Abstract
The synthesis of steroid hormones occurs in specific cells and tissues in the body in response to trophic hormones and other signals. In order to synthesize steroids de novo, cholesterol, the precursor of all steroid hormones, must be mobilized from cellular stores to the inner mitochondrial membrane (IMM) to be converted into the first steroid formed, pregnenolone. This delivery of cholesterol to the IMM is the rate-limiting step in this process, and has long been known to require the rapid synthesis of a new protein(s) in response to stimulation. Although several possibilities for this protein have arisen over the past few decades, most of the recent attention to fill this role has centered on the candidacies of the proteins the Translocator Protein (TSPO) and the Steroidogenic Acute Regulatory Protein (StAR). In this review, the process of regulating steroidogenesis is briefly described, the characteristics of the candidate proteins and the data supporting their candidacies summarized, and some recent findings that propose a serious challenge for the role of TSPO in this process are discussed.
Keywords: Steroidogenesis, Mitochondria, Translocator Protein (TSPO), Steroidogenic Acute Regulatory Protein (StAR), Conditional knockout, Global knockout
1. Introduction
Steroidogenesis is the process by which important steroid hormones are synthesized by specific tissues and cells in the body. Examples of these important steroids are the glucocorticoids that are synthesized in the adrenal cortex, the mineralocorticoids that are synthesized in the adrenal glomerulosa, the ovarian and placental progestins and estrogens, the testicular androgens and several neurosteroids such as pregnenolone, progesterone, 5α-dihydroprogesterone, allopregnanolone and DHEA, that are synthesized in the brain. The adrenal glucocorticoids serve to regulate carbohydrate metabolism and manage stress and the mineralocorticoids are involved in salt balance and the maintenance of blood pressure. Ovarian progesterone and estrogen are involved in the maintenance of female secondary sex characteristics and reproductive function while testicular testosterone is involved in maintaining male secondary sex characteristics and is essential for male fertility. Neurosteroid functions include stimulation of GABAergic responses, modulation of the response of Purkinje cells to excitatory amino acids and the enhancement of memory function. Other tissues and cells have also been reported to have the capacity for de novo steroid synthesis, but the localization of where steroids are synthesized and their respective functions are not the main focus of this review. Rather, this review will concentrate on the manner in which the synthesis of steroid hormones are regulated and the history of the efforts that have been made to uncover the components and the mechanisms involved in this regulation. This history dates back approximately six decades when it was first observed that the synthesis of steroid hormones in vitro could be stimulated with trophic hormones and that this synthesis required the production of a new protein(s), as will be described later in this review. This singular observation formed the basis for what has been a long and most interesting search for the putative regulatory protein(s). We will briefly summarize the early studies that were performed in the search for this regulatory protein(s), the necessary characteristics of the candidates required to perform this function and some of the controversies that have arisen along the way, and indeed, remain to the present time. To be sure, this has been an interesting undertaking by a number of investigators in the field and it would seem safe to say that at this juncture in time, the entire story of what factors are involved in the acute regulation of steroid hormone biosynthesis and how they function is not yet completely understood.
2. Characteristics of the regulation of steroid hormone biosynthesis
The initial step in steroidogenesis is the conversion of cholesterol to the first steroid formed, pregnenolone, which occurs in all steroidogenic tissues (Miller, 1988, Pescador et al., 1997). This conversion is a result of the action of the cytochrome P450 side-chain cleavage enzyme (P450scc; CYP11A1), that is part of the cholesterol side chain cleavage system that resides on the matrix side of the inner mitochondrial membrane (Farkash et al., 1986). Pregnenolone then exits the mitochondria and is converted to progesterone and other steroids in the microsomal compartment and, in some cases, downstream steroids re-enter the mitochondrial compartment to be converted to the final product dependent upon the complement of steroidogenic enzymes present within specialized cells in those tissues (Miller, 1998). Understanding the enzymes involved in steroidgenesis and, most importantly, their intracellular location, is key to understanding how the synthesis of steroid hormones is regulated.
