Abstract
In a tractable model for cell invasion, the Caenorhabditis elegans anchor cell migrates through basement membranes towards a polarity cue provided by netrin. A new study reveals that the anchor cell polarity network can break symmetry and oscillate in the absence of netrin, suggesting the presence of interlinked positive and negative feedback loops, which are common in polarity pathways.
Establishment of cell polarity and guidance of cell growth or movement are critical processes for proper development of a multicellular organism. However, because of the difficulty in detecting and manipulating such processes in vivo, most studies on polarity mechanisms have focused on single-cell systems. A new study by Wang et al. [1] published in the Journal of Cell Biology exploits the transparent nematode worm Caenorhabditis elegans to demonstrate that polarity control principles recently discovered in single-cell systems also act in a multicellular context.
In order to initiate uterine–vulval attachment, the C. elegans anchor cell polarizes toward a netrin spatial cue and invades through the basement membranes separating the uterine and vulval tissues (Figure 1Ai) [2]. Netrin, a laminin-related protein, is secreted by the ventral nerve cord and is sensed by the anchor cell through the netrin receptor UNC-40 (the ortholog of the vertebrate deleted in colorectal cancer receptor, DCC). In addition to anchor cell invasion, netrin directs several other polarization events in C. elegans, including dendritic and axon outgrowth [3,4], synaptogenesis [5], and distal tip cell migration [6].
Figure 1.
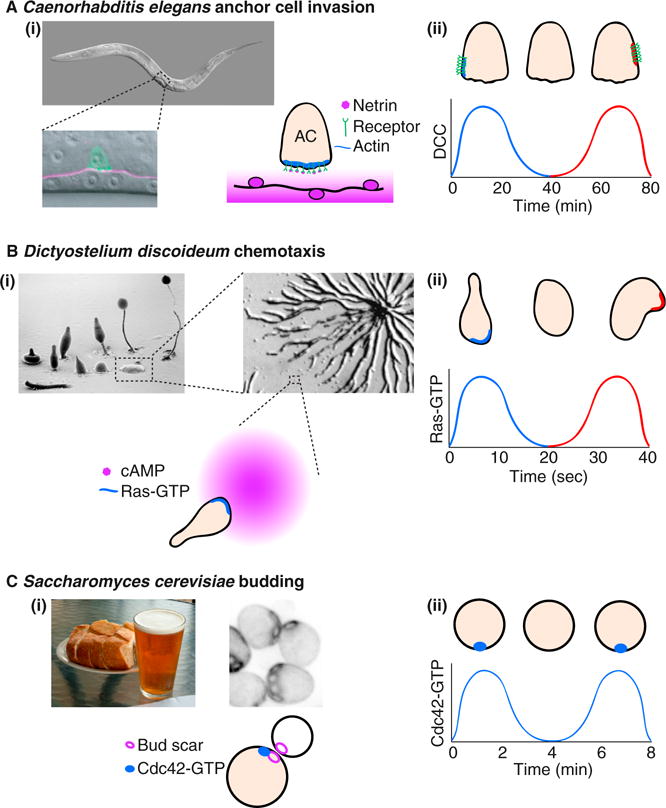
Removal of spatial cues reveals a potential for oscillatory behavior in several polarity systems.
(Ai) The C. elegans anchor cell (AC) invades through the underlying basement membrane to initiate uterine–vulval attachment (pictures courtesy of Judith Kimble, University of Wisconsin-Madison, and [17]). The anchor cell polarizes towards the spatial cue, netrin, which is released by the ventral nerve cord. (Aii) In animals without netrin, the anchor cell netrin receptors cluster at a random location, disperse, and then repolarize at a different site. The graph represents the concentration of netrin receptor (UNC-40/DCC) at the blue and red sites in the picture [1]. (Bi) D. discoideum cells migrate up the cAMP gradient released by other cells as they aggregate (pictures courtesy of M.J. Grimson and R.L. Blanton, Texas Tech University, and [18]). (Bii) In uniform cAMP, the cells polarize transiently, then depolarize and repolarize in a random direction. The graph represents the local concentration of the polarity regulator Ras-GTP [7]. Note the faster time scale of clustering and disassembly. (Ci) S. cerevisiae cells polarize towards an internal spatial cue at the bud scar. (Cii) In mutants lacking the spatial cue, cells cluster Cdc42 at a random location in an oscillatory manner. The graph represents the local concentration of Cdc42-GTP in the cluster [8].
