Abstract
Background
In patients with primary hyperoxaluria (PH), oxalate overproduction can result in recurrent urolithiasis and nephrocalcinosis, which in some cases results in a progressive decline in renal function, oxalate retention, and systemic oxalosis involving bone, retina, arterial media, peripheral nerves, skin, and heart. Oxalosis involving the myocardium or conduction system can potentially lead to heart failure and fatal arrhythmias.
Methods and Results
A retrospective review of our institution’s database was conducted for all patients with a confirmed diagnosis of PH between 1/1948 and 1/2006 (n=103). Electrocardiogram (ECG) and echocardiography were used to identify cardiac abnormalities. Ninety-three patients fulfilled the inclusion criteria, 58% were male. Mean follow-up was 11.9 (median 8.8) years. In 38 patients who received an ECG or echocardiography, 31 were found to have any cardiac abnormalities. Cardiac findings correlated with decline in renal function.
Conclusions
Our data suggests that physicians caring for patients with PH should pay close attention to cardiac status, especially if renal function is impaired.
Keywords: Cardiovascular disease, Chronic kidney failure, Diagnosis, Mineral metabolism
The primary hyperoxalurias (PH) are rare autosomal recessive disorders of oxalate metabolism. PH type 1 (PH 1). is caused by a deficiency of the peroxisomal liver enzyme alanine-glyoxylate aminotransferase (AGT), which results in overproduction of oxalate. Oxalate cannot be metabolized. It is cleared by the kidneys but urine super saturation for calcium oxalate occurs rapidly and results in nephrocalcinosis and recurrent urolithiasis.1, 2 Progressive renal injury caused by calcium oxalate deposition leads to renal failure. Reduced urinary oxalate excretion in turn results in marked increases in plasma oxalate concentrations and the consequence of systemic calcium oxalate deposition (oxalosis). While kidney transplantation corrects the kidney failure, definitive correction of the metabolic defect in PH 1 is liver transplantation. PH type 2 (PH2) results from a deficiency in the cytosolic enzyme glyoxylate reductase/hydroxypyruvate reductase (GRHPR). This defect, similar to PH1, results in an increased glyoxylate pool, which leads to enhanced synthesis of oxalate, hyperoxaluria and recurrent urolithiasis, nephrocalcinosis.1,2 As in PH1, systemic oxalosis follows development of renal failure. Patients with marked hyperoxaluria and similar clinical features, but with normal AGT and GRHPR have been described. The cause of the hyperoxaluria remains to be elucidated. Patients with these findings are referred to as non 1, non 2 PH.3
Oxalosis involving the myocardium or conduction system can potentially lead to heart failure and fatal arrhythmias. The frequency, and severity of cardiac oxalosis in PH are poorly described, with no organized data available beyond scattered literature reports. Current information is limited to case reports and small case series, which are restricted to cardiomyopathy, vulvular disease and conduction abnormalities.4–6 We aim to review our institution’s database to identify cardiac abnormalities by electrocardiogram (ECG) and echocardiography found in PH patients between 1/1948 and 1/2006.
Methods
All medical records and the secure, web-based. International Registry for PH at Mayo Clinic Rochester were scanned for cases with PH between 1948 and January 2006.7 Medical charts included inpatient and outpatient records such as physicians’ clinical notes, discharge summaries, and subspecialty consultation notes; these were reviewed for clinical, demographic, diagnostic, and treatment data. A standardized, data abstraction form was developed with clear definition of clinical, laboratory, diagnostic procedures, treatment modalities, and patient outcomes. Autopsy records from the same period were reviewed. The medical records of all potential PH cases were confirmed from the International Registry for PH. The study was approved by the institutional review boards at Mayo Clinic.
Inclusion Criteria: Algorithm for Selection
All PH patients were included in the study if PH was a confirmed diagnosis by genetic testing or enzymatic analysis.
1. Identification of PH Patients
Patients presenting with recurrent calcium oxalate stone formation, renal failure, hematuria, dysuria, urinary tract infection, renal colic, or other manifestations of urolithiasis and confirmed hyperoxaluria by either enzymatic or genetic analysis confirming PH were included.
2. Degree of Hyperoxaluria or Hyperoxalemia Suggestive of PH
Twenty-four hour urine oxalate excretion rates that exceed twice the upper limit of normal (normal <0.45 mmol · I.73 m−2·24 h−1) and subsequently confirmed by enzymatic or genetic analysis.