The biosynthesis of steroid hormones are mostly regulated by pituitary trophic hormones such as adrenocorticotropin (ACTH), luteinizing hormone (LH) and follicle stimulating hormone (FSH) and this regulation occurs in two phases. The acute phase occurs very rapidly (within minutes) and is responsible for the production of steroids in response to immediate needs (Stocco and Clark, 1996). In addition, chronic regulation (on the order of hours) also occurs and consists of the longer-term expression of the mRNAs and proteins for the steroidogenic pathway enzymes to provide for continuing steroid synthesis following the acute phase (Miller, 1988, Simpson et al., 1992, Simpson and Waterman, 1988, Waterman and Simpson, 1985). This review will focus only on elements involved in the acute phase of steroid hormone biosynthesis. Like most biosynthetic pathways, the steroidogenic pathway has a rate-limiting step and following a number of years of speculation, it was experimentally determined that the regulated and rate-limiting step in steroidogenesis was the delivery of the substrate cholesterol from the outer mitochondrial membrane (OMM) to the inner mitochondrial membrane (IMM) where the P450scc enzyme is located (Black et al., 1994, Farkash et al., 1986). This step was rate-limiting because the hydrophobic cholesterol could not cross the aqueous mitochondrial intermembrane space to the relatively cholesterol poor IMM within the time frame that was observed for the acute synthesis of steroids. There followed a significant period of investigation to determine the nature of the acutely regulated step. Early investigations were performed using adrenal perfusions in vitro and it was observed that ACTH could stimulate the biosynthesis of steroids (Stone and Hechter, 1954) and importantly, that acute steroid production had an absolute requirement for the synthesis of new proteins (Ferguson, 1962, 1963, Garren et al., 1965, Garren et al., 1966, Garren, 1968). Subsequent studies demonstrated that the putative regulator protein in all likelihood functioned at the level of the delivery of cholesterol to the P450scc enzyme in the IMM, the regulated step (Arthur and Boyd, 1974, Davis and Garren, 1968, Ohno et al., 1983, Privalle et al., 1983, Simpson and Boyd, 1966). The overall significance of all these early observations was that the search for the putative regulator now had a specific target on which to focus, namely, a newly synthesized protein.
The discovery and characterization of the putative protein regulator of steroidogenesis was the goal of several laboratories and over the ensuing decades candidate proteins for the acute regulator emerged. These candidates included the Sterol Carrier Protein 2 (SCP2) (Vahouny et al., 1987), the Steroidogenesis-Activator Polypeptide (SAP) (Pedersen and Brownie, 1983, 1987), the Peripheral Benzodiazepine Receptor/Translocator Protein (PBR/TSPO) and the Steroidogenic Acute Regulatory protein (StAR), and all were placed in contention for being the protein responsible for the acute regulation of cholesterol transfer to the IMM and thus, steroidogenesis.
Experimental evidence revealed that each of these proteins appeared to have characteristics that rendered them as viable candidates. However, after the initial studies demonstrating that SCP2 could enhance steroid synthesis in isolated mitochondria, further research on this candidate based on observations in knockout mice ruled out an involvement for SCP2 in steroidogenesis (Seedorf et al., 1998). Also, while first isolated as a small molecular weight peptide (Pedersen and Brownie, 1983, 1987), SAP was later identified as a fragment of the much larger glucose-regulated protein 78 (GRP78) (Li et al., 1989). GRP78 is a regulator of the unfolded protein response (UPR) (Wang et al., 2009), and it was demonstrated that GRP78 knockout mice died at an early embryonic stage (Luo et al., 2006). Later studies examining GRP78 conditional knockouts indicated a phenotype in oncogenic signaling (Wey et al., 2012), and examination of GRP78 structure showed that it contained a nucleotide-binding domain and a peptide substrate-binding domain (Wisniewska et al., 2010), but had no characteristics that indicated the potential for lipid transport. Therefore, a function for GRP78 in the acute regulation of cholesterol transport appeared unlikely. In contrast to SCP2 and SAP, the candidate proteins TSPO and StAR have continued to be studied in great detail.
