Bjorklund et al28 expression matrix. Expression levels of the PTGER genes in different innate lymphoid cell (ILC) subsets and natural killer (NK) cells were interpreted as violin plots by using R software.
Statistical analyses
In all experiments n represents the number of individual donors used for ILC2 isolation. Differences between 2 groups were analyzed by using the matched-pairs t test, and 3 or more groups were compared by means of 1-way ANOVA for repeated measurements and the Dunnett multiple comparisons test. Analyses were performed with GraphPad Prism 6 software (GraphPad Software, La Jolla, Calif).
Results
PGE2 suppresses IL-5 and IL-13 production in human tonsillar ILC2s
ILC2s were sort purified from tonsillar mononuclear cells as Lin−CD127+CD161+ chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2)+ lymphocytes (Fig 1, A). Because of the relative rareness of ILC2s in tonsils, we expanded freshly sorted ILC2s by culturing the cells with irradiated feeder cells for 2 weeks in the presence of IL-2 (100 U/mL), IL-4 (25 ng/mL), and PHA (1 μg/mL). Compared with freshly isolated ILC2s (see Fig E1, A, in this article's Online Repository at www.jacionline.org), expanded ILC2s maintained their phenotype and expressed high levels of CD161 and CRTH2 while lacking CD3 and CD56 expression (see Fig E1, B). To assess how PGE2 regulates ILC2 function, we incubated tonsillar ILC2s with cytokines known to drive human ILC2 function (ie, IL-33, TSLP, IL-25, and IL-2) in the presence or absence of PGE2 for 24 hours. Control treatment of ILC2s contained only IL-2. We chose this time point because there is no detectable ILC2 proliferation occurring in these cultures within 24 hours, allowing us to assess the proliferation-independent effects of PGE2 on ILC2s (see Fig E2, A, in this article's Online Repository at www.jacionline.org). As expected, cytokine-activated ILC2s secreted high amounts of IL-5 and IL-13, as determined by means of ELISA (Fig 1, B and C). Incubation of ILC2s with PGE2 alone did not induce any changes in cytokine production (Fig 1, D); however, PGE2 inhibited cytokine production of activated ILC2s in a concentration-dependent manner (see Fig E3, A and B, in this article's Online Repository at www.jacionline.org). In all subsequent experiments we used 30 nmol/L PGE2, which caused, on average, 70% inhibition of cytokine secretion (Fig 1, B and C). Similar results were obtained when we assessed the IL-5 and IL-13 production on a per-cell basis because PGE2 significantly reduced the percentage of ILC2s expressing intracellular IL-5 and IL-13 after cytokine stimulation (Fig 1, E-G).
Fig 1.
PGE2 suppresses IL-5 and IL-13 production of human tonsillar ILC2s. A, Gating strategy for sort-purifying human tonsillar ILC2s. Sorted and expanded tonsillar ILC2s in the presence of IL-2 (10 U/mL) were stimulated with IL-33, IL-25, and TSLP (50 ng/mL each), shortened as IL, or left nonstimulated for 24 hours. PGE2 (30 nmol/L) was added 10 minutes before the cytokines. B-D, Concentrations of released IL-5 (Fig 1, B and D) and IL-13 (Fig 1, C and D) in ILC2 supernatants were assessed by means of ELISA, and graphs show individual concentrations (n = 9-10). ****P < .0001. E, Intracellular cytokine expression of IL-5 and IL-13 was determined by means of flow cytometry. F and G, Graphs present individual percentages of IL-5+ (Fig 1, F) and IL-13+ (Fig 1, G) cells (n = 6). **P < .01 and ***P < .001. H, Expression of GATA-3 was analyzed by using flow cytometry. I and J, Graphs show individual geometric mean fluorescence intensity (Fig 1, I) and geometric mean fluorescence intensity normalized to nonstimulated cells (Fig 1, J) of GATA-3 expression (n = 5). *P < .05. ns, Nonstimulated.
Fig E1.
Expanded human tonsillar ILC2s retain their phenotype. A, Gating of ILC2s from lineage-depleted alive freshly isolated human tonsillar mononuclear cells. B, Sorted and expanded ILC2s retain their phenotype after 2 weeks of culture expressing CD161 and CRTH2 and lacking expression of CD3 and CD56, as analyzed by using flow cytometry.
Fig E2.
PGE2 does not affect ILC2 viability and proliferation after 24 hours. ILC2s were stimulated as indicated for 24 hours. A, Proliferation of CellTrace violet dye–loaded ILC2s after 24 hours was analyzed by using flow cytometry. Representative histograms are shown (n = 2). B, Cell viability was determined as a percentage of singlet cells, which are negative for dead cell markers. Data are shown as means + SEMs (n = 4). ns, Nonstimulated.
Fig E3.
PGE2 concentration-dependently inhibits cytokine production of ILC2s. A and B, Sorted and expanded tonsillar ILC2s were stimulated with a combination of cytokines (IL-33, TSLP, and IL-25 plus IL-2) for 24 hours. PGE2 was added in different concentrations (3, 10, 30, 100, and 300 nmol/L) 10 minutes before the cytokines. Concentrations of IL-5 (Fig E3, A) and IL-13 (Fig E3, B) in ILC2 supernatants were determined by means of ELISA, and data are shown as mean ± SEM concentrations (n = 4). *P < .05, **P < .01, and ***P < .001. ns, Nonstimulated. C and D, ILC2s were stimulated with a cocktail of cytokines or individual cytokines in addition to IL-2. Graphs show individual IL-5 (Fig E3, C) and IL-13 (Fig E3, D) concentrations (n = 3).
Of note, as previously demonstrated,29 we observed that the combination of all 3 cytokines (IL-33, IL-25, and TSLP) generated higher ILC2 responses than the individual cytokines alone (see Fig E3, C and D). Interestingly, IL-33 and, to a lesser extent, IL-25 and TSLP alone could induce only moderate IL-5 and IL-13 production. Importantly, PGE2 inhibited ILC2 activation in response to the combination of all cytokines, as well as in response to IL-33, IL-25, and TSLP alone (see Fig E3, C and D).
The transcription factor GATA-3 is crucial in ILC2 function because it regulates production of type 2 cytokines.13, 30 Intracellular protein detection of GATA-3 revealed that it is highly expressed in resting ILC2s and further enhanced after 24 hours of cytokine stimulation (Fig 1, H-J). We show that addition of PGE2 caused a statistically significant decrease in GATA-3 expression (Fig 1, H-J). Cell viability was not affected by PGE2 (see Fig E2, B). Taken together, PGE2 reduces the production and release of IL-5 and IL-13 by stimulated ILC2s, likely through downregulation of GATA-3.