3. Genetic Screening
AGT is a pyridoxine 5’-phosphate-dependent enzyme that is liver specific and usually located within the peroxisome. This enzyme allows efficient removal of potentially toxic glyoxylate via conversion to glycine. In the absence of AGT, glyoxylale accumulates and is converted to oxalate and leads to hyperoxaluria type I.8 The AGXT gene is the gene encoding the AGT protein. Glyoxylate reductase/hydroxypyruvate reductase, which is found primarily in the liver, plays a role in preventing the buildup of a potentially harmful glyoxylate by converting it to glycolatc. This enzyme is encoded by the GRHPR gene. Hyperoxaluria type II is associated with mutations in this gene.9 Most common mutations in the AGXT gene are c.33_34insC, c.508G>A and c.731T>C while GRHPR gene is c.103delG.10
DNA of common mutations of the gene coding for AGT gene (PH type I) or (GRHPR gene PH type II) are screened. However, if a patient lacks homozygosity or compound heterozygosity for the mutations screened, the diagnosis must be confirmed with liver biopsy.
4. Liver Biopsy for Enzyme Analysis as Definitive Testing
Measurement of enzyme activity in a biopsy sample of liver is definitive for the diagnosis. In unclassified PH (non-type I, non-type II): patients with clinical features of PH, but with normal AGT and GRHPR, the diagnosis is one of exclusion.
We followed the algorithm of the International Registry for Primary Hyperoxaluria.7
- Presence of definite enzyme or genetic confirmation.
-
–Liver biopsy confirmation of deficient AGT activity (PH1), GRHPR activity (PH2).
-
–Congenital homozygous or heterozygous for a known mutation in the causative genes.
-
–
- In the absence of definitive enzyme or genetic data
-
-–Urinary oxalate excretion >0.8 mmol ·1.73 m−2·24 h−1 in the absence of known causes such as enteric hyperoxaluria.
-
–Family history of PH in a sibling will be supportive.
-
–A history or current finding of urolithiasis and/or nephrocalcinosis will be supportive.
-
–Increase glycolate excretion will be suggestive of PH1, increase L-glycerate excretion will be suggestive of PH2 (not be required for diagnosis).
-
-–
Exclusion Criteria
Patients who had clinical and/or laboratory evidence of PH but lacked precise criteria on genetic or enzymatic testing.
Identification of Cardiac Involvement
ECG abnormalities were identified as any abnormality defined by the Minnesota Code Manual for Electrocardiographic Findings.11 Echocardiographic criteria based on the American Society of Echocardiography guidelines for normal patients were used to identify abnormalities.12
Statistical Analysis
Frequencies or percentages were used to describe categorical variables. Continuous variables were described as mean ± standard deviation or median. Ninety-five percent confidence intervals based on the binomial distribution were computed for all variables, P<0.05 were considered statistically significant. Log-rank test was used for statistical analysis and P<0.05 was considered significant. All analyses were performed using JMP statistical software, version 5.2 (SAS Institute Inc, Cary, NC, USA).
Results
Ninety three of 103 patients fulfilled the inclusion criteria, 8 patients (8.6%) with genetic analysis, 46 patients (49.5%) with liver biopsy and 39 (40.9%) patients with urinary oxalate level. Thirty-three patients had ECG and 26 had echocardiography data available for analysis.
Baseline Characteristics
Baseline characteristics are summarized in Table 1. Among 93 patients, mean age at diagnosis was 16.5 years (0–74 years). A total of 86 patients (92.5%) were aged ≤40 years and 59 patients (63.4%) were aged ≤20 years at the time of PH diagnosis. The mean duration of follow-up was I 1.9 years (median 8.8). There were 54 (58.1%) male and 39 (41.9%) female participants. Eighty-five percent of the patients were Caucasian. PH type I had the highest prevalence, 78 patients (83.0%), while type II and unknown type were 8 (8.6%) and 7 (7.5%) respectively. Hypertension history was present in 22.6%. Mean blood pressure was in the normal range. 87 mmHg (range 48–126 mmHg). Average plasma oxalate level was (43.4 umol/L). Plasma oxalate in patients with dialysis was higher (86.5 umol/L) than in patients not on dialysis (6.8 umol/L). Renal function was reduced in the entire cohort when averaged, with serum creatinine (Cr), 3.39 mg/dl and glomerular filtration rate (GFR). 72.3 ml/min. However, in PH patients not on dialysis, renal function was nearly normal with GFR, 99.9 ml/min serum and Cr, 0.89 mg/dl; while renal function was markedly reduced in PH patients on dialysis GFR, 37.7 ml/min and Cr 6.79. Among PH patients pre-kidney transplantation, the GFR was lowest at 23.0 ml/min.
Table 1.
