Abstract
Efficient control of attentional resources and high-acuity vision are both fundamental for survival. Shifts in visual attention are known to covertly enhance processing at locations away from the center of gaze, where visual resolution is low. It is unknown, however, whether selective spatial attention operates where the observer already looks, i.e., within the high-acuity foveola, the small, yet disproportionally important rod-free region of the retina. Using new methods for precisely controlling retinal stimulation, here we show that covert attention flexibly improves and speeds-up both detection and discrimination at loci only a fraction of a degree apart within the foveola. These findings reveal a surprisingly precise control of attention and its involvement in fine spatial vision. They show that the commonly studied covert shifts of attention away from the fovea are the expression of a global mechanism that exerts its action across the entire visual field.
Covert attention is essential for visual perception. Among its many advantages, covert allocation of attentional resources increases contrast sensitivity and spatial resolution, speeds information accrual and reaction times [1] [2] [3] [4], and also alters the signal at the target location during saccade preparation [5] [6] [7]. Covert attention has been studied sometimes in the parafovea (1°–5°), and mostly in the perifovea (5°–10°) and periphery (>10° of eccentricity), i.e., far outside the foveola, the high-acuity region of the retina at the center of gaze [1] [2] [3] [4] [8] [9]. This is the rod and capillary-free region of the fovea where cones are most densely packed, an area that covers only ~1° of visual angle [10], about the size of a full moon in the sky.
At first sight, it may appear that studying covert attention in the foveola makes little sense. This anatomical region is commonly identified with “where the observer looks” [11], and covert attention is traditionally regarded as a process that focuses neural resources outside this portion of the visual field. In addition, small eye movements incessantly move stimuli across the foveola during fixation [12] [13] [14], so that the conceptual distinction between covert and overt responses is unclear at this scale. Furthermore, it is commonly assumed that the spatial scale of attention is too coarse to selectively process information within subregions of the foveola [15] [16]. However, the opposite hypothesis that high-resolution control of covert attention could be beneficial around the center of gaze has also been raised [17] [18] [19]. This proposal is feasible considering the large representation devoted to the fovea in striate and extrastriate cortical areas, as well as the recent observation that microscopic eye movements precisely position the line of sight [20].
Until recently, it would have been impossible to investigate covert attention within the foveola because of technical difficulties. But these challenges have now been overcome [20] [21], and we report here the results of four experiments, which examined, for the first time, the selective deployment and control of attention at the very center of gaze. In this study, we first established a baseline by conducting a detection task in the parafovea (Exp. 1), as in previous studies. We then investigated the consequences of attention on both detection (Exp. 2) and discrimination (Exps. 3 and 4) tasks within the foveola.
Results
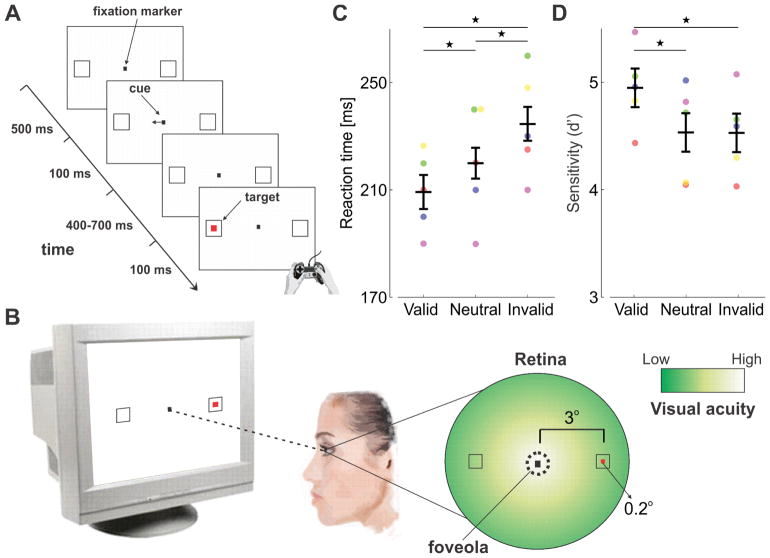
Experiment 1 consisted of a central spatial cueing task with parafoveal stimuli (Fig. 1A–B), a standard procedure to study endogenous (voluntary) covert attention. As expected, compared to the neutral condition, attention resulted in a benefit—faster detection— at the attended location, and a cost—slower detection— at the unattended location (Fig. 1C, ANOVA F(2,4) = 23.6; p=0.0004). This effect is not the consequence of speed-accuracy trade-offs, as accuracy remained high with shorter reaction times (in fact, it slightly increased; Fig. 1D, ANOVA F(2,4) = 14.9; p=0.002), and is consistent with previous studies in which stimuli were presented many degrees away from the center of gaze. Such enhancement is highly beneficial for extrafoveal vision, because various visual functions gradually deteriorate with increasing eccentricity, and attention can effectively attenuate these effects by improving extrafoveal performance [1] [2] [3] [4].
Figure 1. Attention control in the parafovea (Exp. 1).