PGE2 reduces CD25 expression and cell proliferation of ILC2s
We observed that cytokine-stimulated ILC2s upregulated expression of IL-2 receptor α (CD25; Fig 2, A-F). After coincubation with PGE2 for 24 hours, ILC2s showed significantly reduced expression of CD25 compared with cells stimulated only with the cytokines (Fig 2, A-C). On culturing ILC2s for 72 hours, we could observe an even more pronounced inhibitory effect of PGE2 on CD25 expression (Fig 2, D-F). Because IL-2 strongly stimulates ILC2 proliferation,31 we set out to assess the effects of PGE2 on ILC2 proliferation. Cytokine stimulation of ILC2s for 72 hours induced up to 4 rounds of cell division. Furthermore, as predicted based on the PGE2-induced suppression of CD25 expression, PGE2 blocked cytokine-driven ILC2 proliferation, with the majority of ILC2s remaining in the resting phase or the first round of cell division similar to the nonstimulated control (Fig 2, G and H). PGE2 treatment did not affect cell viability after 72 hours of incubation (Fig 2, I).
Fig 2.
PGE2 prevents cytokine-induced CD25 upregulation and ILC2 proliferation. ILC2s were stimulated as indicated for 24 (A-C) or 72 (D-I) hours. Fig 2, A and D, Expression of CD25 was analyzed by using flow cytometry, and representative histograms are shown. Graphs show individual geometric mean fluorescence intensity of CD25 expression after 24 (n = 8; Fig 2, B) and 72 (n = 6; Fig 2, E) hours and geometric mean fluorescence intensity normalized to nonstimulated cells of CD25 expression after 24 (n = 8; Fig 2, C) and 72 (n = 6; Fig 2, F) hours of culture. *P < .05 and **P < .01. Fig 2, G, Proliferation of CellTrace violet dye–loaded ILC2s after 72 hours of incubation was analyzed by means of flow cytometry. Fig 2, H, Data are shown as percentages of ILC2s in different division phases (D0-D4; n = 4). ∗∗∗P < .001. Fig 2, I, Cell viability was determined as the percentage of singlet cells that are negative for dead cell markers. Data are shown as means + SEMs (n = 4). ns, Nonstimulated.
Longer culture time generated higher concentrations of released IL-5 and IL-13 in ILC2 supernatants compared with that at 24 hours (Fig 3, A and B), which could be due to the intensive cell proliferation seen at this time point. Strikingly, PGE2 significantly suppressed the cytokine-stimulated IL-5 and IL-13 secretion of ILC2s after 72 hours of culture (Fig 3, A and B). Furthermore, GATA-3 expression was also inhibited significantly by PGE2 treatment after 72 hours of incubation similar to what was seen after 24 hours (Fig 1, H-J, and Fig 3, C-E).
Fig 3.
PGE2 reduces GATA-3 upregulation. ILC2s were stimulated as indicated for 72 hours. A and B, Concentrations of IL-5 (Fig 3, A) and IL-13 (Fig 3, B) in ILC2 supernatants were determined by means of ELISA, and data are shown as individual concentrations (n = 10). ***P < .001 and ****P < .0001. C, Expression of GATA-3 was analyzed by using flow cytometry. D and E, Graphs show individual geometric mean fluorescence intensity (Fig 3, D) and geometric mean fluorescence intensity normalized to nonstimulated cells (Fig 3, E) of GATA-3 expression (n = 8). **P < .01. ns, Nonstimulated.
In summary, these data indicate not only that PGE2 acts on the “per-cell basis” (ie, by suppressing cytokine production of individual ILC2) but also that PGE2 limits ILC2 function by inhibiting cell proliferation, most likely through negatively affecting CD25 and GATA-3 expression.
PGE2 reduces IL-5 and IL-13 secretion from freshly sorted blood and tonsillar ILC2s
Because our initial experiments were performed with in vitro–expanded ILC2s, we wanted to confirm the suppressive effect of PGE2 on freshly sorted human ILC2s. For this purpose, we analyzed freshly sorted ILC2s from buffy coats (Fig 4, A-E) and tonsils (Fig 4, F and G). Because we have previously observed that freshly isolated ILC2s produce less cytokines on a per-cell basis,29 we incubated ILC2s for 5 days. Freshly isolated blood ILC2s (Fig 4, A and B) and tonsillar ILC2s (Fig 4, F and G) released comparable amounts of IL-5 and IL-13 after 5 days of culture. PGE2 significantly reduced IL-5 and IL-13 production of blood ILC2s (Fig 4, A and B). Additionally, PGE2 significantly reduced IL-13 production of tonsillar ILC2s to approximately 60% and also slightly decreased IL-5 release (Fig 4, F and G).
Fig 4.
PGE2 reduces IL-5 and IL-13 secretion from fresh blood and tonsillar ILC2s. Freshly sorted human blood (A-E) and tonsillar (F and G) ILC2s were incubated as indicated for 5 days. Concentrations of released IL-5 from blood ILC2s (Fig 4, A) and tonsillar ILC2s (Fig 4, F) and IL-13 from blood ILC2s (Fig 4, B) and tonsillar ILC2s (Fig 4, G) were measured by means of ELISA, and graphs show individual concentrations (n = 6 for blood ILC2s and n = 4 for tonsillar ILC2s). *P < .05 and **P < .01. Fig 4, C, Expression of CD25 in freshly sorted blood ILC2s was analyzed by using flow cytometry, and representative histograms are shown. Fig 4, D, Graph shows individual geometric mean fluorescence intensity of CD25 expression in blood ILC2s after 5 days of culture (n = 4). *P < .05. Fig 4, E, Images of freshly sorted blood ILC2s after 5 days of treatments (×10 magnification). Scale bar = 100 μm. ns, Nonstimulated.
Furthermore, cytokine stimulation upregulated CD25 expression in freshly sorted blood ILC2s, which was significantly decreased by PGE2 treatment (Fig 4, C and D). To assess the effects of PGE2 on ILC2 proliferation, we captured images of ILC2s after 5 days of culture. These images, as evaluated by eye, revealed that ILC2s cultured in the presence of PGE2 formed a smaller cell mass compared with ILC2s cultured in the presence of only cytokines (Fig 4, E).
These results indicate that freshly isolated blood and tonsillar ILC2s also respond to PGE2 with repressed activity and confirm the inhibitory effects of PGE2 we observed on expanded tonsillar ILC2s.
PGE2 suppresses IL-5 and IL-13 production of ILC2s by acting on EP2 and EP4 receptors
PGE2 exerts its physiologic effects by acting on 4 different G protein–coupled receptors: EP1 to EP4. To determine which receptors mediate the PGE2-induced inhibitory effects on ILC2s, we assessed expression of the EP receptors on human tonsillar ILC2s. In our previously published single-cell RNA-seq data generated from freshly isolated tonsillar ILC subsets,28 we detected the EP2 and EP4 receptor–encoding transcripts PTGER2 and PTGER4 in ILC2s. In contrast, ILC2s lacked expression of transcripts for EP1 and EP3 receptors (Fig 5, A). RT-qPCR experiments performed with expanded tonsillar ILC2s revealed that cultured ILC2s retain the same EP receptor expression pattern as fresh ILC2s and express exclusively EP2 and EP4 receptors (Fig 5, A and B). Of note, EP2 and EP4 mRNA expression was not affected by cytokine stimulation of expanded tonsillar ILC2s (Fig 5, C).
Fig 5.