3. The Steroidogenic Acute Regulatory Protein
A candidate protein for the acute regulator of steroidogenesis was first described by Orme-Johnson and her colleagues as an ACTH-induced 30 kDa phosphoprotein in hormone-treated rat and mouse adrenocortical cells, and as an LH-induced protein in rat corpus luteum cells and mouse Leydig cells (Alberta et al., 1989, Epstein and Orme-Johnson, 1991a,b, Krueger and Orme-Johnson, 1983, Pon et al., 1986a,b, Pon and Orme-Johnson, 1988). These studies demonstrated that a close relationship existed between the appearance of the 30 kDa proteins and steroid hormone biosynthesis and that their synthesis was sensitive to cycloheximide. Our laboratory was engaged in similar studies in hormone-stimulated MA-10 mouse Leydig tumor cells and described a family of proteins which were identical to those described by Orme-Johnson (Stocco and Kilgore, 1988, Stocco and Chaudhary, 1990, Stocco and Chen, 1991, Stocco and Sodeman, 1991, Stocco, 1992, Stocco and Ascoli, 1993, Stocco et al., 1995). These proteins were found localized to the mitochondria and consisted of several forms of a newly synthesized 30 kDa protein. The proteins, as identified by 2-dimensional polyacrylamide gel electrophoresis (2-D PAGE), are shown in Fig. 1. Later studies determined that the 30 kDa mitochondrial proteins were processed from a 37 kDa precursor protein that contained a mitochondrial signaling sequence in its N-terminus (Epstein and Orme-Johnson, 1991a, b, Stocco and Sodeman, 1991). In general, all of the studies that were performed demonstrated tight correlations between the synthesis of steroids and the synthesis of the 30 kDa mitochondrial proteins and thus, they represented good candidates for the regulatory protein (Alberta et al., 1989, Epstein and Orme-Johnson, 1991a, b, Krueger and Orme-Johnson, 1983, Pon et al., 1986a, b, Pon and Orme-Johnson, 1988, Stocco and Kilgore, 1988, Stocco and Chaudhary, 1990, Stocco and Chen, 1991, Stocco and Sodeman, 1991, Stocco, 1992, Stocco and Ascoli, 1993, Stocco et al., 1995). The purification of the 30 kDa protein, the cloning of the cDNA for the 37 kDa protein precursor and its sequencing were successfully accomplished in 1994 (Clark et al., 1994). Both the nucleic acid sequence of the cDNA and amino acid sequence of the 37 kDa protein were found to be unique, indicating it represented a novel protein. Transient transfection experiments demonstrated that expression of the cDNA-derived protein in both MA-10 mouse Leydig tumor cells and COS-1 monkey kidney cells co-transfected with the P450scc enzyme system to render them steroidogenic resulted in a significant increase in steroid production in the absence of hormone stimulation (Fig. 2). As a result of these findings, this protein was named the Steroidogenic Acute Regulatory Protein or StAR (Clark et al., 1994). Also, transient transfection of COS-1 cells (that had been rendered steroidogenic by transfection with the cholesterol side chain system proteins), with the cDNA for the 37 kDa protein resulted in a several fold increase in the conversion of cholesterol to pregnenolone (Lin et al., 1995, Stocco and Clark, 1996, Sugawara et al., 1995). Taken together, these results indicated a direct role for the 37-30 kDa proteins in hormone-regulated steroid production.
Fig. 1.
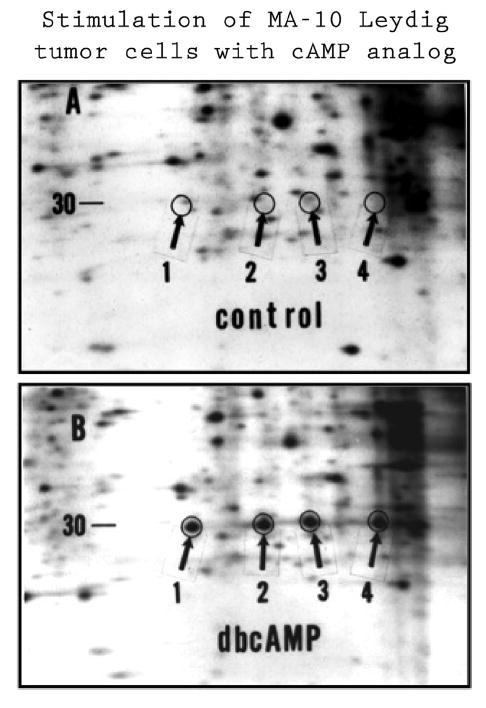
Illustration of Newly Synthesized StAR Proteins. Representative fluorograms of two-dimensional polyacrylamide gel electrophoresis (2-D PAGE) of 35S-methionine labeled mitochondrial proteins isolated from control and stimulated MA-10 mouse Leydig tumor cells. Cells were incubated in the presence of 1 mCi/ml 35S-methionine and either in the presence or absence of 1 mM dibutyryl cAMP (dbcAMP) for 6 h. Mitochondria were isolated and the proteins were prepared for 2-D PAGE. Following electrophoresis the gels were dried and placed in cassettes with X-ray film. After suitable periods of time, the films were developed and the radioactive proteins could be seen. Shown are selected areas of fluorograms from control (A) and stimulated (B) cells. The arrows (1–4), illustrate the positions of the 30 kDa StAR protein forms. Proteins 3 and 4 represent the phosphorylated forms of proteins 1 and 2 respectively. Adapted from Stocco and Clark (1993).
Fig. 2.