(A) A spatial cueing task. Observers (n=5) maintain fixation on a central marker and report the appearance of a target (red square) at one of two locations (black empty squares) as quickly and accurately as possible. A central cue always precedes the target, indicating its most likely location (76% cue validity). The delay between cue and target onsets ensures the deployment of voluntary attention. (B) Targets are presented far from the center of gaze (at 3° eccentricity), so that observers need to covertly shift attention away from the foveola, the region of highest visual acuity. (C) Average reaction times for correct responses and (D) accuracy expressed as index of sensitivity (d′), in the three types of trials in which the cue correctly predicted the target location (valid trials), predicted the wrong location (invalid trials), and had no predictive value (neutral trials). Error bars are 95% CI. Asterisks indicate statistically significant differences (Tukey HSD post-hoc tests. Reaction times: valid trials vs. neutral trials, p=0.0476; valid vs. invalid, p=0.0003; neutral vs. invalid, p=0.0105. Sensitivity: valid vs. neutral, p=0.0034; valid vs. invalid, p=0.0041; neutral vs. invalid p=0.9878). Dots represent data from individual observers, each marked by a different color. To ensure optimal visual stimulation, all analyses reported in this study are based on trials without blinks, saccades, and/or microsaccades (see Methods).
Foveal control of attention facilitates detection
What happens when the attended location is not in the visual periphery, but already at the center of gaze, i.e., within the foveola? Can attention be fine-tuned to selectively enhance vision at specific locations in this tiny portion of the visual field?
Studying attentional control within the foveola is extremely challenging (Fig. 2A). It requires high accuracy in localizing the position of a stimulus with respect to the preferred retinal locus of fixation, a requirement that is beyond the limits of standard video-oculography. These methods typically yield an area of uncertainty that is about 1 deg2 [22], approximately the size of the entire foveola. Furthermore, it requires dealing with the retinal motion resulting from incessant fixational eye movements [13], which continually shift the retinal projection of the attended location across the foveola [23] [24](Fig. 2B,D). Thus, whereas standard procedures to study covert attention work well when stimuli are presented far from the center of gaze, where visual resolution is lower, they cannot be applied to examine selective attention within the high-resolution portion of the visual field.
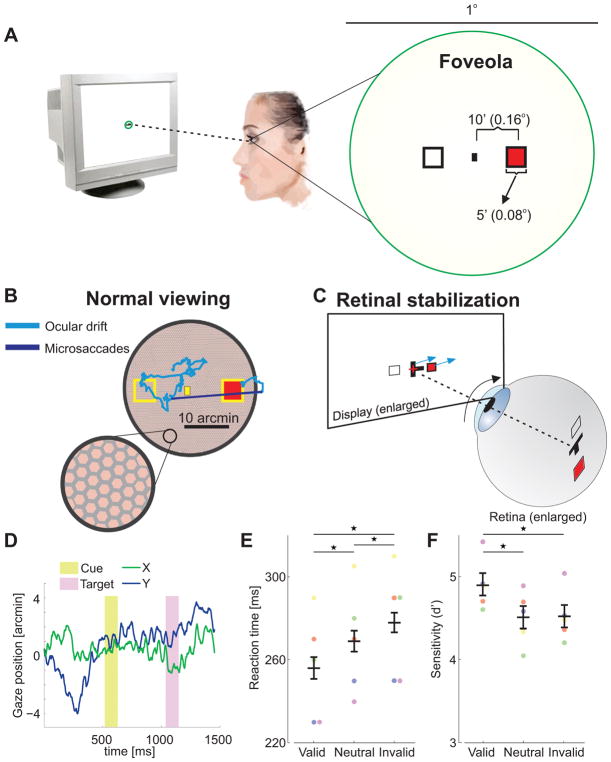
Figure 2. Attention control within the foveola (Exp. 2).
(A) A spatial cueing task in the foveola requires precise presentation of stimuli at nearby locations. (B) This requirement is challenged by incessant small eye movements, which normally displace the retinal image over the photoreceptors mosaic by an area as large as the foveola itself. (C) Stimuli were maintained at the desired eccentricities by moving them in real time, under computer control (cyan arrows), to compensate for the observer’s eye movements (black arrow). (D) An example of eye movements during the course of a trial. (E) Average reaction times and (F) accuracy for different trial types across observers (n=5). Differences in reaction times between valid and invalid trials were statistically significant for all individual observers (two-tailed Wilcoxon rank sum tests. S1: p=0.004; S2: p=0.022; S3: p=0.039; S4: p=0.0009; S5: p=0.0009). Error bars are 95% CI. Asterisks indicate statistically significant differences (Tukey HSD post-hoc tests. Reaction times: valid trials vs. neutral trials, p=0.0074; valid vs. invalid, p=0.0003; neutral vs. invalid, p=0.0472. Sensitivity: valid vs. neutral, p=0.0135; valid vs. invalid, p=0.0128; neutral vs. invalid p=0.9992). Conventions are as in Fig. 1.
To circumvent these limitations, we relied on a state-of-the-art system for gaze-contingent display control [21] coupled with a Dual Purkinjie Image eyetracker, a device with very high spatial and temporal resolution. This enabled use of: (a) a gaze-contingent calibration procedure that effectively improves localization of the center of the preferred retinal locus of fixation by more than one order of magnitude compared to standard methods [20] [25]; and (b) retinal stabilization [20] to ensure that visual stimuli always remained at the desired foveal eccentricities despite the continual presence of fixational eye movements (see Fig. 2C). Using these techniques, in Experiment 2 we tested performance in a spatial cueing task similar to the one shown in Fig. 1, but for targets appearing well within the foveola, only a few arcminutes away from the center of gaze (1 arcmin =1/60 of a degree). In this experiment, the stimuli were scaled down in size so that their cortical representations approximately matched those of the stimuli used in Experiment 1 [26].