Human tonsillar ILC2s selectively express EP2 and EP4 receptors. A, EP receptor transcripts of freshly sorted human tonsillar ILC2s were detected by using single-cell RNA-seq. Expression distribution (violin plots) are shown for genes of PGE2 receptors (PTGER1 to PTGER4) in each ILC subset (group 1 to group 3 ILCs and NK cells), where expression patterns were obtained as reads per kilobase gene model and million mappable reads. B, mRNA expression of EP receptors (PTGER1 to PTGER4) in sorted and expanded tonsillar ILC2s was determined by using RT-qPCR (n = 5). C, mRNA expression of EP2 and EP4 receptors after 24 hours of stimulation normalized to nonstimulated cells. Graphs show mean + SEM expression (n = 5).
Having identified expression of EP2 and EP4 receptor mRNAs in tonsillar ILC2s, we set out to determine whether these receptors were responsible for the inhibitory effects of PGE2. We pretreated ILC2s with selective EP2 (PF-04418948) and EP4 (ONO AE3-208) receptor antagonists before cytokine and PGE2 treatments (Fig 6). Assessment of IL-5 and IL-13 production revealed that selective antagonism of EP2 or EP4 receptor only slightly prevented the suppressive effects of PGE2. Interestingly, simultaneous EP2 and EP4 blockade largely abolished the PGE2-induced reduction of IL-5 and IL-13 release after 24 hours of culture (Fig 6, A and B).
Fig 6.
PGE2 reduces IL-5 and IL-13 production in ILC2s through activation of EP2 and EP4 receptors. Sorted and expanded tonsillar ILC2s were stimulated as indicated for 24 hours. The EP2 receptor antagonist PF-04418948 (PF; 1 μmol/L) and the EP4 receptor antagonist ONO AE3-208 (ONO; 1 μmol/L) were added separately or together 20 minutes before PGE2. A and B, Concentrations of released IL-5 (Fig 6, A) and IL-13 (Fig 6, B) in ILC2 supernatants were determined by means of ELISA. Bar graphs show means + SEMs. (n = 6-7). *P < .05 and **P < .01. C, Representative dot plots show intracellular expression of IL-5 and IL-13 assessed by using flow cytometry. D and E, Graphs show mean + SEM percentages of IL-5+ (Fig 6, D) and IL-13+ (Fig 6, E) ILC2s (n = 6). *P < .05 and **P < .01. Expression of GATA-3 was analyzed by using flow cytometry. F and G, Graphs show geometric mean + SEM fluorescence intensity (Fig 6, F) and geometric mean fluorescence intensity normalized to nonstimulated cells + SEM (Fig 6, G) of GATA-3 expression (n = 5). *P < .05. H and I, Released IL-5 (Fig 6, H) and IL-13 (Fig 6, I) are shown after 10 minutes of pretreatment with the EP2 receptor agonist butaprost (But) and the EP4 receptor agonist L-902,688 (L9) separately or together in 100 nmol/L concentrations. Concentrations were determined by means of ELISA and are shown as means ± SEMs (n = 4; compared to interleukin treatment). *P < .05 and **P < .01.
Because antagonists used separately in 1 μmol/L concentrations did not reverse the effect of PGE2, we examined whether higher concentrations would be more efficient. Using antagonists in the concentration range of 0.1 to 3 μmol/L, we demonstrated that concentrations of greater than 1 μmol/L did not have a more profound reversal effect on PGE2-induced inhibition of ILC2 function (see Fig E4, A and B, in this article's Online Repository at www.jacionline.org).
Fig E4.
Dual engagement of EP2 and EP4 receptors is essential for the PGE2-induced reduction of ILC2 cytokine production. Sorted and expanded tonsillar ILC2s were stimulated as indicated for 24 hours. Additionally, the EP2 receptor antagonist PF-04418948 (PF) and the EP4 receptor antagonist ONO AE3-208 (ONO) were added separately or together in different concentrations (100 nmol/L, 300 nmol/L, 1 μmol/L, and 3 μmol/L) 20 minutes before PGE2. A and B, Concentrations of released IL-5 (Fig E4, A) and IL-13 (Fig E4, B) in ILC2 supernatants were determined by using ELISA and shown as means ± SEM (n = 4). C and D, Sorted and expanded tonsillar ILC2s were treated with the EP2 receptor agonist butaprost (But) and the EP4 receptor agonist L-902,688 (L9) separately or together in different concentrations (10 nmol/L, 30 nmol/L, 100 nmol/L, 300 nmol/L, and 1 μmol/L) 10 minutes before the stimulatory cytokines. Concentrations of released IL-5 (Fig E4, C) and IL-13 (Fig E4, D) in ILC2 supernatants were determined by using ELISA and are shown as means ± SEMs (n = 4; compared with IL treatment). *P < .05 and **P < .01. ns, Nonstimulated.
We observed the same effects on the per-cell basis, revealing that only dual EP2 and EP4 antagonism reversed the PGE2-induced reduction of IL-5 and IL-13 intracellular expression (Fig 6, C-E). Joint antagonism of EP2 and EP4 receptors also reversed the PGE2-induced reduction of GATA-3 expression after incubation for 24 hours (Fig 6, F and G).
Additionally, we addressed the role of selective activation of EP2 and EP4 receptors in regulating the cytokine production of ILC2s by means of preincubation with the specific agonists butaprost (EP2) and L-902,688 (EP4) in the concentration range of 10 to 1000 nmol/L (see Fig E4, C and D). Using this approach, we confirmed that dual engagement of the EP2 and EP4 receptors is required for mimicking the inhibitory effects of PGE2 on ILC2 cytokine production. We observed a tendency for more pronounced inhibition by selectively engaging the EP4 receptor compared with the EP2 receptor, although these effects did not reach statistical significance. In direct comparison with PGE2, we show that the selective EP2 and EP4 agonists in 100 nmol/L concentration only partly reduced cytokine production, whereas their simultaneous activation completely blocked ILC2 stimulation comparably with the effect of PGE2 (Fig 6, H and I).
Next, we aimed to determine whether antagonism of EP2 and EP4 receptors is able to effectively reverse the long-term ILC2 blocking effects of PGE2 after 72 hours of incubation. Because PGE2 almost completely abolished proliferation of ILC2s induced by cytokine stimulation, we examined the role of EP2 and EP4 receptors in ILC2 proliferation. Indeed, combined EP2 and EP4 antagonism reversed the PGE2-suppressed ILC2 proliferation by keeping a large proportion of ILC2s in the first and second division phases (Fig 7, A and B). Dual EP2 and EP4 blockade also reversed the PGE2-caused suppression of ILC2 cytokine production (Fig 7, C and D) and GATA-3 expression at this time point (Fig 7, E and F), further confirming the role of both receptors in mediating the long-term inhibitory effects of PGE2 in ILC2 function.
Fig 7.