Cells that orient towards specific cues generally develop a stable polarity axis with a clear ‘front’. But what happens when the spatial cue is removed? Wang et al. [1] found that, in the absence of netrin, UNC-40 and its effectors cluster at a random site in the anchor cell. Using high-resolution time-lapse microscopy, the authors found that these polarity clusters were dynamic: they disassembled and then reassembled at a new site in an oscillatory manner (Figure 1Aii). Clustering at random sites is suggestive of the existence of positive feedback, such that small asymmetries in polarity protein concentration are amplified to promote clustering. Subsequent cluster disassembly is suggestive of delayed negative feedback, which counteracts positive feedback.
The presence of interlinked positive and negative feedback loops has been inferred from similar oscillatory or excitable behavior in other systems. In appropriate environments, cells of the social amoeba Dictyostelium discoideum aggregate to form fruiting bodies. The cells migrate towards each other following gradients of the chemoattractant cyclic AMP (cAMP) and such cells stably concentrate the polarity regulators Ras-GTP and phosphatidylinositol-3,4,5-triphosphate (PIP3) at the front (Figure 1Bi). But when D. discoideum cells are deprived of a spatial cue, Ras-GTP and PIP3 spontaneously cluster at random locations. The polarity clusters then disperse and reform at other sites (Figure 1Bii) [7]. In another example, cells of the budding yeast Saccharomyces cerevisiae stably polarize Cdc42 and associated polarity factors at preselected bud sites demarcated by a system of inherited bud-site-selection cues (Figure 1Ci). But in mutants lacking such cues, polarity factors spontaneously cluster at a random location on the cell cortex. These polarity clusters also disperse and reform, often, though not always, at the same site (Figure 1Cii) [8]. Similar oscillatory behaviors have also been noted for Cdc42 and related GTPases in T cells [9], fission yeast [10,11], and pollen tubes [12]. Although these various examples utilize different molecules and occur on different timescales (Figure 1Aii,Bii,Cii), they appear to reflect a common underlying architecture of interlinked positive and negative feedback loops [13].
Studies on biological oscillators, like those controlling circadian rhythms and the cell cycle, have established that interlinked positive feedback and delayed negative feedback loops constitute an excellent way to construct a robust oscillator [14,15]. But there is no obvious need for a polarity system to oscillate. Indeed, oscillatory or excitable behaviors of polarity regulators were only revealed when experimenters deprived polarizing cells of their normal spatial cues (Figure 1Aii,Bii,Cii). So, what benefits would interlinked positive and negative feedback provide for polarity networks?
One hypothesis on the potential role of feedback derives from the observation that, in their physiological contexts, polarizing cells are often called upon to track very shallow chemical gradients in a noisy environment. Positive feedback provides an effective way to amplify a shallow gradient signal into a strongly polarized response that can orient cell motility or growth. However, the resulting hair-trigger polarization may lead cells to polarize in the wrong direction, and, once polarized, positive feedback would make it hard to change direction. Theoretical studies showed that addition of a slower negative feedback loop could counteract these problems, allowing error correction and effective gradient tracking [16]. Thus, the circuit design revealed by oscillatory behavior in the absence of spatial information may have been optimized to allow effective gradient tracking when spatial cues are present. Alternatively, it has been speculated that negative feedback may serve to restrain the spreading of polarity clusters by positive feedback, making polarity systems more robust [8].