All observers exhibited the classical attention effects, even though attended and unattended locations were now only 20′ (0.33°) apart (Fig. 2E). As with stimuli outside the foveola, observers were faster at detecting targets presented at the attended location (ANOVA F(2,4) = 25.5; p=0.0003). Notably, the effect of attention did not change with retinal distance. The difference between reaction times in valid and invalid trials was similar to the one measured in Experiment 1 (foveola: 22 ms ± 5 ms; parafovea: 25 ms ± 10 ms; two-tailed paired t-test, p=0.31), but the distance between attended and unattended locations was now approximately twenty times smaller. Again, no speed-accuracy trade-off was present, i.e., faster reaction times did not come at the cost of accuracy; accuracy remained very high in all conditions, and it even slightly increased in the valid trials (Fig. 2F, ANOVA F(2,4) = 9.6; p=0.008). Also note that despite retinal stabilization, eye movement characteristics were virtually identical to those measured in Experiment 1 (Supplementary Figure 1). These data show that attention facilitates detection at attended locations at the expense of unattended ones in the foveola, just as in the rest of the visual field.
Interestingly, observers were slower at detecting targets presented within the foveola than in the parafovea (p<0.0001, two-tailed paired t-test; compare reaction times in Fig. 1C and in Fig. 2E), even though the stimuli were equally detectable in both experiments (p>0.77 in all three trial types, two-tailed paired t-tests). On average, across all types of trials, the increment in reaction time was 46 ± 3 ms. These findings parallel the eccentricity-dependent effects reported outside the fovea [27], i.e., the slower information accrual for stimuli presented in the parafovea than in the perifovea, and point at qualitative differences in the processing of foveal and parafoveal stimuli.
Oculomotor reaction times also follow a similar trend; it takes longer to generate a microsaccade toward a stimulus already within the fovea, than a larger saccade toward a more eccentric stimulus [28]. In fact, we found similar results when observers were asked to report the target by performing a microsaccade toward its location rather than by pressing a button (Supplementary Fig. 2). These effects might originate from various factors, such as the characteristics of cone photoreceptors in the fovea and in the visual periphery [29], the different proportions of parvocellular and magnocellular neurons in the two regions [30] [31], and/or influences from the rostral part of the superior colliculus, where foveal space is represented [32]. Yet, despite these differences, attention facilitates detection in a similar way within and outside the foveola.
Foveal control of attention enhances fine spatial discrimination
The data in Fig. 2 show that attention speeds up detection of foveal stimuli. Can attention also enhance discrimination of fine detail? This is a critical question given that many daily activities require examination of fine patterns, and the finding of attentional control within the foveola in detection (Experiment 2) suggests that microscopic shifts of attention may play an important role in high-acuity vision. To investigate this hypothesis, in Experiment 3, we used a spatial cueing discrimination task. Observers were asked to report the orientation of a tiny bar that could appear at four possible locations, all at the same eccentricity within the foveola (Fig. 3A).
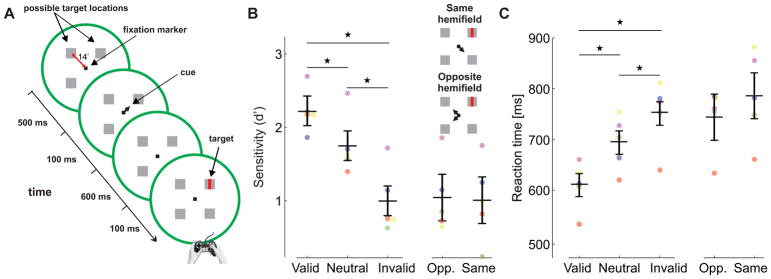
Figure 3. Attention and fine spatial discrimination (Exp. 3).
(A) Observers reported whether a tiny bar, which could appear in one of four boxes 14′ away from the fixation marker, was tilted vertically or horizontally. The target was preceded by a central cue that indicated its most likely location (76% cue validity). (B-C) Experimental results, measured as (B) accuracy (d′), and (C) reaction times for different trial types across observers (n=5). Differences between valid and invalid trials were statistically significant for all individual (Sensitivity, two-tailed z-tests: S1: p=1.9 10−7; S2: p=0.01; S3: p=4.9 10−4; S4: p=0.019; S5: p=2.9 10−12. Reaction times, two-tailed Wilcoxon rank sum tests: S1: p=1.6 10−9; S2: p=6.4 10−16; S3: p=2.1 10−10; S4: p=1.7 10−11; S5: p=8 10−24). Accuracy and reaction times are also shown separately for the invalid trials in which the cue and the target were presented in the same and opposite hemifield, respectively. Error bars are 95% CI. Asterisks indicate statistically significant differences (Tukey HSD post-hoc tests. Sensitivity: valid trials vs. neutral trials, p=0.0123; valid vs. invalid, p=0.00004; neutral vs. invalid, p=0.0008. Reaction times: valid vs. neutral, p=0.0009; valid vs. invalid, p=0.00004; neutral vs. invalid p=0.0084). Conventions are as in Fig. 1.
As shown in Fig. 3B, discrimination accuracy, expressed as the index of sensitivity d′, was significantly higher in the valid trials, when the cue correctly predicted the target location, than in the neutral and invalid trials (ANOVA F(2,4) = 49.9; p<0.0001). Reaction times were also faster in valid trials (ANOVA F(2,4) = 51; p<0.0001, Fig. 3C). Thus, for both accuracy and reaction times, significant benefits and costs occurred, respectively, in the valid- and invalid-cue trials relative to the neutral trials. Moreover, the cost resulting from focusing attention at the “wrong” location was similar, irrespective of whether this location fell within the same or opposite hemifield as the target (Fig. 3B–C, p=0.83 two-tailed paired t-test). Therefore, attentional shifts retained a very high degree of selectivity even for locations separated by only 20′ within the same hemifield.