Activation of EP2 and EP4 receptors inhibits ILC2 cytokine release, likely through preventing ILC2 proliferation. Sorted and expanded tonsillar ILC2s were stimulated as indicated for 72 hours. Additionally, the EP2 receptor antagonist PF-04418948 (PF; 1 μmol/L) and the EP4 receptor antagonist ONO AE3-208 (ONO; 1 μmol/L) were added separately or together 20 minutes before PGE2. A, Proliferation of CellTrace violet dye–loaded ILC2s after 72 hours of incubation was analyzed by using flow cytometry. B, Data are shown as percentages of ILC2s in different division phases (D0-D4; n = 4). *P < .05 and ***P < .001. C and D, Concentrations of released IL-5 (Fig 7, C) and IL-13 (Fig 7, D) in ILC2 supernatants were detected by means of ELISA, and bars show means + SEMs (n = 7). *P < .05 and **P < .01. Expression of GATA-3 was analyzed by using flow cytometry. E and F, Graphs show geometric mean fluorescence intensity + SEM (Fig 7, E) and geometric mean fluorescence intensity normalized to nonstimulated cells + SEM (Fig 7, F) of GATA-3 expression (n = 7). *P < .05 and **P < .01.
Taken together, PGE2 suppresses IL-5 and IL-13 production and blocks ILC2 proliferation through engagement of both the EP2 and EP4 receptors.
Discussion
In this study we describe PGE2 as a potent negative regulator of human ILC2 function by inhibiting IL-5 and IL-13 production, as well as ILC2 proliferation. The cellular mechanism of the PGE2-caused ILC2 inhibition involved suppression of CD25 and GATA-3 expression in ILC2s. Furthermore, we found that PGE2 mediated these effects through engagement of both EP2 and EP4 receptors, which are expressed by ILC2s.
ILC2s are important in initiating type 2 inflammation, as shown in mouse models of asthma and allergy.3, 16 In line with these observations, studies of human subjects showed increased numbers of ILC2s in peripheral blood and sputum of patients with allergic asthma32, 33 and lung fibrosis,34 in nasal tissue of patients with chronic rhinosinusitis,35 and in the skin of patients with atopic dermatitis.36 PGE2 is present abundantly in the human lung and produced by alveolar epithelial and endothelial cells, as well as by macrophages and dendritic cells,37 whereas under inflammatory conditions, PGE2 levels are increased further.38 Therefore our data first indicate that PGE2 might serve as an endogenous messenger for restriction of uncontrolled signals mediated by ILC2s. Second, the findings support the proposal that pharmacologic targeting of the PGE2-EP2/EP4 pathway might be an efficient novel way to modulate ILC2 function as a therapeutic strategy in patients with allergy and asthma.
Importantly, earlier clinical studies showed that inhalation of PGE2 prevented bronchoconstriction in healthy and asthmatic subjects.22, 39, 40, 41 Additionally, PGE2 inhibited human TH2 cell activation,42 eosinophil trafficking,24, 43 and mast cell–dependent constriction of human bronchi.44 In mouse models of asthma and allergy, PGE2 has been shown to be protective by preventing airway sensitization.21, 45 Because ILC2s are important initiators of allergic responses, we now extend these findings by showing that PGE2 directly suppresses ILC2 function.
Our single-cell RNA-seq and RT-qPCR data demonstrated that human tonsillar ILC2s exclusively express EP2 and EP4 receptors. Interestingly, selective blockade of EP2 or EP4 receptors resulted in only partial reversion of the inhibitory effect of PGE2, whereas their combined antagonism abolished the inhibitory effect of PGE2. Accordingly, joint EP2 and EP4 receptor agonism was necessary to completely inhibit ILC2 activation. Selective EP4 receptor activation appeared to have more profound suppressive effects than EP2. This might be due to differences in expression levels of the EP2 and EP4 receptors because we observed that freshly isolated and in vitro–expanded ILC2s expressed higher levels of PTGER4 than PTGER2 mRNA. However, only simultaneous engagement of EP2 and EP4 receptors could mimic the inhibitory effect of PGE2 in ILC2 function. This suggests an interesting mechanism in which PGE2 requires engagement of both EP2 and EP4 receptors to exert its full inhibitory effect on ILC2 function.
In line with our findings, previous studies described anti-inflammatory roles of the Gs protein–coupled EP2 and EP4 receptors.21, 25, 46, 47 Furthermore, PGE2 was shown to control immunologic responses in which such cooperation of EP2 and EP4 receptors was essential, such as in the PGE2-induced inhibition of antigen-specific T-cell responses of human peripheral blood TH2 cells.42 Similarly, EP2 and EP4 receptor engagement suppressed human alveolar macrophages.48 In contrast, the PGE2-induced inhibition of mast cells and the consequent bronchoprotection,49 as well as the cytokine production of human nasal polyp cells,46 were mediated only by the EP2 receptor. In addition, PGE2-EP2 signaling was impaired in patients with aspirin-exacerbated respiratory disease.50, 51 Another study showed that although EP2 receptor activation induced bronchodilation in several animal models, only EP4 receptor was responsible for inducing relaxation of human isolated bronchi.52 Bronchorelaxation, together with EP2- and EP4-induced inhibition of the immune cells involved in the allergic response, would be an additional beneficial effect of treatments with these agonists. In addition to these protective effects, EP2 and EP4 receptor activation induces vasodilation and decreases blood pressure.53, 54 In addition, activation of these receptors upregulates production of vascular endothelial growth factor.55, 56 Therefore local administration of EP2 and/or EP4 agonists in the airways might be beneficial to minimize systemic side effects.
Although both EP2 and EP4 receptors are Gs protein–coupled receptors and activate cyclic AMP (cAMP)/protein kinase A pathways, the EP4 receptor can trigger pathways independent of cAMP signaling. It was shown that EP4 activation can inhibit nuclear factor κB activation in human macrophages57 and that EP4 engagement attenuates human eosinophil migration through a cAMP-independent pathway.26 Thus these previously described mechanisms support our findings about the dual requirement of EP2 and EP4 receptors in mediating the PGE2-induced suppression of ILC2 function.
In addition to PGE2, some other lipid mediators are emerging as novel inhibitors of ILC2 function.28 Human ILC2s were inhibited by the proresolving lipid mediator lipoxin A4,58 and PGI2 was shown to reduce mouse lung ILC2 activity.59 In contrast, PGD2 activates ILC2s by binding to the CRTH2 receptor.18 CRTH2 is a Gi protein–coupled receptor and inhibits cAMP-mediated signaling. Previously, it was shown that PGD2, through activating the CRTH2 receptor, stimulates other immune cells, such as TH2 cells, basophils, and eosinophils.60, 61 As we previously mentioned, EP2 and EP4 have been shown to act in an inhibitory manner during allergic inflammation. We demonstrate here that PGE2 also negatively regulates human ILC2 function.
We observed that PGE2 inhibited the ILC2 response to each of the stimulatory cytokines, IL-33, IL-25, and TSLP, as well as to their combination, which was necessary for complete ILC2 stimulation. Therefore we assessed the mechanism of the PGE2-induced inhibition of ILC2 function by using the combination of these activating cytokines. Importantly, we demonstrated that PGE2 suppressed both freshly isolated blood and tonsillar ILC2 function, although the inhibitory effects of PGE2 were more pronounced in expanded ILC2s.