Baseline Characteristics
| Total population (n=93) | N/Mean (%) |
|---|---|
| Gender | |
| Male | 54 (58.1%) |
| Female | 39 (41.9%) |
| Age* (years) | 16.5 |
| Race | |
| Caucasian | 79 (85.0%) |
| Non Caucasian | 14 (15.1%) |
| PH type | |
| I | 78 (83.0%) |
| II | 8 (8.6%) |
| Unknown | 7 (7.5%) |
| Date of PH Dx | |
| 1960 s | 8 (9.0%) |
| 1970 s | 17 (18.0%) |
| 1980 s | 16 (17.0%) |
| 1990 s | 32 (34.0%) |
| 2000 s | 20 (22.0%) |
| BP* | |
| History of hypertension | 21 (22.6%) |
| SBP (mmHg) | 119 |
| DBP (mmHg) | 71 |
| Plasma oxalate* (umol/L) | 43.4 |
| Plasma oxalate in patients with dialysis | 86.5 |
| Plasma oxalate in patients without dialysis | 6.8 |
| Serum creatinine* (mg/dl) | 3.39 |
| At time of diagnosis with out dialysis (n=46) | 0.89 |
| At time of diagnosis with dialysis (n=39) | 6.79 |
| GFR* (ml/min) | 72.3 |
| At time of diagnosis with out dialysis (n=51) | 99.9 |
| At time of diagnosis with dialysis (n=39) | 37.7 |
| Pre-kidney transplantation (n=20) | 23.0 |
PH, primary hyperoxalurias; BP, blood pressure; SBP, systolic BP; DBP, diastolic BP; GFR, glomerular filtration rate.
At time of PH diagnosis.
Characteristics of PH patients who had an ECG and an echocardiogram performed are shown in Table 2. Approximately 70% had both investigations performed as part of a symptom driven pre-transplant evaluation. Symptoms at the time of renal transplant evaluation was dyspnea, chest pain, palpitations and syncope with 10.5%, 7.9%, 7.9% and 2.6% respectively. A family history of hyperoxaluria was found in 2 patients (5.3%) in this group. Cardiomegaly, pulmonary venous hypertension and pleural effusion were found by chest X-ray in 18.4%., 5.3% and 2.6% respectively. Calcified coronary arteries were found in 3 patients (7.9%) with 1 patient diagnosed with high grade stenosis of the right coronary artery at coronary angiography. Combined liver and kidney, renal and liver transplantation were done in 44.7%, 26.3% and 2.6% respectively.
Table 2.
Characteristics of Hyperoxaluria Patients Performing ECG or Echocardiogram
| Total population (n=38) | N/Mean (%) |
|---|---|
| Symptoms | |
| Dyspnea | 4 (10.5%) |
| Chestpain | 3 (7.9%) |
| Palpitation | 3 (7.9%) |
| Syncope | 1 (2.6%) |
| Family history of hyperoxaluria | 2 (5.3%) |
| Chest X-ray abnormalites | |
| Cardiomegaly | 7 (18.4%) |
| Pulmonary venous hypertension | 2 (5.3%) |
| Pleural effusion | 1 (2.6%) |
| Cardiac CT abnormality | |
| Calcified coronary artery | 3 (7.9%) |
| Transplantation | |
| Combined liver and kidney | 17(44.7%) |
| Renal | 10 (26.3%) |
| Liver | 1 (2.6%) |
ECG, electrocardiogram; CT, computed tomography.
PH and Cardiac Abnormalities
The mean age at the time of cardiac manifestation was 40 years. Table 3 lists the ECG and echocardiographic abnormalities. Thirty-eight patients had either an ECG or echocardiography or both tests performed. Left ventricular hypertrophy (LVH). bundle branch block and atrioventricular block pattern were found in 5.3%, 7.9% and 5.3% respectively or 6.1%, 9.1% and 6.1% of all available ECG respectively. Increased left ventricular mass index (LVMI), left atrium enlargement, pulmonary hypertension and diastolic dysfunction were the main cardiac abnormalites occurring with frequencies of 28.9%, 21.1%, 15.8% and 10.5% or 42.3%, 30.8%, 23.1% and 15.4% of available echocardiographic studies. Other cardiac abnormalities were poor ejection fraction (EF), infiltrative process and valve pathology and impaired right ventricular (RV) function found in 11.5%, 7.7%, 7.7% and 7.7% respectively. Two patients (7.7%) had significant valve abnormalities, one had moderate tricuspid regurgitation and other moderate pulmonary regurgitation. Diastolic dysfunction was noted in 4 patients (15.4%). Three patients had grade II diastolic dysfunction and 1 had grade III diastolic dysfunction. Mean Cr in patients with cardiac abnormalities was 7.16 (0.9–33.2) mg/dl, while patients with no cardiac abnormalities had a mean Cr of 1.93 (0.3–21.6) mg/dl. Of the patients with cardiac findings, 77.8% had a history of end-stage renal failure requiring dialysis or kidney transplantation, while in the total cohort 28% had received a kidney transplant, 23% a combined liver kidney transplant, and 44% patients had required dialysis. Plasma oxalate was 79.4µmol/L in those with cardiac findings, compared to 7.3µmol/L in those without.