Testing whether these concepts hold in the anchor cell will require elucidation of the mechanisms of positive and negative feedback, so that they can be surgically removed. Wang et al. [1] show that UNC-40 and its effectors localize to the polarity clusters, and that the UNC-40 receptor itself is required for polarity cluster oscillations. This suggests that the receptor is active even in the absence of netrin, and may itself participate in the posited feedback mechanisms. Given the polarizing roles of netrin/DCC in other cell types, it will be very interesting to see whether similar feedback loops are common in other developmental contexts. More generally, an understanding of the feedback mechanisms in different polarity systems will allow us to appreciate whether they are indeed employed to hone gradient tracking or whether they provide additional benefits.
References
- 1.Wang Z, Linden LM, Naegeli KM, Ziel JW, Chi Q, Hagedorn EJ, Savage NS, Sherwood DR. UNC-6 (netrin) stabilizes oscillatory clustering of the UNC-40 (DCC) receptor to orient polarity. J Cell Biol. 2014;206:619–633. doi: 10.1083/jcb.201405026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziel JW, Hagedorn EJ, Audhya A, Sherwood DR. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat Cell Biol. 2009;11:183–189. doi: 10.1038/ncb1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teichmann HM, Shen K. UNC-6 and UNC-40 promote dendritic growth through PAR-4 in Caenorhabditis elegans neurons. Nat Neurosci. 2011;14:165–172. doi: 10.1038/nn.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler CE, Fetter RD, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colon-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 7.Huang CH, Tang M, Shi C, Iglesias PA, Devreotes PN. An excitable signal integrator couples to an idling cytoskeletal oscillator to drive cell migration. Nat Cell Biol. 2013;15:1307–1316. doi: 10.1038/ncb2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell AS, Jin M, Wu CF, Zyla TR, Elston TC, Lew DJ. Negative feedback enhances robustness in the yeast polarity establishment circuit. Cell. 2012;149:322–333. doi: 10.1016/j.cell.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinai P, Nguyen C, Schatzle JD, Wulfing C. Transience in polarization of cytolytic effectors is required for efficient killing and controlled by Cdc42. Proc Natl Acad Sci USA. 2010;107:11912–11917. doi: 10.1073/pnas.0913422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das M, Drake T, Wiley DJ, Buchwald P, Vavylonis D, Verde F. Oscillatory dynamics of Cdc42 GTPase in the control of polarized growth. Science. 2012;337:239–243. doi: 10.1126/science.1218377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendezu FO, Martin SG. Cdc42 explores the cell periphery for mate selection in fission yeast. Curr Biol. 2013;23:42–47. doi: 10.1016/j.cub.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Hwang JU, Gu Y, Lee YJ, Yang Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell. 2005;16:5385–5399. doi: 10.1091/mbc.E05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CF, Lew DJ. Beyond symmetry-breaking: competition and negative feedback in GTPase regulation. Trends Cell Biol. 2013;23:476–483. doi: 10.1016/j.tcb.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai TY, Choi YS, Ma W, Pomerening JR, Tang C, Ferrell JE., Jr Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science. 2008;321:126–129. doi: 10.1126/science.1156951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novak B, Tyson JJ. Design principles of biochemical oscillators. Nat Rev Mol Cell Biol. 2008;9:981–991. doi: 10.1038/nrm2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meinhardt H. Orientation of chemotactic cells and growth cones: models and mechanisms. J Cell Sci. 1999;112:2867–2874. doi: 10.1242/jcs.112.17.2867. [DOI] [PubMed] [Google Scholar]
- 17.Hagedorn EJ, Sherwood DR. Cell invasion through basement membrane: the anchor cell breaches the barrier. Curr Opin Cell Biol. 2011;23:589–596. doi: 10.1016/j.ceb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang W, Gomer RH. Combining experiments and modelling to understand size regulation in Dictyostelium discoideum. J Roy Soc Interface. 2008;5(Suppl 1):S49–S58. doi: 10.1098/rsif.2008.0067.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]