Because PGE2 reduced cytokine production of ILC2s, we investigated whether PGE2 could affect signaling pathways essential for ILC2 activation. The transcription factor GATA-3 was shown to be crucial for ILC2 development, function, and activation.13 In our work PGE2 reduced upregulation of GATA-3 on stimulatory cytokine treatment, suggesting that PGE2 interferes with GATA-3 induction. To the best of our knowledge, we provide the first evidence for PGE2 modulating GATA-3 expression in immune cells. Additionally, we observed that PGE2 inhibited ILC2 proliferation. IL-2 is an essential coactivator of human ILC2s.32 A recent study showed that IL-2 is required for ILC2 function in type 2 lung inflammation in mice.62 Because IL-2 upregulates CD25 expression through a positive feedback mechanism, it might serve as an indicator of ILC2 activation. CD25 expression was increased on cytokine stimulation, whereas PGE2 prevented cytokine-induced CD25 upregulation, indicating the effect of this lipid mediator on ILC2 activation and at later time points also on ILC2 proliferation. These data are in line with previous reports demonstrating that PGE2 reduces CD25 expression and, consequently, IL-2 signaling in human primary peripheral blood T cells63 and bovine CD4+ T cells.64 Similarly, PGE2 was previously shown to induce genes that block the cell cycle and proliferation of human primary CD4+ T cells.42, 65
In conclusion, our findings reveal that PGE2 hampers fresh and expanded ILC2 activity by reducing production of the type 2 cytokines IL-5 and IL-13. We demonstrated that both EP2 and EP4 receptors are expressed on ILC2s and are critical in mediating the PGE2-caused abolishment of ILC2 activation. We showed that PGE2 interferes with molecular pathways involved in type 2 cytokine production, preventing upregulation of CD25 and GATA-3 expression. Because ILC2s are essential in the initiation and amplification of type 2 inflammation, our data propose PGE2 and selective or even dual EP2/EP4 agonists as promising therapeutic tools for allergic diseases by directly limiting human ILC2 activation.
Key messages.
-
•
PGE2 inhibits cytokine production and proliferation of stimulated human ILC2s through the EP2 and EP4 receptors.
-
•
PGE2 interferes with CD25 and GATA-3 expression in stimulated ILC2s.
-
•
EP2 and EP4 agonists could prove beneficial for targeting ILC2 function in allergy therapy.
Acknowledgments
We thank the Department of Medicine, Huddinge Flow Cytometry Facility at Karolinska Institutet, for allowing us to use the cell sorters.
Footnotes
Supported by the Swedish Research Council (521-2013-2791), the Swedish Cancer Society (130396), the Foundation for Strategic Research (ICA12-0023), and the Swedish Society for Medical Research (to J.M.) and the Austrian Science Fund FWF (grant P25531-B23; to V.K.).
Disclosure of potential conflict of interest: S.-E. Dahlen has received grants from the Swedish Research Council (521-2013-2791), the Swedish Cancer Society (130396), the Foundation for Strategic Research (ICA12-0023), and AstraZeneca; has a board membership with RSPR Pharma AB; and has consultant arrangements with AstraZeneca, GlaxoSmithKline, Merck, Novartis, Regeneron/Sanofi, RSPR Pharma AB, and Teva. A. Heinemann has received consultant arrangements AstraZeneca, Bayer, and Amgen; has received grants from Austrian Science Funds; and has received payment for lectures from Eli Lilly. V. Konya has received a grant from the Austrian Science Fund (FWF). J. Mjösberg has received grants from the Swedish Research Council, the Swedish Cancer Foundation, the Knut and Alice Wallenberg Foundation, the Swedish Foundation for Strategic Research, and the Swedish Society for Medical Research. The rest of the authors declare that they have no relevant conflicts of interest.
Contributor Information
Viktoria Konya, Email: viktoria.konya@medunigraz.at.
Jenny Mjösberg, Email: jenny.mjosberg@ki.se.
Methods
Isolation and flow cytometric sorting of tonsillar and blood ILC2s
Human tonsils were freshly obtained from tonsillectomies of patients with obstructive sleep apnea syndrome at the Ear Nose Throat Clinic at the Karolinska University Hospital Huddinge, Sweden. The collection and use of tonsils were approved by the regional ethical board at Karolinska Institutet. Buffy coats were provided by the blood bank at the Karolinska University Hospital Huddinge and were collected according to the approval of the regional ethical board at Karolinska Institutet.
Cell isolation from tonsils was performed, as previously described.E1 Briefly, tonsils were dissected into small pieces, ground through a filter, and centrifuged. Mononuclear cells were isolated by using Lymphoprep centrifugation and depleted from lineage-positive cells by using directly conjugated anti-CD3, anti-CD19, and anti-CD14 microbeads (markers for T cells, B cells, and monocytes, respectively) and LD MACS columns (Miltenyi Biotec, Bergisch Gladbach, Germany). Lineage-depleted cells from buffy coats were additionally enriched with the CD127 MicroBead Kit (Miltenyi Biotec). Lineage-depleted cells were incubated with the following specific mAbs: fluorescein isothiocyanate–conjugated anti-CD14 (TÜK4; Dako, Glostrup, Denmark); anti-CD3 (SK7), anti-FcεRIα (AER-37 CRA-1), anti-CD34 (581), anti-CD123 (6H6), anti-CD1a (HI149), anti–T-cell receptor αβ (IP26), anti–T-cell receptor γδ (B1), anti-CD94 (DX22; all from BioLegend, San Diego, Calif), anti-BDCA2 (AC144; Miltenyi Biotech), and anti-CD19 (4G7; BD Biosciences, San Jose, Calif). Additionally, we used Brilliant Violet 605–conjugated anti-CD161 (HP-3G10), Brilliant Violet 711–conjugated anti-CD56 (HCD56; both from BioLegend); phycoerythrin (PE)-CF594-conjugated anti-CRTH2 (BM16), V500-conjugated anti-CD45 (HI30; both from BD Biosciences); PE-Cy7–conjugated anti-CD127 (R34.34), PE-Cy5.5–conjugated anti-CD117 (104D2D1), and PE-Cy5–conjugated anti-NKp44 (Z231; all from Beckman Coulter, Fullerton, Calif). In addition, cells were stained with the LIVE/DEAD Fixable Green Dead Cell Stain Kit (Life Technologies, Grand Island, NY). ILC2s were sort-purified based on their specific phenotype as Lin−CD127+CD161+CRTH2+ cells by using FACSAria Fusion cell sorter (BD Bioscience) equipped with FACSDiva software, version 8.
Culture and expansion of ILC2s
Freshly sorted ILC2s were cultured in Iscove modified Eagle medium (IMDM) medium (Gibco) with Yssel supplement and 1% normal human serum (NHS; Invitrogen, Carlsbad, Calif) with penicillin (100 U/mL; HyClone, South Logan, Utah), and streptomycin (0.1 mg/mL; HyClone). Sorted tonsillar ILC2s were expanded for 2 weeks in culture with a feeder cell mixture of PBMCs irradiated at 25 Gy and JY cells irradiated at 50 Gy, with IL-2 (100 U/mL), IL-4 (25 ng/mL), and PHA (1 μg/mL), as previously described.E2 Freshly sorted ILC2s were kept in Yssel medium/1% NHS with IL-2 (10 U/mL) in 96-well plate (2000 cells per sample) and treated the following day.