Table 3.
Primary Hyperoxalurias and Cardiac Abnormalities
| Cardiac abnormalities | No | % in each Lab |
% of total patients with ECG and/or echocardiography (N=38) |
|---|---|---|---|
| ECG (N=33) | |||
| LVH | 2 | 6.1 | 5.3 |
| BBB | 3 | 9.1 | 7.9 |
| AV block | 2 | 6.1 | 5.3 |
| Echocardiography (N=26) | |||
| Valve pathology | 2 | 7.7 | 5.3 |
| Impair EF (<50%) | 3 | 11.5 | 7.9 |
| Large LVMI (women >44–88, men >50–102 g/m2) | 11 | 42.3 | 28.9 |
| LA enlargement | 8 | 30.8 | 21.1 |
| Impair RV function | 2 | 7.7 | 5.3 |
| PH (RVSP≤35 mmHg) | 6 | 23.1 | 15.8 |
| Diastolic dysfunction | 4 | 15.4 | 10.5 |
| Infiltrative process | 2 | 7.7 | 5.3 |
Echocardiographic Parameter in Hyperoxaluria Patients (Table 4)
Table 4.
Echocardiographic Parameter in Hyperoxaluria
| Dialysis (N=21) | No-dialysis (N=5) | P value | |
|---|---|---|---|
| LVMI (g/m2) (50–102) | 114.73±43.29 | 89.75±16.62 | 0.0946 |
| IVS (mm) (6–10) | 10.81±2.10 | 11,00±1.41 | 0.8377 |
| LVPSW (mm) (6–10) | 10.31 ±2.57 | 10.50±1.29 | 0.8412 |
| LVEDD (mm) (42–59) | 50.94±6.03 | 48.20±2.59 | 0.1684 |
| LVESD (mm) (29–33) | 32.33±5.79 | 30.40±2.19 | 0.2942 |
| LA (mm) (30–40) | 44.08±8.53 | 36.33±7.64 | 0.2123 |
| EF (%) (≥55%) | 61.70±8.22 | 63.20±4.49 | 0.5922 |
| E (m/s) (0.5–1.0) | 1.00±0.82 | 0.95±0.07 | 0.5071 |
| A (m/s) (0.3—0.7) | 0.57±0.06 | 0.80±0.00 | 0.0198 |
| E/A (1.0–2.0) | 1.73±0.21 | 1.19±0.08 | 0.0108 |
| E/e’ (4.6–11.3) | 11.72±3.92 | 13.00±0.00 | 0.4587 |
| RVSP (mmHg) (<35) | 36.63±13.95 | 32.33±3.79 | 0.3153 |
IVS, interventricular septum thickness; LVPSW, LV posterior wall thickness; LVEDD, LV end-diastolic dimension; LVESD, LV end-systolic dimension; E, peak transmitral flow velocities at early filling phase; A, peak transmitral flow velocities at late filling phases; e’, early diastolic myocardial peak velocity septal annulus. Other abbreviations see in Tables 1–3.
LVMI in PH patients was higher compared to the predicted normal value. LVMI was also higher in hyperoxaluria patients undergoing dialysis, compared to PH patients not on dialysis. The interventricular septum (IVS) and left ventricular posterior septum wall thickness (LVPSW) was increased compared the predicted normal value but was not statically different between dialysis and non-dialysis groups. Both left ventricular (LV) end diastolic and systolic dimension did not change significantly but left atrial size increased in the dialysis group. Mean EF was in the normal range for both groups. Peak A-wave and E-wave velocity did not change significantly but the E/A, E/e’ ratio showed an increasing trend in PH patients compared to normals, suggesting an impairment in LV diastolic function in hyperoxaluria patients. RV systolic pressure was elevated in PH patients on dialysis.
Discussion
Our study shows that distinct cardiac abnormalities were found in PH. This is the first systematic and long-term study describing cardiac abnormalities in PH. Findings are consistent with some case reports showing a relation of PH with cardiac abnormalities. The study also identifies the need for a more organized cardiac assessment in PH patients.