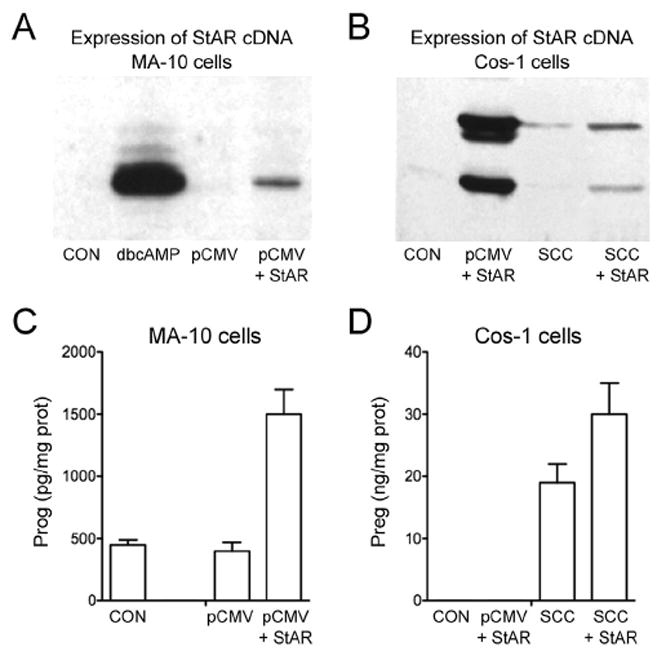
In vitro Expression of StAR Protein and Steroid Production in MA-10 and COS-1 Cells. A, MA-10 cells were stimulated with dbcAMP and subjected to Western blot analysis as were unstimulated MA-10 cells that had been transfected with pCMV vector alone or pCMV containing the 37 kDa cDNA for StAR. C, Progesterone production in control and StAR transfected MA-10 cells were measured by RIA 16 h post transfection. B, COS-1 cells rendered steroidogenic through transfection with the proteins for the cholesterol side chain cleavage enzyme system (SCC) were transfected with control pMCV and pCMV containing 37 kDa StAR cDNA and western blot analysis was performed. D, pregnenolone synthesis was measured in cells transfected with empty vector, StAR alone, SCC proteins alone and cells containing both StAR and the SCC system. It can clearly be seen that in both MA-10 and COS-1 cells, expression of the StAR protein is required for the cells to increase steroid synthesis.
Shortly following the cloning of the StAR cDNA, collaborative studies with Walter Miller and Jerry Strauss demonstrated that mutations in the StAR gene resulted in the disease congenital lipoid adrenal hyperplasia (lipoid CAH) (Lin et al., 1995). Lipoid CAH is a lethal condition characterized by an almost complete inability of the newborn to synthesize steroids. These afflicted patients also have large adrenals containing high levels of cholesterol and cholesterol esters and an increased amount of lipid accumulation in testicular Leydig cells indicating an inability to transfer cholesterol to the P450scc enzyme for conversion to pregnenolone. A recent review provides an up-to-date summary of the mutations in StAR that have been uncovered as of the present time (Miller, 2016). These observations added compelling evidence for the essential role of this protein in the regulation of steroidogenesis since, in essence, with lipoid CAH nature provided a StAR knockout and the phenotype had all of the expected characteristics.
At approximately that same time we also performed collaborative studies with Keith Parker and his colleagues. In one of these studies we were able to demonstrate that during the course of embryonic development in the mouse, StAR mRNA expression was tightly correlated with the appearance of steroidogenic cells and the timing of steroidogenesis in the adrenal gland and the testis (Clark et al., 1995). These studies clearly demonstrated the presence of StAR transcripts in the adrenal cortex but not the adrenal medulla and in the testicular Leydig cells, but not the seminiferous tubules during embryonic development. As expected, there were no StAR transcripts in the ovary during the course of development, as estrogen is not produced in this organ until puberty. In another study in collaboration with Dr. Parker’s laboratory, targeted disruption of the StAR gene in mice was used to successfully produce StAR knockout mice (Caron et al., 1997). All StAR knockout mice had female external genitalia, failed to grow normally and died within a short period of time, presumably as a result of adrenocortical insufficiency. Serum levels of corticosterone and aldosterone were depressed while levels of ACTH and CRH were elevated indicating impaired production of adrenal steroids with an accompanying loss of feedback regulation at the level of the hypothalamus or pituitary. The adrenal glands had a normal medulla but an abnormal cortex, that displayed a disrupted fascicular zone, and staining with oil red-O revealed elevated lipid deposits in the cortex of the StAR knockout mouse. Images depicting grossly elevated lipid deposits in both the adrenal cortex and in the Leydig cells in the interstitial compartment of the testis are shown in Fig. 3. Thus, the StAR knockout mouse demonstrated characteristics very similar to human lipoid CAH and further substantiated the necessity for StAR action in steroid biosynthesis.