Similar results were also obtained when the discrimination task of Experiment 3 (Fig. 3) was repeated with normal, non-stabilized, fixation (Experiment 4, Fig. 4). Also in this case there were significant accuracy (ANOVA F(2,4) = 79; p<0.0001) and reaction time (ANOVA F(2,4) = 98; p<0.0001) effects. Compared to the neutral condition, attention resulted in a benefit—higher and faster discrimination— at the attended location, and a cost—lower and slower discrimination— at the unattended location. As under retinal stabilization, perceptual enhancements were not the consequence of fixational eye movements repositioning the stimulus on a preferred retinal locus [20]. These effects were also present in the trials without microsaccades (Supplementary Fig. 3), as well as in trials in which the center of gaze—determined by means of our high-resolution localization procedure [20] [25]—remained relatively far from the target (e.g., distance from target >14′; ANOVA F(2,4) = 52; p<0.0001) or close to the fixation marker (e.g., distance from marker <7′; ANOVA F(2,4) = 19; p=0.0009). Thus, fine attentional control continues to operate in the presence of the physiological motion of the retinal image, when stimuli move across the foveola because of natural fixational instability (Supplementary Fig. 4B).
Figure 4. Fine attentional control during normal retinal image motion (Exp. 4).
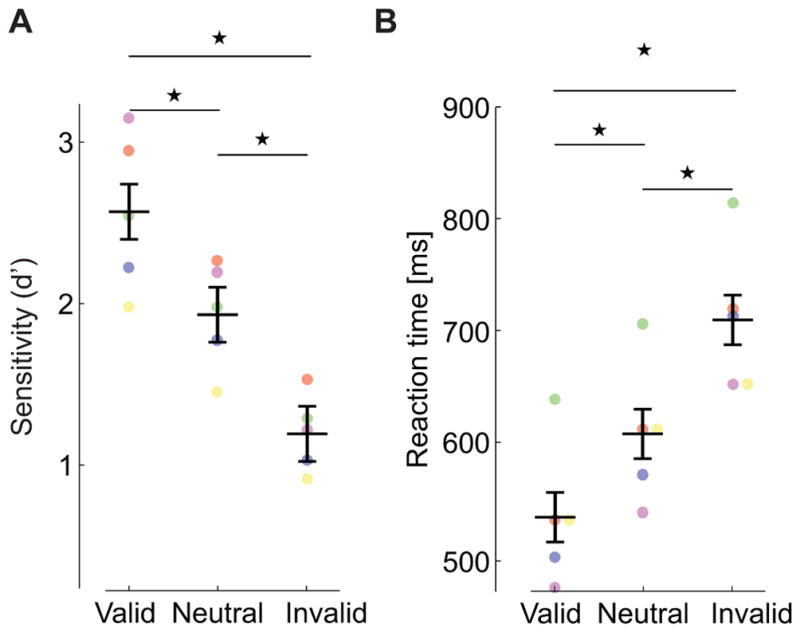
Results of a control experiment identical to Experiment 3 (Fig. 3), but without retinal stabilization. Stimuli moved on the retina because of the physiological instability of fixation. (A) Accuracy (d′); and (B) reaction times for different trial types across observers (n=5). Error bars are 95% CI. Asterisks indicate statistically significant differences (Tukey HSD post-hoc tests. Sensitivity: valid trials vs. neutral trials, p=0.0010; valid vs. invalid, p=0.00004; neutral vs. invalid, p=0.0004. Reaction times: valid vs. neutral, p=0.0010; valid vs. invalid, p=0.00004; neutral vs. invalid p=0.0001). Conventions are as in Fig. 1.
Discussion
The results of Experiments 2 and 3 (Figs. 2–3) show that attention is much more fine-grained and flexible than hitherto assumed: selective enhancement of visual processing can be restricted to very narrow regions and shifted across locations separated by only a few minutes of arc at the center of gaze. That is, contrary to the widespread assumption [1] [2] [3] [4] [8] [15] [33], spatial attention is not uniformly distributed within the region of highest acuity, but its allocation varies according to the demands of the task and the characteristics of the observed stimulus. These findings reveal that the intuitive view of covert attention as a process that operates only far from the fovea is misleading. Covert attention acts as a selection mechanism that modulates information throughout the visual field, and can be controlled with surprising precision at the very center of gaze.
An important direct consequence of our findings is that covert attentional mechanisms contribute to fine pattern vision. Covert shifts of attention are not only useful for enhancing low-resolution vision at peripheral locations, but also for improving high-acuity performance at selected locations where the observer already looks. It has been argued that an important function of covert attention is to attenuate gaps in visual function between the fovea and the periphery [1] [4]. Lack of homogeneity at both the anatomical [10] and functional levels [20], has also been reported in the foveola. Thus, our results suggest that attentional deployment may serve a similar balancing function within the foveola, by tempering uneven performance at nearby retinal locations [20]. Furthermore, by prioritizing and enhancing selected foveal sub-regions, similar to what occurs extrafoveally [34], covert attention may also help alleviate the processing challenges posed by crowded visual stimuli [35], which are typical of natural scenes. In sum, together with previous findings on the contributions of very small eye movements to high-acuity tasks [36] [37], our results indicate that fine spatial vision cannot be regarded as a purely sensory accomplishment, but as the outcome of a complex visuomotor interaction in which precise control of covert attention plays a critical role.