PH is a rare genetic disease with metabolic abnormalities that have protean extra-renal manifestations and a high prevalence of cardiac involvement.13, 14 The majority of patients have PH type I (83%) in our study. Published reports supports this high prevalence which occurs in 0.1 1 to 0.26 per 100,000 births.15 In our cohort, age at diagnosis is 16.5 years and two-thirds are ≤20 years old. Latta et al found that PH type 1 presents for the first time before age 1 in 15% and before age 5 in 50%.16 The infantile form is characterized by chronic renal failure with massive parenchymal oxalosis but do not develop renal calculi.17 In our study group, there was a higher prevalence of patients that required dialysis or renal transplantation. It should be mentioned that renal dysfunction is considered to be a poor prognostic factor in patients with chronic heart failure in both preserved and reduced LVEF.18,19
Older PH patients are likely to present with symptoms of urolithiasis and in some cases with resultant acute renal failure.20 Although PH type II is rare and presents with less severe symptoms, the age of symptom onset and initial serum Cr and oxalate concentrations are similar to that found in PH type I.21 Cardiac symptoms most frequently present in our cohort are dyspnea, chest pain palpitations and syncope (Table 2). A total of 70% of the patients had either an ECG and/or echocardiogram performed because of symptoms in the pre-transplanatation evaluation. In the absence of cardiac related symptoms, no investigations were performed.
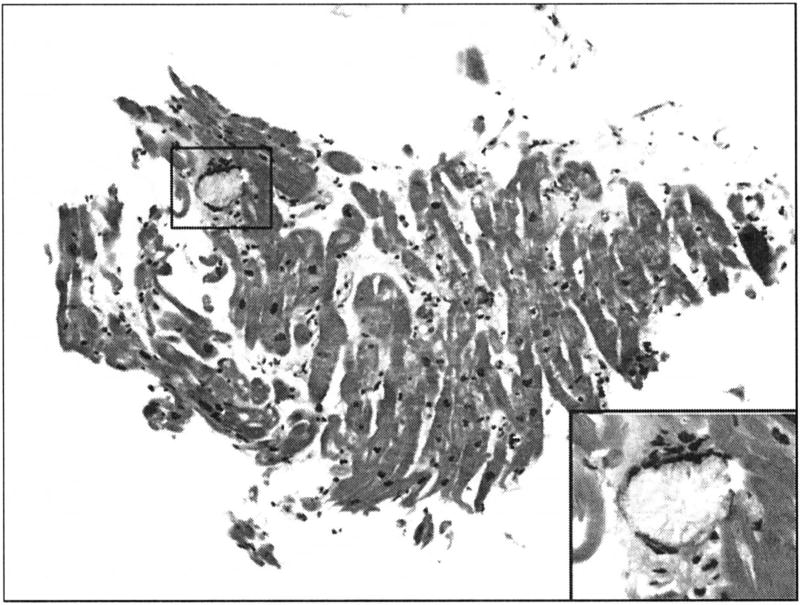
The critical saturation point for calcium oxalate in plasma is 30–45/µmol/L, which is easily exceeded as a result of compromised renal function and subsequent extra-renal oxalate deposition.10,22,17 Large LVMI, impaired LV and RV function, diastolic dysfunction, left atrium enlargement, increased wall thickness suggestive of myocardial infiltration and rhythm abnormalities are most likely due to calcium oxalate deposits in cardiac tissue. Cardiac abnormalities are best determined at autopsy or RV biopsy, but this procedure is undertaken infrequently because of the inherent risks associated with the procedure. Hormonal and metabolic derangement associated with end-stage renal disease might result in pulmonary vascular abnormalities and result in pulmonary hypertension in PH; however, direct oxalate deposition within the pulmonary vasculature might also be a factor. Direct deposition in cardiac muscle might lead to cardiomyopathy.13,14,23 Case reports and limited case series describe some cardiac abnormalities such as increased wall thickness due to myocardial oxalate deposition.5,6 restrictive cardiomyopathy,24 heart failure,6 tricuspid and mitral regurgitation.4,6 conduction defeels,4,25–29 ventricular tachycardia,30 impaired LV and RV function.4 In our study hyperoxaluria patients have higher LVMI, IVS and LVPSW than normal, this might be due to myocardial oxalate deposition. RV biopsy from one of the PH patients confirm calcium oxalate deposition in cardiac muscle (Figure). PH also tend to have diastolic dysfunction as measured by echocardiography. We should keep in mind that other factors such as renal failure and hypertension that coexist in PH patients could also affect cardiac thickness and diastolic dysfunction. As noted in the LV mass and diastolic assessments, these measures appeared to be a little worse in PH patients on dialysis than those not on dialysis, suggesting at least that more advanced PH disease might have this effect. Recent guidelines on myocardial disease from both American Heart Association and European Society of Cardiology do not include calcium oxalate as a cause of cardiomyopathy.31,32 Sakurabayashi et al report that supplementation with L-carnitine might induce regression of LVH in patients with LVH on hemodialysis, even with normal systolic function.33 While cardiac manifestations can be fatal, they are reversed following successful liver transplantation.34–37 The true epidemiology of cardiac involvement is unknown and case reports are not ideal sources for best evidence. This is the first paper that systematically investigates cardiac abnormalities in a large population of PH from a single academic center over a 6 decade period. The recently developed International Registry for Primary Hyperoxaluria provides an opportunity for a more systematic understanding of disease manifestations, including the extra-renal manifestations of PH.7
Figure.