Treatment of ILC2s
ILC2s were expanded for 2 weeks and then rested in Yssel medium/1% NHS with IL-2 (2 U/mL) for 3 days before the experiments. Cells were seeded in 96-well U-bottom plates at 5 × 104 cells/well. ILC2s were treated with PGE2 (30 nmol/L; Cayman Chemicals, Ann Arbor, Mich) for 10 minutes before adding IL-2 (10 U/mL) with or without IL-33 (PeproTech, Rocky Hills, NJ), TSLP (PeproTech), and IL-25 (R&D Systems, Minneapolis, Minn) at 50 ng/mL each. The EP2 receptor antagonist PF-04418948 and the EP4 receptor antagonist ONO AE3-208 (both from Cayman Chemicals) were added 20 minutes before PGE2 in 1 μmol/L concentration. ILC2s were incubated at 37°C for 24 or 72 hours. For addressing concentration-dependent effects of PGE2, the following concentrations were used: 3, 10, 30, 100, and 300 nmol/L. Dose-response effects of EP2 and EP4 receptor antagonists were analyzed by using the following concentrations: 100 nmol/L, 300 nmol/L, 1 μmol/L, and 3 μmol/L. To confirm the involvement of EP2 and EP4 receptors in suppression of ILC2 function, we used the EP2 receptor–specific agonist butaprost and the EP4 receptor–specific agonist L-902,688 (both from Cayman Chemicals) in the following concentrations: 10 nmol/L, 30 nmol/L, 100 nmol/L, 300 nmol/L, and 1 μmol/L.
For RT-qPCR, expanded ILC2s were seeded at 1 × 105 cells/well and incubated for 24 hours with IL-2 (10 U/mL) with or without a combination of IL-33, TSLP, and IL-25 (50 ng/mL each).
Freshly sorted ILC2s were kept in the presence of IL-2 (10 U/mL) and control treated or treated with the combination of IL-33, TSLP, and IL-25 (50 ng/mL each) for 5 days. PGE2 (100 nmol/L) was added 10 minutes before cytokines. Supernatants were collected after 5 days.
Flow cytometric staining and analysis of intracellular cytokines and transcription factors
Cell-surface staining of expanded tonsillar ILC2s was assessed by using PE-Cy7–conjugated anti-CD127, PE-Cy5.5–conjugated anti-CD117, Brilliant Violet 605–conjugated anti-CD161, PE-CF594–conjugated anti-CRTH2, and Brilliant Violet 711–conjugated anti-CD56. Dead cells were excluded with the LIVE/DEAD Fixable Green Dead Cell Stain Kit (Life Technologies). CD25 cell-surface expression was determined by using Brilliant Violet 650–conjugated anti-CD25 (BC96; BioLegend).
For intracellular cytokine detection, ILC2s were stimulated for 24 hours, as described above, with GolgiPlug and GolgiStop (BD Biosciences) added after 18 hours. Thereafter, ILC2s were fixed (2% paraformaldehyde), permeabilized (FACS Permeabilizing Solution 2; BD Biosciences); and stained for intracellular IL-13 (allophycocyanin-conjugated anti–IL-13; JES10-5A2) and IL-5 (PE-conjugated anti–IL-5; TRFK5) with antibodies from BD Biosciences.
Intracellular expression of GATA-3 was determined after fixation and permeabilization (Foxp3/transcription factor staining buffer set; eBioscience) by using allophycocyanin-conjugated anti–GATA-3 (clone TWAJ; eBioscience).
All samples were measured on an LSR Fortessa flow cytometer (BD Biosciences) and equipped with FACSDiva software, version 8, and data were analyzed with FlowJo software (Tree Star, Ashland, Ore).
Proliferation assay
For assessing cell proliferation, ILC2s were incubated in 500 μL of IMDM with 1 μL of CellTrace violet dye (Invitrogen) for 20 minutes at 37°C. Cells were washed twice with IMDM/10% FCS. Thereafter, cells were resuspended in Yssel medium/1% NHS and seeded into 96-well plates. Cells were treated with PGE2 (30 nmol/L) for 10 minutes and thereafter with IL-2 (10 U/mL) with or without IL-33, TSLP, and IL-25 (50 ng/mL each) for 72 hours. EP2 and EP4 receptor antagonists (1 μmol/L) were added 20 minutes before PGE2. All samples were measured on an LSR Fortessa flow cytometer, as described above. Cell viability was determined as the percentage of all singlet cells lacking staining of the LIVE/DEAD cell marker.
ELISA
IL-5 and IL-13 concentrations in ILC2 supernatants after the indicated treatments were assessed by means of ELISA. The Human IL-5 ELISA Duo-Set Kit was purchased from R&D Systems, and the human IL-13 ELISA Kit was from Sanquin.
RT-qPCR
Total RNA from expanded tonsillar ILC2s was isolated with the RNeasy Micro Kit (Qiagen). iScript cDNA Synthesis Kit (Bio-Rad Laboratories) was used for cDNA synthesis and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories) for PCR reactions. Primers were from the PrimePCR SYBR Green assay (PTGER1-PTGER4) for human EP1 to EP4 receptors and the PrimePCR SYBR Green assay (human RPS18) for the housekeeping gene RPS18 (all primers were from Bio-Rad Laboratories). RT-qPCRs were performed in the CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories).
RNA-seq analysis
Single-cell RNA-seq expression patterns were obtained as reads per kilobase gene model and million mappable reads from the Björklund et alE1 expression matrix (Gene Expression Omnibus no. GSE70580). Expression levels of the PTGER genes in the different ILC subsets and NK cells were interpreted as violin plots by using R software.
Statistical analyses
In all experiments n represents the number of individual donors used for ILC2 isolation. Differences between 2 groups were analyzed by using the matched-pairs t test, and 3 or more groups were compared by using 1-way ANOVA for repeated measurements and the Dunnett multiple comparisons test. Analyses were performed with GraphPad Prism 6 software.