Our results bear multiple implications for the neuronal processes responsible for controlling attention. These processes must (a) include dedicated high-resolution representations (saliency maps) of the central region of the visual field; (b) include mechanisms for continually updating these representations during fixational instability; and (c) possess much higher spatial specificity than that generally attributed to the neural mechanisms held responsible for the coarse control of attention at farther eccentricities. One possibility is that the commonly postulated attentional mechanisms, like a convergence of neuronal receptive fields toward the attended location [4] [38], changes in the synchronization [39] and/or the general correlated structure [40] [41] of neural responses, may reach the required resolution thanks to cortical magnification, which expands the dedicated cortical area with decreasing eccentricity [26] [42]. However, given that, during fixation, the eyes move by amounts that are relevant at this scale [43], spatial registration of the attended foveal locations is necessary even in the intervals between saccades. The need for such process is emphasized by the data in Supplementary Fig. 3, showing that observers retain attentional specificity even though the eye movements covered distances comparable to the separation between attended and unattended locations (Supplementary Fig. 4B). This capability calls for mechanisms of spatial updating qualitatively similar to those studied during saccades in the visual periphery [7] [11] [44], but with much higher resolution as it needs to account for changes in retinal positions caused by fixational eye movements.
During visual exploration, shifts of attention are known to precede the execution of saccades and enhance vision at the saccade target locations [5] [6] [7]. Attentional modulations have also been observed in the presence of microsaccades [45], small saccades that keep the stimulus within the foveola. These modulations, however, have been measured far outside the fovea during maintained fixation. It has been recently observed that microsaccades precisely center the line of sight on task-relevant features when fixation is not enforced [25]. We therefore hypothesize that microscopic shifts in attention similar to those reported here could also precede overt responses carried out by microsaccades, which then would center the stimulus of interest on the preferred foveal locus to further enhance fine pattern vision [20]. Critically, although microsaccades have been claimed to mediate attention effects [46], our results show that covert attention shifts are not necessarily tied to microsaccades; attentional tradeoffs—benefits at the attended location and costs at the unattended locations— in the foveola and the parafovea are present even in the absence of these oculomotor events.
To conclude, our finding that shifts in covert attention occur even within the high-acuity portion of the visual field at the center of gaze, call for a generalization of the very concept of covert attention. Covert attention is not a process that facilitates perceptual computations away from where the observer looks, but it is the manifestation of a more general mechanism evolved for selectively improving and accelerating processing throughout the visual field, including the region of highest acuity.
Online Methods
Observers
A total of thirteen emmetropic human observers, all naïve about the purpose of the study, participated in the experiments (age range 20–29). Five observers (2 males and 3 females) took part in Experiments 1 and 2 (Figs. 1–2), four of these subjects took part in the experiment of Supplementary Figure 2, five (2 males and 3 females) in Experiment 3 (Fig. 3), and five observers (2 males and 3 females) in the control experiment of Supplementary Figure 4. One observer participated in all experiments. Only emmetropic observers were enrolled in this study to ensure optimal stabilization of the stimulus on the retina. Informed consent was obtained from all participants following procedures approved by the Boston University Charles River Campus Institutional Review Board.
Stimuli and gaze-contingent control apparatus
Stimuli were displayed on a fast-phosphor CRT monitor (Iyama HM204DT) at a vertical refresh rate of 150 Hz and spatial resolution of 768×1024 pixels. Observers performed the task monocularly with their right eye while the left eye was patched. A dental-imprint bite bar and a head-rest prevented head movements. The movements of the right eye were measured by means of a Generation 6 Dual Purkinje Image (DPI) eyetracker (Fourward Technologies), a system with an internal noise of ~20″ and a spatial resolution of 1′ [47]. Vertical and horizontal eye positions were sampled at 1 kHz and recorded for subsequent analysis.
Stimuli were rendered by means of EyeRIS [48], a custom-developed system based on dedicated hardware, which allows flexible gaze-contingent display control. This system acquires eye movement signals from the eyetracker, processes them in real time, and updates the stimulus on the display according to the desired combination of estimated oculomotor variables. Precise foveal stimulation was achieved by means of retinal stabilization. The stimulus moved in real time, under EyeRIS control, to compensate for the observer’s eye movements, ensuring that both the cue and target remained at fixed foveal eccentricities with respect to the estimated center of gaze. The delay of the system in these experiments was 10 ms (the time required to render 1.5 frames on the display), which resulted in a stabilization error of approximately 1′, as measured a posteriori by comparing the recorded oculomotor traces to the coordinates of the stabilized stimulus saved by EyeRIS during the experiments. All trials in which the delay exceeded 14 ms (two frames) were discarded (less than 10 trials per observer).