Right ventricular endomyocardial biopsy from cardiac oxalosis with hematoxylin and eosin (H&E) stain patient showing a giant cell containing crystalline material consistent with calcium oxylate. The inset shows the giant cell and crystals on higher power.
Cardiac findings are associated with a decline in renal function and appear to correlate with the plasma oxalate level in our study. We have very few patients that had echocardiography and ECG post-operatively, because these tests were symptom driven pre-operatively and post-operatively at follow-up. We do not have ECG or echocardiographic data to compare at the time of diagnosis of PH and the onset of cardiac manifestation. This would be with in the scope of a prospective dataset. Concerning oxalate levels and cardiac abnormalities, this might be due to a cutoff level beyond which cardiac involvement occurred. The only time point in which there was sufficient plasma oxalate data to analyze from a statistical standpoint was at the time of diagnosis. It is possible that if more time points were available a relationship would be identified.
The next step would be a prospective study at PH patients in a more systematic manner for cardiac abnormalities using novel echocardiographic techniques that might have greater sensitivity and specificity in diagnosing cardiac abnormalities in PH patients, eg, vector velocity imaging and strain of both the LV and the RV.38–41
Imaging modalities such as cardiac computed tomography (CT) or magnetic resonance imaging might add information on cardiac involvement and provide evidence of vascular involvement, without the need for invasive transjugular myocardial biopsy. While myocardial biopsy remains the gold standard, it is not routinely used and does pose a definable risk. In addition to assessing myocardial involvement by either CT or cardiac magnetic resonance, an observation can be made on a dose response of oxalate deposition with higher serum levels of oxalate causing higher levels of cardiac dysfunction and whether liver and kidney transplant result in regression of crystal deposition in these tissues. This concept of non-invasive cardiac imaging would be consistent with other infiltrative cardiomyopathies such as hemochromatosis and cardiac amyloidosis.
Study Limitations
This retrospective study focuses on the cardiac manifestations of PH from a single center in the LISA, however the International Registry provides opportunities for a more systematized and co-ordinated effort in further understanding this disease. A single time point for lab investigation such as serum calcium oxalate precludes a dose-response assessment on the deleterious effects of oxalate crystals in extra-renal tissues. Further studies are needed to demonstrate the time point at diagnosis for PH and when cardiac abnormalities begin to manifest. While the cardiac findings observed could potentially be caused by cardiac calcium oxalate deposition in the myocardium or conduction system, certain changes can also be seen among patients with renal failure of any cause as well as primary cardiac disease. But the relatively young age would suggest that oxalate is likely responsible for these cardiac findings.
Conclusion
Our data suggests that physicians caring for patients with PH should pay close attention to cardiac status, especially if renal function is impaired. This study also suggests that prospective and systematic assessment of patients with PH using non-invasive strategies noted above be deployed routinely to allow better insights and understanding of the cardiac manifestations of PH. Early intervention with definitive therapies such as liver or combined liver kidney transplant might avoid the challenges associated with these surgical interventions if there is co-existent cardiac or pulmonary involvement. In fact, progressive cardiac or pulmonary involvement might serve as an indication for liver transplantation if a reliable non-invasive screening tool is used as a routine by clinicians caring for such patients.
Acknowledgments
We thank Dr Henry D. Tazelaar for the pathology slides. This work was supported in part by the Primary Hyperoxaluria Foundation and the International Primary Hyperoxaluria Registry for providing the database.
Footnotes
Disclosure
The authors declared no competing of interests.