References
- 1.Fort M.M., Cheung J., Yen D., Li J., Zurawski S.M., Lo S. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 2.Hurst S.D., Muchamuel T., Gorman D.M., Gilbert J.M., Clifford T., Kwan S. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 3.Halim T.Y., Krauss R.H., Sun A.C., Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Barlow J.L., Bellosi A., Hardman C.S., Drynan L.F., Wong S.H., Cruickshank J.P. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198. doi: 10.1016/j.jaci.2011.09.041. e1-4. [DOI] [PubMed] [Google Scholar]
- 5.Bartemes K.R., Iijima K., Kobayashi T., Kephart G.M., McKenzie A.N., Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y.J., DeKruyff R.H., Umetsu D.T. The role of type 2 innate lymphoid cells in asthma. J Leukoc Biol. 2013;94:933–940. doi: 10.1189/jlb.0313127. [DOI] [PubMed] [Google Scholar]
- 7.Monticelli L.A., Sonnenberg G.F., Abt M.C., Alenghat T., Ziegler C.G., Doering T.A. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith S.G., Chen R., Kjarsgaard M., Huang C., Oliveria J.P., O'Byrne P.M. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86.e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 9.Mjösberg J., Eidsmo L. Update on innate lymphoid cells in atopic and non-atopic inflammation in the airways and skin. Clin Exp Allergy. 2014;44:1033–1043. doi: 10.1111/cea.12353. [DOI] [PubMed] [Google Scholar]
- 10.Mjösberg J., Bernink J., Peters C., Spits H. Transcriptional control of innate lymphoid cells. Eur J Immunol. 2012;42:1916–1923. doi: 10.1002/eji.201242639. [DOI] [PubMed] [Google Scholar]
- 11.Duerr C.U., Fritz J.H. Regulation of group 2 innate lymphoid cells. Cytokine. 2016;87:1–8. doi: 10.1016/j.cyto.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Hazenberg M.D., Spits H. Human innate lymphoid cells. Blood. 2014;124:700–709. doi: 10.1182/blood-2013-11-427781. [DOI] [PubMed] [Google Scholar]
- 13.Mjösberg J., Bernink J., Golebski K., Karrich J.J., Peters C.P., Blom B. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Lambrecht B.N., Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 15.Li B.W., Hendriks R.W. Group 2 innate lymphoid cells in lung inflammation. Immunology. 2013;140:281–287. doi: 10.1111/imm.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halim T.Y., Hwang Y.Y., Scanlon S.T., Zaghouani H., Garbi N., Fallon P.G. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2016;17:57–64. doi: 10.1038/ni.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fanning L.B., Boyce J.A. Lipid mediators and allergic diseases. Ann Allergy Asthma Immunol. 2013;111:155–162. doi: 10.1016/j.anai.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue L., Salimi M., Panse I., Mjösberg J.M., McKenzie A.N., Spits H. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konya V., Mjösberg J. Lipid mediators as regulators of human ILC2 function in allergic diseases. Immunol Lett. 2016;179:36–42. doi: 10.1016/j.imlet.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Machado-Carvalho L., Roca-Ferrer J., Picado C. Prostaglandin E2 receptors in asthma and in chronic rhinosinusitis/nasal polyps with and without aspirin hypersensitivity. Respir Res. 2014;15:100. doi: 10.1186/s12931-014-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaslona Z., Okunishi K., Bourdonnay E., Domingo-Gonzalez R., Moore B.B., Lukacs N.W. Prostaglandin E(2) suppresses allergic sensitization and lung inflammation by targeting the E prostanoid 2 receptor on T cells. J Allergy Clin Immunol. 2014;133:379–387. doi: 10.1016/j.jaci.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavord I.D., Wong C.S., Williams J., Tattersfield A.E. Effect of inhaled prostaglandin E2 on allergen-induced asthma. Am Rev Respir Dis. 1993;148:87–90. doi: 10.1164/ajrccm/148.1.87. [DOI] [PubMed] [Google Scholar]
- 23.Sreeramkumar V., Fresno M., Cuesta N. Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol. 2012;90:579–586. doi: 10.1038/icb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturm E.M., Schratl P., Schuligoi R., Konya V., Sturm G.J., Lippe I.T. Prostaglandin E2 inhibits eosinophil trafficking through E-prostanoid 2 receptors. J Immunol. 2008;181:7273–7283. doi: 10.4049/jimmunol.181.10.7273. [DOI] [PubMed] [Google Scholar]
- 25.Konya V., Ullen A., Kampitsch N., Theiler A., Philipose S., Parzmair G.P. Endothelial E-type prostanoid 4 receptors promote barrier function and inhibit neutrophil trafficking. J Allergy Clin Immunol. 2013;131:532–540. doi: 10.1016/j.jaci.2012.05.008. e1-2. [DOI] [PubMed] [Google Scholar]
- 26.Luschnig-Schratl P., Sturm E.M., Konya V., Philipose S., Marsche G., Frohlich E. EP4 receptor stimulation down-regulates human eosinophil function. Cell Mol Life Sci. 2011;68:3573–3587. doi: 10.1007/s00018-011-0642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacKenzie K.F., Clark K., Naqvi S., McGuire V.A., Noehren G., Kristariyanto Y. PGE(2) induces macrophage IL-10 production and a regulatory-like phenotype via a protein kinase A-SIK-CRTC3 pathway. J Immunol. 2013;190:565–577. doi: 10.4049/jimmunol.1202462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Björklund A.K., Forkel M., Picelli S., Konya V., Theorell J., Friberg D. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17:451. doi: 10.1038/ni.3368. [DOI] [PubMed] [Google Scholar]
- 29.Mjösberg J.M., Trifari S., Crellin N.K., Peters C.P., van Drunen C.M., Piet B. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 30.Hoyler T., Klose C.S., Souabni A., Turqueti-Neves A., Pfeifer D., Rawlins E.L. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirchandani A.S., Besnard A.G., Yip E., Scott C., Bain C.C., Cerovic V. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 32.Bartemes K.R., Kephart G.M., Fox S.J., Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–678.e4. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagakumar P., Denney L., Fleming L., Bush A., Lloyd C.M., Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol. 2016;137:624–626.e6. doi: 10.1016/j.jaci.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Hams E., Armstrong M.E., Barlow J.L., Saunders S.P., Schwartz C., Cooke G. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014;111:367–372. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho J., Bailey M., Zaunders J., Mrad N., Sacks R., Sewell W. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015;45:394–403. doi: 10.1111/cea.12462. [DOI] [PubMed] [Google Scholar]
- 36.Salimi M., Barlow J.L., Saunders S.P., Xue L., Gutowska-Owsiak D., Wang X. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harizi H. Reciprocal crosstalk between dendritic cells and natural killer cells under the effects of PGE2 in immunity and immunopathology. Cell Mol Immunol. 2013;10:213–221. doi: 10.1038/cmi.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rumzhum N.N., Ammit A.J. Cyclooxygenase 2: its regulation, role and impact in airway inflammation. Clin Exp Allergy. 2016;46:397–410. doi: 10.1111/cea.12697. [DOI] [PubMed] [Google Scholar]
- 39.Melillo E., Woolley K.L., Manning P.J., Watson R.M., O'Byrne P.M. Effect of inhaled PGE2 on exercise-induced bronchoconstriction in asthmatic subjects. Am J Respir Crit Care Med. 1994;149:1138–1141. doi: 10.1164/ajrccm.149.5.8173753. [DOI] [PubMed] [Google Scholar]