Fig. 3.
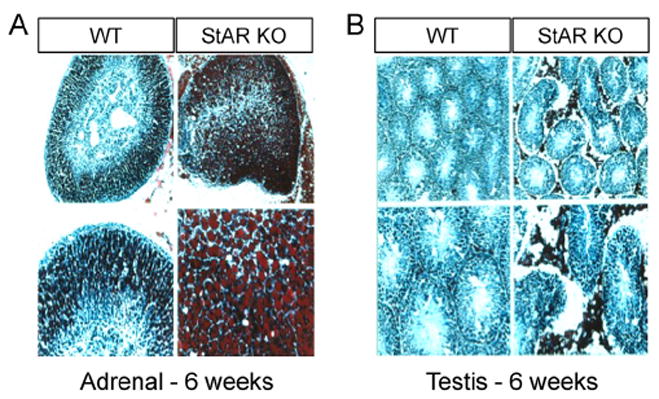
Adrenal Glands and Testes in StAR Knockout Mice. Adrenal glands and testes were isolated from wild type and StAR knockout mice at six weeks after birth. Frozen sections from each tissue were stained with oil red O for the detection of neutral lipids. A, the adrenal glands from StAR null mice contained significantly higher levels of lipid than did wild type animals. In the upper panels of A, the whole gland is shown, and a higher magnification of the gland is shown in the lower panels. B, similar procedures were performed in the testis and in the interstitial areas, the location of the steroidogenic Leydig cells, significantly higher deposits of lipid can be seen at both the lower and higher magnifications.
With the availability of StAR reagents, results obtained with methodologies such a Western analysis, Northern analysis, in situ hybridization, immunocytochemistry, RNAse protection assays and RT PCR have all been employed to show that StAR expression is essentially confined to steroidogenic tissues. Importantly, these studies were conducted in many different laboratories and were able to successfully show that StAR was present in the steroidogenic cells of various steroidogenic tissues. Thus, StAR expression has been demonstrated in the adrenal cortical layers (both glomerulosa and fasciculata-reticularis cells) (Brand et al., 1998, Cherradi et al., 1998, Fleury et al., 1998, Lehoux et al., 1999, Liu et al., 1996, Nicol et al., 1998, Nishikawa et al., 1996, Peters et al., 1998, Pollack et al., 1997), ovarian theca cells (Brand et al., 1998, Cherradi et al., 1998, Fleury et al., 1998, Kiriakidou et al., 1996, Lehoux et al., 1999, Nicol et al., 1998, Peters et al., 1998, Pollack et al., 1997), ovarian granulosa cells (Balasubramanian et al., 1997, Bao et al., 1998, Kerban et al., 1999, Kiriakidou et al., 1996, LaVoie et al., 1999, Pescador et al., 1996, Ronen-Fuhrmann et al., 1998, Sekar et al., 2000, Thompson et al., 1999), ovarian corpora lutea cells (Chen et al., 1999, Chung et al., 1998, Juengel et al., 2000, Kiriakidou et al., 1996, Mamluk et al., 1999, Pescador et al., 1996, 1999, Pollack et al., 1997, Townson et al., 1996), fetal mouse giant trophoblast cells (Arensburg et al., 1999), both fetal and adult testis, ovary and adrenal tissues (Pilon et al., 1997), adrenal tumors (Liu et al., 1996) and testicular Leydig cells (Bosmann et al., 1996, Kanzaki and Morris, 1999, Leers-Sucheta et al., 1999, Lejeune et al., 1998, Lin et al., 1998, Luo et al., 1998, Mauduit et al., 1998, Pollack et al., 1997). Since those early studies and using more sensitive technologies StAR protein has been detected in a wider variety of tissues where its roles are, so far, not completely known, but have been subjected to most interesting discussion that is beyond the scope of this review (Anuka et al., 2013).