References
- 1.Leumann E, Hoppe B. The primary hyperoxalurias. J Am Soc Nephrol. 2001;12:1986–1993. doi: 10.1681/ASN.V1291986. [DOI] [PubMed] [Google Scholar]
- 2.Milliner DS. The primary hyperoxalurias: An algorithm for diagnosis. Am J Nephrol. 2005;25:154–160. doi: 10.1159/000085407. [DOI] [PubMed] [Google Scholar]
- 3.Monico CG, Persson M, Ford GC, Rumsby G, Milliner DS. Potential mechanisms of marked hyperoxaluria not due to primary hyperoxaluria I or II. Kidney Int. 2002;62:392–400. doi: 10.1046/j.1523-1755.2002.00468.x. [DOI] [PubMed] [Google Scholar]
- 4.Palka P, Duhig F, Carey L, Galbraith A. Primary oxalosis with cardiac involvement: Echocardiographic features of an unusual form of cardiomyopathy. Circulation. 2001;103:E122–E123. doi: 10.1161/hc2401.092123. [DOI] [PubMed] [Google Scholar]
- 5.Yoshioka J, Park YD, Tanaka Y, Kobayashi Y, Miyajima M, Tani A, et al. Echocardiographic features in a patient with primary oxalosis. Echocardiography. 2001;18:599–602. doi: 10.1046/j.1540-8175.2001.00599.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Driessche L, Dhondt A, De Sutter J. Heart failure with mitral valve regurgitation due to primary hyperoxaluria type I. Case report with review of the literature. Acta Cardiol. 2007;62:202–206. doi: 10.2143/AC.62.2.2020243. [DOI] [PubMed] [Google Scholar]
- 7.Lieske JC, Monico CG, Holmes WS, Bergstralh EJ, Slezak JM, Rohlinger AL, et al. International registry for primary hyperoxaluria. Am J Nephrol. 2005;25:290–296. doi: 10.1159/000086360. [DOI] [PubMed] [Google Scholar]
- 8.Williams E, Rumsby G. Selected exonic sequencing of the AGXT gene provides a genetic diagnosis in 50% of patients with primary hyperoxaluria type 1. Clin Chem. 2007;53:1216–1221. doi: 10.1373/clinchem.2006.084434. [DOI] [PubMed] [Google Scholar]
- 9.Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP. The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with primary hyperoxaluria type II. Hum Mol Genet. 1999;8:2063–2069. doi: 10.1093/hmg/8.11.2063. [DOI] [PubMed] [Google Scholar]
- 10.Rumsby G, Williams E, Coulter-Mackie M. Evaluation of mutation screening as a first line test for the diagnosis of the primary hyperoxalurias. Kidney Int. 2004;66:959–963. doi: 10.1111/j.1523-1755.2004.00842.x. [DOI] [PubMed] [Google Scholar]
- 11.Prineas RJ, Crow RC, Blackburn H. The Minnesota Code Manual for Electrocardiographic Findings. Boston: John Wright PSG, Inc; 1982. [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Danpure CJ. Molecular and clinical heterogeneity in primary hyperoxaluria type I. Am J Kidney Dis. 1991;17:366–369. doi: 10.1016/s0272-6386(12)80624-x. [DOI] [PubMed] [Google Scholar]
- 14.Watts RW. Primary hyperoxaluria type I. Qjm. 1994;87:593–600. [PubMed] [Google Scholar]
- 15.Levy M, Feingold J. Estimating prevalence in single-gene kidney diseases progressing to renal failure. Kidney Int. 2000;58:925–943. doi: 10.1046/j.1523-1755.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 16.Latta K, Brodehl J. Primary hyperoxaluria type I. Eur J Pediatr. 1990;149:518–522. doi: 10.1007/BF01957682. [DOI] [PubMed] [Google Scholar]
- 17.Langman CB. The optimal approach to the patient with oxalosis. Adv Ren Replace Ther. 2001;8:214–222. doi: 10.1053/jarr.2001.26354. [DOI] [PubMed] [Google Scholar]
- 18.Miyagishima K, Hiramitsu S, Kimura H, Mori K, Ueda T, Kato S, et al. Long term prognosis of chronic heart failure: Reduced vs preserved left ventricular ejection fraction. Circ J. 2009;73:92–99. doi: 10.1253/circj.cj-07-1016. [DOI] [PubMed] [Google Scholar]
- 19.Shiba N, Matsuki M, Takahashi J, Tada T, Watanabe J, Shimokawa H. Prognostic importance of chronic kidney disease in Japanese patients with chronic heart failure. Circ J. 2008;72:173–178. doi: 10.1253/circj.72.173. [DOI] [PubMed] [Google Scholar]
- 20.Hoppe B, Langman CB. A United Stales survey on diagnosis, treatment, and outcome of primary hyperoxaluria. Pediatr Nephrol. 2003;18:986–991. doi: 10.1007/s00467-003-1234-x. [DOI] [PubMed] [Google Scholar]
- 21.Milliner DS, Wilson DM, Smith LH. Phenotypic expression of primary hyperoxaluria: Comparative features of types I and II. Kidney Int. 2001;59:31–36. doi: 10.1046/j.1523-1755.2001.00462.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoppe B, Kemper MJ, Bokenkamp A, Langman CB. Plasma calcium-oxalate saturation in children with renal insufficiency and in children with primary hyperoxaluria. Kidney Int. 1998;54:921–925. doi: 10.1046/j.1523-1755.1998.00066.x. [DOI] [PubMed] [Google Scholar]