Procedure and experimental tasks
Data were collected by means of multiple experimental sessions at various times of the day. Each session lasted approximately one hour, and each subject completed on average 12 sessions. Every session started with preliminary setup operations that lasted a few minutes, which involved comfortably positioning the observer in the apparatus, tuning the eyetracker for optimal performance, and executing a two-step gaze-contingent calibration procedure to map the eyetracker’s output into visual angles. This procedure improves localization of the preferred retinal locus of fixation by approximately one order of magnitude over standard methods [49]. In the first phase (automatic calibration), observers sequentially fixated on each of the nine points of a 3×3 grid, as it is standard in oculomotor experiments. These points were located 1.32° apart from each other on both the horizontal and vertical axes. In the second phase (manual calibration), observers confirmed or refined the voltage-to-pixel mapping given by the automatic calibration by fixating again on each of the nine points of the grid while the location of the line of sight estimated on the basis of the automatic calibration was displayed in real time on the screen. Observers used a joypad to fine-tune the predicted gaze location, if necessary. The manual calibration procedure was repeated for the central position before each trial to compensate for possible drifts in the electronics as well as microscopic head movements that may occur even on a bite-bar. Note that the center of gaze estimated in this way corresponds to the preferred retinal locus of fixation and does not necessarily coincide with the foveal locus of highest cone density [50] [51].
Detection experiments
Data in Experiments 1 and 2 (Figs. 1 and 2) were collected using a standard spatial cueing detection task. The target (a red square) appeared at one of two possible locations, either to the left or to the right of the point of fixation and, in all trials, was preceded by a cue (a horizontal bar at the center of the display), which pointed toward the target (valid trials) or in the opposite direction (invalid trials), or toward both directions (neutral trials). Both the cue and the target were displayed for 100 ms, with an inter-stimulus interval randomly alternating between 400 and 700 ms. Observers were instructed to press one of two keys on a joypad as soon as the target was detected. Four types of trials were presented: valid trials with congruent cue direction and target location (47% of all trials and 76% of the directional cue trials); invalid trials with incongruent cue direction and target location (15% of all trials); neutral trials in which the cue pointed in both directions (19%); and catch trials in which a directional or neutral cue was presented without a following target (19%). In the catch trials, observers were instructed not to press any button. In Experiment 1 (Fig. 1), the target (10′×10′ in size) was presented in the parafovea, 3° away from the center of gaze. In Experiment 2 (Fig. 2), the target (5′×5′) appeared at only 10′ eccentricity, well within the foveola.
Discrimination experiment
In Experiment 3 (Fig. 3), observers reported whether a small bar (7′×2′) was tilted vertically or horizontally, by pressing one of two keys on the joypad. The bar could appear in one of four boxes (7′×7′) surrounding a central fixation point, each at 14′ distance from the fixation point. A central cue preceded the target by 600 ms. Three types of trials were presented; valid (congruent cue direction and target location; 53% of all trials and 76% of directional trials), invalid (incongruent cue direction and target location; 17% of all trials), neutral (a cue pointed in all four directions; 30% of all trials).
In all three experiments, observers were instructed to maintain their gaze at the center of the display throughout the course of the trial, and presentation of different trials types was randomized. Although visual fading can occur under prolonged exposure to retinally stabilized stimuli, the brief duration and high contrast of visual stimulation in Experiments 2 and 3 did not allow enough time for visual fading to occur within the fovea. Stimuli were maintained on the display for less than 2 s, and both the cue and the target were transiently presented for only 100 ms. In all experiments, reaction times were measured relative to the target’s offset.
In the experiment of Supplementary Figure 2, the stimuli were the same as in the detection experiments (Experiments 1 and 2), but observers reported the target by making a saccade rather than pressing a button. Observers were instructed to look at the target as soon as it appeared. As in the manual detection experiments, the target was presented either in the parafovea or in the foveola and preceded by a neutral cue. In the latter condition, the fixation marker and the boxes indicating the possible target location were retinally stabilized as in Experiment 2. However, stabilization was turned off at the time of target appearance to allow the saccade to normally shift gaze.
In Experiment 4 (Fig. 4), procedures were identical to those of Experiment 3 (Fig. 3), but stimuli were not stabilized on the retina. They remained immobile at their fixed locations at the center of the display and moved on the retina because of fixational eye movements.
Detailed information about the experimental design can be found in the Life Sciences Reporting Summary.
Data analysis
Performance was evaluated over trials with good quality of retinal stabilization. To ensure that stimuli remained at fixed locations on the retina, we discarded all trials with sub-optimal eye-tracking and/or in which the eye moved too fast for the stabilization apparatus. These included: trials with blinks and/or saccades at any point during the trial, trials with microsaccades at any time later than 150 ms before cue presentation (altogether 25% of the total trials); and trials in which the ocular span—defined as the radius of the smallest circle encompassing the eye trajectory—exceeded 0.5° during the period between cue and target onset (7% of the total trials). We also discarded trials in which the observers were not engaged in the task, as revealed by their anticipation or delays in responding (<100 ms and >1000 ms in detection; <200 ms and >2000 ms in discrimination; less than 10 trials excluded per observer, ≈ 1% of the total trials). The same criteria were also applied in Experiment 1 to enable rigorous comparison between data from the two experiments (Figs. 1 and 2). Supplementary Figure 1 shows that eye movements did not differ in these two experiments.
Approximately the same amounts of trials were discarded across subjects in different experiments. However, because of well-known individual variability in fixational eye movements, the proportion of selected valid, invalid, and neutral trials varied slightly across observers. Results did not change when weighted averages, based on the number of trials available per participant, were used and/or when less conservative selection criteria were applied, e.g. when trials with microsaccades were included in the analyses. On average, performance was evaluated over 194 trials per trial type per observer.
In the control experiment of Supplementary Figure 3, in which stimuli were not stabilized on the retina, trials with microsaccades were also included in the analysis. Again, results did not change when we eliminated these trials, as shown in Supplementary Figure 3 by the data of two subjects, who were run extensively to collect large pools of drift-only trials. Results also did not change when we selected trials in which the line of sight remained far from the target or close to the fixation marker at the center of the display.