Uncovering the role of StAR in the mechanism of cholesterol transport to the IMM has so far proved to be quite elusive even after more than two decades of effort. An early model depicted that import of the StAR protein into the mitochondrial matrix temporarily formed contact sites between the OMM and IMM and might thus allow the hydrophobic cholesterol to transfer between the membranes (Stocco and Clark, 1996). A later model, resulting from solving the crystal structure of a StAR homolog, MLN64, suggested that StAR mediated cholesterol transport by shuttling cholesterol molecules to the IMM (Tsujishita and Hurley, 2000). It was also proposed that StAR might alter the mitochondrial membranes to allow for the passage of cholesterol to the IMM, before it is transported to the mitochondrial matrix (Stocco, 2001). However, it was soon found that these models for StAR action would have to be modified when it was shown that deletion of 62 amino acids at the N-terminus of StAR prevented mitochondrial import, but did not affect cholesterol transfer and steroid production (Arakane et al., 1996, Wang et al., 1998). Further demonstrating that StAR need not enter the mitochondria to be active in cholesterol transfer, an OMM protein, TOM20, when fused to StAR resulted in a complex that could not enter the matrix but could still induce steroidogenesis (Bose et al., 2002). In an effort to explain what might be occurring at the level of the OMM Miller and colleagues performed a series of biophysical studies, which demonstrated that StAR undergoes a conformational alteration when it binds to cholesterol (Baker et al., 2005, Bose et al., 1999, Christensen et al., 2001). This alteration, which is caused by an acid-induced breaking of hydrogen bonds, appears to be required for StAR activity and is a result of StAR’s transition to a molten globule form (Bose et al., 1999, Christensen et al., 2001). This observation was confirmed in another laboratory (Rajapaksha et al., 2013). Recent studies have revealed involvement of the voltage-dependent anion channel 2 (VDAC2) and TOM22 for StAR activity at the OMM membrane (Prasad et al., 2015) (Rajapaksha et al., 2016). In addition to the involvement of VDAC2 in this process, it has also been shown that the OMM proteins voltage-dependent anion channel 1 (VDAC1) and phosphate carrier protein (PCP) are also required for the production of steroids (Bose et al., 2008). The authors demonstrated that phosphorylation of StAR by protein kinase A requires PCP followed by the interaction of phospho-StAR with VDAC1 in order to promote cholesterol transfer and steroidogenesis. In spite of these efforts, there has yet to arise a model that adequately explains the role of StAR in the transfer of cholesterol into the IMM for conversion to pregnenolone.
By using the TOM20-StAR fusion protein, work performed by Papadopoulos and colleagues demonstrated that knockdown of PBR using antisense oligonucleotides resulted in an inhibition of steroid synthesis (Hauet et al., 2005). These findings suggested that that there existed a cooperation between StAR and PBR that mediated mitochondrial cholesterol transport and a model in which StAR carried cholesterol from cellular stores to the OMM where PBR acted as a protein tunnel for the transfer of cholesterol to the IMM was proposed (Papadopoulos and Miller, 2012).
4. The Peripheral Benzodiazepine Receptor (PBR)/Translocator Protein (TSPO)
The Peripheral Benzodiazepine Receptor (PBR), whose name was recently changed to the Translocator Protein (TSPO; hereafter referred to as TSPO in this review) was first described in the late 1970s (Gavish et al., 1999). TSPO was demonstrated to have high binding affinity for benzodiazepines but was a distinctly different receptor from the central benzodiazepine receptor, the γ-aminobutyric acid type A receptor/GABAA receptor. Since that time there have been numerous studies involving TSPO that have attempted to characterize its pharmacological and physiological functions (Gavish et al., 1999, Rupprecht et al., 2010). Shortly after the discovery of TSPO, it was found that it was present in many different tissues, it appeared to be most highly concentrated in steroidogenic cells and that it was localized to the mitochondria in those cells (Mukhin et al., 1989, Papadopoulos et al., 1990). In those studies it was further demonstrated that treatment of adrenocortical and testicular Leydig cells with the TSPO ligands PK11195 and Ro5-4864 could stimulate steroid synthesis.