- 40.Szczeklik A., Mastalerz L., Nizankowska E., Cmiel A. Protective and bronchodilator effects of prostaglandin E and salbutamol in aspirin-induced asthma. Am J Respir Crit Care Med. 1996;153:567–571. doi: 10.1164/ajrccm.153.2.8564099. [DOI] [PubMed] [Google Scholar]
- 41.Gauvreau G.M., Watson R.M., O'Byrne P.M. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med. 1999;159:31–36. doi: 10.1164/ajrccm.159.1.9804030. [DOI] [PubMed] [Google Scholar]
- 42.Okano M., Sugata Y., Fujiwara T., Matsumoto R., Nishibori M., Shimizu K. E prostanoid 2 (EP2)/EP4-mediated suppression of antigen-specific human T-cell responses by prostaglandin E2. Immunology. 2006;118:343–352. doi: 10.1111/j.1365-2567.2006.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konya V., Philipose S., Balint Z., Olschewski A., Marsche G., Sturm E.M. Interaction of eosinophils with endothelial cells is modulated by prostaglandin EP4 receptors. Eur J Immunol. 2011;41:2379–2389. doi: 10.1002/eji.201141460. [DOI] [PubMed] [Google Scholar]
- 44.Säfholm J., Manson M.L., Bood J., Delin I., Orre A.C., Bergman P. Prostaglandin E2 inhibits mast cell-dependent bronchoconstriction in human small airways through the E prostanoid subtype 2 receptor. J Allergy Clin Immunol. 2015;136:1232–1239.e1. doi: 10.1016/j.jaci.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Birrell M.A., Maher S.A., Dekkak B., Jones V., Wong S., Brook P. Anti-inflammatory effects of PGE2 in the lung: role of the EP4 receptor subtype. Thorax. 2015;70:740–747. doi: 10.1136/thoraxjnl-2014-206592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okano M., Fujiwara T., Haruna T., Kariya S., Makihara S., Higaki T. Prostaglandin E(2) suppresses staphylococcal enterotoxin-induced eosinophilia-associated cellular responses dominantly through an E-prostanoid 2-mediated pathway in nasal polyps. J Allergy Clin Immunol. 2009;123:868–874.e13. doi: 10.1016/j.jaci.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 47.Konya V., Maric J., Jandl K., Luschnig P., Aringer I., Lanz I. Activation of EP4 receptors prevents endotoxin-induced neutrophil infiltration into the airways and enhances microvascular barrier function. Br J Pharmacol. 2015 doi: 10.1111/bph.13229. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratcliffe M.J., Walding A., Shelton P.A., Flaherty A., Dougall I.G. Activation of E-prostanoid4 and E-prostanoid2 receptors inhibits TNF-alpha release from human alveolar macrophages. Eur Respir J. 2007;29:986–994. doi: 10.1183/09031936.00131606. [DOI] [PubMed] [Google Scholar]
- 49.Kay L.J., Yeo W.W., Peachell P.T. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br J Pharmacol. 2006;147:707–713. doi: 10.1038/sj.bjp.0706664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corrigan C.J., Napoli R.L., Meng Q., Fang C., Wu H., Tochiki K. Reduced expression of the prostaglandin E2 receptor E-prostanoid 2 on bronchial mucosal leukocytes in patients with aspirin-sensitive asthma. J Allergy Clin Immunol. 2012;129:1636–1646. doi: 10.1016/j.jaci.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Cahill K.N., Raby B.A., Zhou X., Guo F., Thibault D., Baccarelli A. Impaired E prostanoid2 expression and resistance to prostaglandin E2 in nasal polyp fibroblasts from subjects with aspirin-exacerbated respiratory disease. Am J Respir Cell Mol Biol. 2016;54:34–40. doi: 10.1165/rcmb.2014-0486OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buckley J., Birrell M.A., Maher S.A., Nials A.T., Clarke D.L., Belvisi M.G. EP4 receptor as a new target for bronchodilator therapy. Thorax. 2011;66:1029–1035. doi: 10.1136/thx.2010.158568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norel X. Prostanoid receptors in the human vascular wall. ScientificWorldJournal. 2007;7:1359–1374. doi: 10.1100/tsw.2007.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Audoly L.P., Tilley S.L., Goulet J., Key M., Nguyen M., Stock J.L. Identification of specific EP receptors responsible for the hemodynamic effects of PGE2. Am J Physiol. 1999;277:H924–H930. doi: 10.1152/ajpheart.1999.277.3.H924. [DOI] [PubMed] [Google Scholar]
- 55.Bradbury D., Clarke D., Seedhouse C., Corbett L., Stocks J., Knox A. Vascular endothelial growth factor induction by prostaglandin E2 in human airway smooth muscle cells is mediated by E prostanoid EP2/EP4 receptors and SP-1 transcription factor binding sites. J Biol Chem. 2005;280:29993–30000. doi: 10.1074/jbc.M414530200. [DOI] [PubMed] [Google Scholar]
- 56.Deacon K., Knox A.J. Human airway smooth muscle cells secrete amphiregulin via bradykinin/COX-2/PGE2, inducing COX-2, CXCL8, and VEGF expression in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2015;309:L237–L249. doi: 10.1152/ajplung.00390.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takayama K., Sukhova G.K., Chin M.T., Libby P. A novel prostaglandin E receptor 4-associated protein participates in antiinflammatory signaling. Circ Res. 2006;98:499–504. doi: 10.1161/01.RES.0000204451.88147.96. [DOI] [PubMed] [Google Scholar]
- 58.Barnig C., Cernadas M., Dutile S., Liu X., Perrella M.A., Kazani S. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra26. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou W., Toki S., Zhang J., Goleniewksa K., Newcomb D.C., Cephus J.Y. Prostaglandin I2 Signaling and Inhibition of Group 2 Innate Lymphoid Cell Responses. Am J Respir Crit Care Med. 2016;193:31–42. doi: 10.1164/rccm.201410-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xue L., Gyles S.L., Wettey F.R., Gazi L., Townsend E., Hunter M.G. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6536. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 61.Sedej M., Schroder R., Bell K., Platzer W., Vukoja A., Kostenis E. D-type prostanoid receptor enhances the signaling of chemoattractant receptor-homologous molecule expressed on T(H)2 cells. J Allergy Clin Immunol. 2012;129:492–500. doi: 10.1016/j.jaci.2011.08.015. e1-9. [DOI] [PubMed] [Google Scholar]
- 62.Roediger B., Kyle R., Tay S.S., Mitchell A.J., Bolton H.A., Guy T.V. IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. J Allergy Clin Immunol. 2015;136:1653–1663. doi: 10.1016/j.jaci.2015.03.043. e1-7. [DOI] [PubMed] [Google Scholar]
- 63.Kolenko V., Rayman P., Roy B., Cathcart M.K., O'Shea J., Tubbs R. Downregulation of JAK3 protein levels in T lymphocytes by prostaglandin E2 and other cyclic adenosine monophosphate-elevating agents: impact on interleukin-2 receptor signaling pathway. Blood. 1999;93:2308–2318. [PubMed] [Google Scholar]
- 64.Maslanka T., Spodniewska A., Barski D., Jasiecka A., Zuska-Prot M., Ziolkowski H. Prostaglandin E(2) down-regulates the expression of CD25 on bovine T cells, and this effect is mediated through the EP4 receptor. Vet Immunol Immunopathol. 2014;160:192–200. doi: 10.1016/j.vetimm.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Chemnitz J.M., Driesen J., Classen S., Riley J.L., Debey S., Beyer M. Prostaglandin E2 impairs CD4+ T cell activation by inhibition of lck: implications in Hodgkin's lymphoma. Cancer Res. 2006;66:1114–1122. doi: 10.1158/0008-5472.CAN-05-3252. [DOI] [PubMed] [Google Scholar]
References
- Björklund A.K., Forkel M., Picelli S., Konya V., Theorell J., Friberg D. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17:451. doi: 10.1038/ni.3368. [DOI] [PubMed] [Google Scholar]
- Mjösberg J.M., Trifari S., Crellin N.K., Peters C.P., van Drunen C.M., Piet B. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]