In all reported experiments, all individual observers exhibited significant differences between valid and invalid trials using non-parametric tests that only assumed independence (Wilcoxon rank sum tests). Results were not affected by the target location (left/right of fixation) nor, in Experiment 2, by the delay between cue and target (400 or 700 ms). Summary statistics are reported over N=5 observers, a sample size chosen to guarantee with p < 0.05 that the effect generalizes to the majority of the population [52]. All figures show average values for each individual observer and summary statistics across observers.
Statistics
Individual observers’ data were examined using two-tailed Wilcoxon rank sum tests. Averages across observers in different trials types were examined by means of one-way within-subjects ANOVAs followed by Tukey post hoc tests. Comparisons between two conditions across observers were tested using two-tailed paired t-tests. Data collection and analysis were not performed blind to the conditions of the experiments.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
All the computer code used to implement the experiments, collect, and analyze data is available from the corresponding author on reasonable request.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation Grants BCS-1534932 (M.P.) and 1420212 (M.R.), and National Institutes of Health Grants R01-EY18363 (M.R.), R01-EY019693 (M.C) and R01-EY016200 (M.C). We thank Mike Landy, Sam Ling, Ernst Niebur, Miriam Spering, Jonathan Victor, Alex White, and Yaffa Yeshurun for helpful comments, and Rania Ezzo for helping with data collection.
Footnotes
Author Contributions: M.P. conceived the study, collected, and analyzed the data. The three authors contributed to the design of the experiments, the interpretation of experimental data, and the writing of the manuscript.
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Carrasco M. Visual attention: The past 25 years. Vision Res. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco M. Covert attention increases contrast sensitivity: Psychophysical, neurophysiological and neuroimaging studies. Prog Brain Res. 2006;154:33–70. doi: 10.1016/S0079-6123(06)54003-8. [DOI] [PubMed] [Google Scholar]
- 4.Anton-Erxleben K, Carrasco M. Attentional enhancement of spatial resolution: Linking behavioural and neurophysiological evidence. Nat Rev Neurosci. 2013;14:188–200. doi: 10.1038/nrn3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- 6.Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44:1453–1467. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Li HH, Barbot A, Carrasco M. Saccade Preparation Reshapes Sensory Tuning. Curr Biol. 2016;26:1564–1570. doi: 10.1016/j.cub.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egeth HE, Yantis S. Visual attention: Control, representation, and time course. Annu Rev Psychol. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- 9.Krauzlis RJ, Lovejoy LP, Zenon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 2013;36:165–182. doi: 10.1146/annurev-neuro-062012-170249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 11.Cavanagh P, Hunt AR, Afraz A, Rolfs M. Visual stability based on remapping of attention pointers. Trends Cogn Sci. 2010;14:147–153. doi: 10.1016/j.tics.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poletti M, Rucci M. A Compact Field Guide to the Study of Microsaccades: Challenges and Functions. Vision Res. 2016;83–97(118) doi: 10.1016/j.visres.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowler E. Eye movements: The past 25 years. Vision Res. 2011;51:1457–1483. doi: 10.1016/j.visres.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rucci M, Poletti M. Control and function of fixational eye movements. Annu Rev Vis Sci. 2015;1:499–518. doi: 10.1146/annurev-vision-082114-035742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksen CW, Hoffman JE. Temporal and spatial characteristics of selective encoding from visual displays. Percept Psychophys. 1972;12(2-B):201–204. [Google Scholar]
- 16.Eriksen C, James JS. Visual attention within and around the field of focal attention: A zoom lens model. Percept Psychophys. 1986;40:225–240. doi: 10.3758/bf03211502. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Res. 1989;29:1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- 18.Intriligator J, Cavanagh P. The spatial resolution of visual attention. Cogn Psychol. 2001;43:171–216. doi: 10.1006/cogp.2001.0755. [DOI] [PubMed] [Google Scholar]
- 19.Barbot A, Carrasco M. Attention modifies spatial resolution according to task demands. Psychological Science. 2017;28:285–296. doi: 10.1177/0956797616679634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poletti M, Listorti C, Rucci M. Microscopic eye movements compensate for nonhomogeneous vision within the fovea. Curr Biol. 2013;23:1691–1695. doi: 10.1016/j.cub.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santini F, Redner G, Iovin R, Rucci M. EyeRIS: A general-purpose system for eye movement contingent display control. Behav Res Methods. 2007;39:350–364. doi: 10.3758/bf03193003. [DOI] [PubMed] [Google Scholar]
- 22.Holmqvist K, Nystrom M, Andersson R, Dewhurst R, Jarodzka H, Van de Weijer J. Eye tracking: A comprehensive guide to methods and measures. Oxford University Press; 2011. [Google Scholar]