There followed over the next decade a series of studies in several different steroidogenic cell types that also indicated that TSPO ligands could increase steroid hormone production (Krueger and Papadopoulos, 1990, Papadopoulos et al., 1991a, b, c). These observations were further strengthened when it was reported that an intracellular TSPO binding protein, the diazepam binding inhibitor (DBI) increased steroid synthesis in steroidogenic cells (Garnier et al., 1993, Papadopoulos et al., 1991a, b, c, 1992). Knockdown technology to inhibit TSPO expression indicated that the presence of this protein was an absolute requirement for steroid synthesis (Boujrad et al., 1993, Papadopoulos et al., 1997a, b). The fact that knockdown of TSPO could, by itself, inhibit steroid biosynthesis indicated that its absence could prevent steroidogenesis while in the presence of the StAR protein. This was interpreted as indication that it acted downstream from StAR and functioned as a cholesterol channel, receiving intracellular cholesterol from StAR to be transported to the IMM (Papadopoulos et al., 1990, Papadopoulos and Miller, 2012). It was not possible to confirm the effects of knocking out TSPO in vivo as attempts to produce TSPO knockout animals was reported to result in early embryonic lethality in the mice (Papadopoulos et al., 1997a, b). Another study that seemed to strongly support the role of TSPO in steroidogenesis was that steroid production in the constitutively steroid synthesizing cell line, the R2C rat Leydig tumor cell line, occurred as a result of the presence of a higher affinity TSPO ligand-binding site in R2C cells (Garnier et al., 1994). In later experiments, knockdown of TSPO in these R2C cells was shown to almost completely inhibit their ability to produce steroid hormones (Papadopoulos et al., 1997a, b). It was also reported that TSPO contained a cholesterol-binding amino acid consensus (CRAC) motif that was described by the authors as providing a mechanism for the binding and transport of cholesterol into the mitochondria (Li et al., 2001). The collective consideration of all of these characteristics of TSPO resulted in the authors concluding that TSPO played an indispensable role in cholesterol transfer to the IMM and steroidogenesis. This conclusion has persisted for over two decades with a number of models having been proposed as to how TSPO functioned in cholesterol transport into the mitochondria. Several recent reviews summarize the current state of the role of TSPO in steroidogenesis (Aghazadeh et al., 2015, Midzak et al., 2015, Papadopoulos et al., 2015).
A more recent model that has been proposed for the role of TSPO in cholesterol transport to the IMM involves its participation in an 800 kDa protein complex (Rone et al., 2012). This complex is composed of TSPO, the voltage dependent anion channel (VDAC), P450scc, the ATPase family AAA domain-containing protein 3A (ATAD3A) and optic atrophy type 1 proteins. Knockdown of ATAD3A or VDAC in this complex results in an inhibition of steroid synthesis, suggesting that an intact form of the complex is required for cholesterol transport. This model also indicated that the addition of StAR to the complex could increase steroid synthesis by mobilizing cholesterol that is bound to TSPO polymers present in the complex. Unfortunately, no knockdown of TSPO was included in these experiments so assessing its absolute requirement or role in this complex in cholesterol transfer and steroidogenesis could not be determined.
5. Controversies in the regulation of cholesterol transfer and steroidogenesis
A brief summary of the studies that have been performed in determining the roles of StAR and TSPO in regulating cholesterol transport to the IMM and thus stimulating steroid biosynthesis is given above. There have been a number of models proposed for the actions of each of these proteins. Some of the models have been challenged as a result of direct experimentation that demonstrated the mechanisms that they proposed to be unlikely, as were the cases with the candidate proteins SCP2 and SAP. However, in spite of the mechanisms that may be involved in their respective roles in transporting cholesterol to the IMM of steroidogenic cells, both protein candidates have been described as being absolutely indispensable for steroid biosynthesis to occur for a number of years. The basis of this belief for TSPO has been grounded in the studies that have shown that binding of various ligands to TSPO have resulted in an increase in steroid synthesis and that knockdown of TSPO using various methodologies have all resulted in very significant decreases in steroidogenesis (Boujrad et al., 1993, Garnier et al., 1993, 1994, Mukhin et al., 1989, Papadopoulos et al., 1990, 1991a, b, c, 1992, 1997a, b). Such results have been deemed to demonstrate the unequivocal requirement for TSPO in order for the acute stimulation of steroidogenesis to occur. For the StAR protein, the belief for its indispensable role in steroidogenesis comes mainly from the observations that mutations in the StAR gene in humans result in an almost complete inability of those individuals to synthesize steroids and from the fact that StAR null mice have essentially the same phenotype as see in the human condition (Caron et al., 1997, Lin et al., 1995). Also, strong evidence for the role of StAR in steroid biosynthesis came from transient transfection experiments that demonstrated expression of the StAR protein resulted in increased steroid production in the absence of hormone stimulation (Clark et al., 1994, Lin et al., 1995). As a result, many attempts have been made to determine the mechanism of action of these two proteins and, if possible, to illustrate how they may work together in cholesterol transfer to the IMM. Despite several models that have been proposed, there remains a huge void in our knowledge of how these proteins might work, either separately or in concert with each other.