- 23.Abdelwhab S, Elshinnawy S. Pulmonary hypertension in chronic renal failure patients. Am J Nephrol. 2008;28:990–997. doi: 10.1159/000146076. [DOI] [PubMed] [Google Scholar]
- 24.Schulze MR, Wachter R, Schmeisser A, Fischer R, Strasser RH. Restrictive cardiomyopathy in a patient with primary hyperoxaluria type II. Clin Res Cardiol. 2006;95:235–240. doi: 10.1007/s00392-006-0362-2. [DOI] [PubMed] [Google Scholar]
- 25.Tonkin AM, Mond HG, Mathew TH, Sloman JG. Primary oxalosis with myocardial involvement and heart block. Med J Aust. 1976;1:873–874. [PubMed] [Google Scholar]
- 26.West RR, Salyer WR, Hutchins GM. Adult-onset primary oxalosis with complete heart block. Johns Hopkins Med J. 1973;133:195–200. [PubMed] [Google Scholar]
- 27.Coltarl DJ, Hudson RE. Primary oxalosis of the heart: A cause of heart block. Br Heart J. 1971;33:315–319. doi: 10.1136/hrt.33.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massie BM, Bharati S, Scheinman MM, Lev M, Desai J, Rubeson E, et al. Primary oxalosis with pan-conduction cardiac disease: Electrophysiologic and anatomic correlation. Circulation. 1981;64:845–852. doi: 10.1161/01.cir.64.4.845. [DOI] [PubMed] [Google Scholar]
- 29.Hamaya K, Ohishi K. Primary oxalosis with cardiac manifestations. Acta Pathol Jpn. 1980;30:451–458. doi: 10.1111/j.1440-1827.1980.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 30.Quan KJ, Biblo LA. Type 1 primary hyperoxaluria: An unusual presentation of ventricular tachycardia. Cardiol Rev. 2003;11:318–319. doi: 10.1097/01.crd.0000065421.50549.21. [DOI] [PubMed] [Google Scholar]
- 31.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. American Heart Association; Council on Clinical Cardiology. Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 32.Elliott P, Andersson B, Arbustini H, Bilinka Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 33.Sakurabayashi T, Miyazaki S, Yuasa Y, Sakai S, Suzuki M, Takahashi S, et al. L-carnitine supplementation decreases the left ventricular mass in patients undergoing hemodialysis. Circ J. 2008;72:926–931. doi: 10.1253/circj.72.926. [DOI] [PubMed] [Google Scholar]
- 34.Detry O, Honore P, DeRoover A, Trimeche M, Demoulin JC, Beaujean M, et al. Reversal of oxalosis cardiomyopathy after combined liver and kidney transplantation. Transpl Int. 2002;15:50–52. doi: 10.1007/s00147-001-0364-y. [DOI] [PubMed] [Google Scholar]
- 35.Rodby RA, Tyszka TS, Williams JW. Reversal of cardiac dysfunction secondary to type 1 primary hyperoxaluria after combined liver-kidney transplantation. Am J Med. 1991;90:498–504. [PubMed] [Google Scholar]
- 36.Fyfe BS, Israel DH, Quish A, Squire A, Burrows L, Miller C, et al. Reversal of primary hyperoxaluria cardiomyopathy after combined liver and renal transplantation. Am J Cardiol. 1995;75:210–212. doi: 10.1016/s0002-9149(00)80085-5. [DOI] [PubMed] [Google Scholar]
- 37.McDonald JC, Landreneau MD, Rohr MS, DeVault GA., Jr Reversal by liver transplantation of the complications of primary hyperoxaluria as well as the metabolic defect. N Engl J Med. 1989;321:1100–1113. doi: 10.1056/NEJM198910193211607. [DOI] [PubMed] [Google Scholar]
- 38.Amundsen BH, Crosby J, Steen PA, Torp H, Slordahl SA, Stoylen A. Regional myocardial long-axis strain and strain rate measured by different tissue Doppler and speckle tracking echocardiography methods: A comparison with tagged magnetic resonance imaging. Eur J Echocardiogr. 2009;10:229–237. doi: 10.1093/ejechocard/jen201. [DOI] [PubMed] [Google Scholar]
- 39.Korinek J, Sengupta PP, Wang J, Romero-Corral A, Boukatina AE, Vitek J, et al. Doppler strain imaging closely reflects myocardial energetic status in acute progressive ischemia and indicates energetic recovery after reperfusion. J Am Soc Echocardiogr. 2008;21:961–968. doi: 10.1016/j.echo.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sengupta PP, Khandhcria BK, Narula J. Twist and untwist mechanics of the left ventricle. Heart Fail Clin. 2008;4:315–324. doi: 10.1016/j.hfc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Goffinet C, Chenot F, Robert A, Pouleur AC, le Polain de Waroux JB, Vancrayenest D, et al. Assessment of subendocardial vs. subepicardial left ventricular rotation and twist using two-dimensional speckle tracking echocardiography: Comparison with tagged cardiac magnetic resonance. Eur Heart J. 2009;30:608–617. doi: 10.1093/eurheartj/ehn511. [DOI] [PubMed] [Google Scholar]