- 23.Poletti M, Aytekin M, Rucci M. Head-Eye Coordination at a Microscopic Scale. Curr Biol. 2015;25:3253–3259. doi: 10.1016/j.cub.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aytekin M, Victor JD, Rucci M. The visual input to the retina during natural head-free fixation. J Neurosci. 2014;34:12701–12715. doi: 10.1523/JNEUROSCI.0229-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko HK, Poletti M, Rucci M. Microsaccades precisely relocate gaze in a high visual acuity task. Nat Neurosci. 2010;13:1549–1553. doi: 10.1038/nn.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virsu V, Rovamo J. Visual resolution, contrast sensitivity, and the cortical magnification factor. Exp Brain Res. 1979;37:475–494. doi: 10.1007/BF00236818. [DOI] [PubMed] [Google Scholar]
- 27.Carrasco M, McElree B, Denisova K, Giordano AM. Speed of visual processing increases with eccentricity. Nat Neurosci. 2003;6:699–670. doi: 10.1038/nn1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyman D, Steinman RM. Latency characteristics of small saccades. Vision Res. 1973;13:2173–2175. doi: 10.1016/0042-6989(73)90195-8. [DOI] [PubMed] [Google Scholar]
- 29.Sinha R, Hoon M, Baudin J, Okawa H, Wong ROL, Rieke F. Cellular and Circuit Mechanisms Shaping the Perceptual Properties of the Primate Fovea. Cell. 2017;168:413–426. doi: 10.1016/j.cell.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azzopardi P, Jones KE, Cowey A. Uneven mapping of magnocellular and parvocellular projections from the lateral geniculate nucleus to the striate cortex in the macaque monkey. Vision Res. 1999;39:2179–2189. doi: 10.1016/s0042-6989(98)00319-8. [DOI] [PubMed] [Google Scholar]
- 31.Malpeli JG, LD, Baker FH. Laminar and retinotopic organization of the macaque lateral geniculate nucleus: Magnocellular and parvocellular magnification functions. J Comp Neurol. 1996;375:363–377. doi: 10.1002/(SICI)1096-9861(19961118)375:3<363::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Goffart L, Hafed ZM, Krauzlis RJ. Visual fixation as equilibrium: Evidence from superior colliculus inactivation. J Neurosci. 2012;32:10627–10636. doi: 10.1523/JNEUROSCI.0696-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He S, Cavanagh P, Intriligator J. Attentional resolution. Trends Cogn Sci. 1997;1:115–121. doi: 10.1016/S1364-6613(97)89058-4. [DOI] [PubMed] [Google Scholar]
- 34.Yeshurun Y, Rashal E. Precueing attention to the target location diminishes crowding and reduces the critical distance. J Vis. 2010;10:1–12. doi: 10.1167/10.10.16. [DOI] [PubMed] [Google Scholar]
- 35.Levi DM, Klein SA, Aitsebaomo AP. Vernier acuity, crowding and cortical magnification. Vision Res. 1985;25:963–977. doi: 10.1016/0042-6989(85)90207-x. [DOI] [PubMed] [Google Scholar]
- 36.Rucci M, Poletti M. Control and Functions of Fixational Eye Movements. Annu Rev Vis Sci. 2015;1:499–518. doi: 10.1146/annurev-vision-082114-035742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rucci M, Iovin R, Poletti M, Santini F. Miniature eye movements enhance fine spatial detail. Nature. 2007;447:852–855. doi: 10.1038/nature05866. [DOI] [PubMed] [Google Scholar]
- 38.Womelsdorf T, Anton-Erxleben K, Treue S. Receptive field shift and shrinkage in macaque middle temporal area through attentional gain modulation. J Neurosci. 2008;28:8934–8944. doi: 10.1523/JNEUROSCI.4030-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 40.Cohen MR, Maunsell JHR. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azzopardi P, Cowey A. Preferential representation of the fovea in the primary visual cortex. Nature. 1993;361:719–721. doi: 10.1038/361719a0. [DOI] [PubMed] [Google Scholar]
- 43.Cherici C, Kuang X, Poletti M, Rucci M. Precision of sustained fixation in trained and untrained observers. J Vis. 2012;12:1–16. doi: 10.1167/12.6.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lisi M, Cavanagh P, Zorzi M. Spatial constancy of attention across eye movements is mediated by the presence of visual objects. Atten Percept Psychophys. 2015;77:1159–1169. doi: 10.3758/s13414-015-0861-1. [DOI] [PubMed] [Google Scholar]
- 45.Yuval-Greenberg S, Merriam DJ, Heeger EP. Spontaneous microsaccades reflect shifts in covert attention. J Neurosci. 2014;34:13693–13700. doi: 10.1523/JNEUROSCI.0582-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hafed ZM. Alteration of visual perception prior to microsaccades. Neuron. 2013;77:775–786. doi: 10.1016/j.neuron.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Crane HD, Steele C. Generation V Dual Purkinje-Image eyetracker. Appl Opt. 1985;24:527–537. doi: 10.1364/ao.24.000527. [DOI] [PubMed] [Google Scholar]
- 48.Santini F, Redner G, Iovin R, Rucci M. EyeRIS: A general-purpose system for eye movement contingent display control. Behav Res Methods. 2007;39:350–364. doi: 10.3758/bf03193003. [DOI] [PubMed] [Google Scholar]
- 49.Poletti M, Rucci M. A compact field guide to the study of microsaccades: Challenges and functions. Vision Res. 2016;118:83–97. doi: 10.1016/j.visres.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Putnam NM, et al. The locus of fixation and the foveal cone mosaic. J Vis. 2005;7:632–639. doi: 10.1167/5.7.3. [DOI] [PubMed] [Google Scholar]
- 51.Li KY, Tiruveedhula P, Roorda A. Intersubject variability of foveal cone photoreceptor density in relation to eye length. Invest Ophthalmol Vis Sci. 2010;51:6858–6867. doi: 10.1167/iovs.10-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson AJ, Vingrys AJ. Small Samples: Does Size Matter? Invest. Ophthalmol Vis Sci. 2001;42:1411–1413. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.