This was the state of affairs until two years ago. Since that time, experiments that demonstrated the requirement for TSPO in regulating cholesterol transport and steroidogenesis have been very seriously challenged. The first of these challenges arose as a result of studies performed by Vimal Selvaraj and colleagues at Cornell University which demonstrated that mice with a conditional knockout of TSPO in testicular Leydig cells were viable, fertile and had no differences in serum testosterone nor in testis weight or seminal vesicle weight from that of wild type animals (Morohaku et al., 2014) (Fig. 4). This observation dealt a serious blow to the earlier observations that TSPO was an absolute requirement for the production of steroids. Soon after the description of the conditional Leydig cell TSPO knockout appeared, a second manuscript from the Selvaraj laboratory was published describing a global knockout of TSPO in mice (Tu et al., 2014). These mice had essentially an identical phenotype as the wild type with regards to steroid synthesis and displayed none of the effects that would be expected if steroids were not being synthesized normally. They were viable, fertile and had normal circulating levels of adrenal (Fig. 5B) and gonadal steroids in spite of TSPO not being present in any of the tissues examined. Further, there were no histological differences found in the steroidogenic tissues of these animals, a sharp contrast to the results seen in the StAR knockout animals (Fig. 5A). In addition to the studies described above, another TSPO knockout mouse was generated separately in an entirely different setting and displayed a phenotype similar to the animals generated by the Selvaraj laboratory (Banati et al., 2014). Also, since many of the TSPO knockdown studies were performed in steroidogenic cell lines, we attempted to duplicate those studies to determine if in vitro studies differed from in vivo studies. Knockdown of TSPO in MA-10 mouse Leydig cells (Fig. 5C) and Y-1 mouse adrenocortical cells (Fig. 5D), resulted in approximately an 80% decrease in TSPO, but had no effect on steroid biosynthesis (Tu et al., 2014). Most interestingly, another cell line, the human adrenocortical H295R cell line, contained no detectable TSPO in wild type cells, yet synthesized steroids abundantly (Fig. 5E) (Tu et al., 2014). These results were completely opposite to those that had earlier been reported for the knockdown of TSPO in steroidogenic cell lines (Boujrad et al., 1993, Li et al., 2001, Papadopoulos et al., 1997a, b). These observations were further supported in studies in which TSPO was completely deleted using CRISPR/Cas9 technology in MA-10 Leydig cells and in the complete absence of TSPO, three separate clones of TSPO knockout cells synthesized steroids at both basal and stimulated levels identical to wild type cells (Tu et al., 2015). In an attempt to discern a possible linkage between TSPO ligands and steroid synthesis, we performed studies in which increasing concentrations of the TSPO ligand PK11195 was added to unstimulated wild type MA-10 cells as well as TSPO deleted cells (Tu et al., 2015). In these studies it was clearly shown that both wild type and TSPO deleted cells demonstrated similar increases in steroid production, indicating that TSPO was not required for these cells to increase steroid output and that the addition of the TSPO ligand in all probability caused off target effects.
Fig. 4.
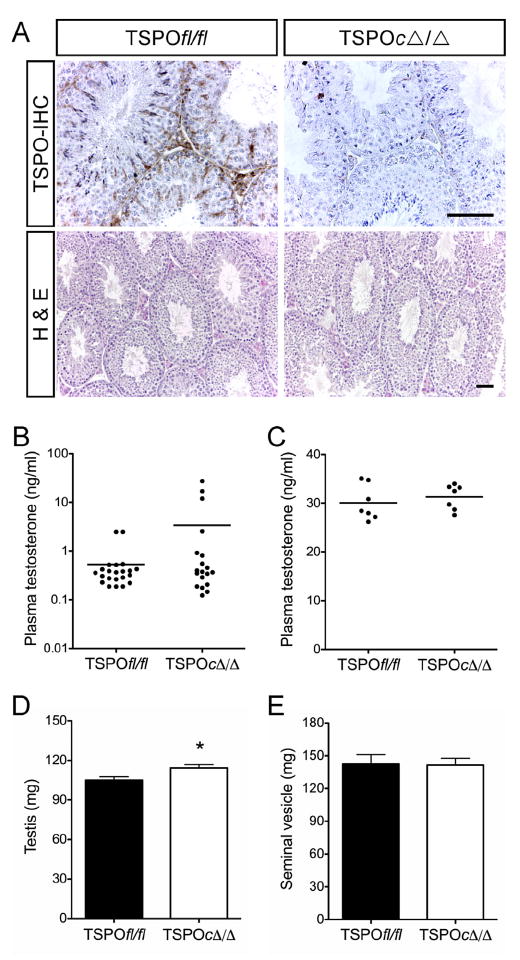
Effects of Conditional Knockout of TSPO in Mouse Testis. A, Immunohistochemical (IHC) localization showing complete absence of TSPO in Leydig and Sertoli cells of TSPOc–/– testes. Hematoxylin and eosin (H&E) staining showing unaltered seminiferous tubule morphology and spermatogenesis in TSPOc–/– testes (n = 5). Scale bars, 50 μm. B, Plasma testosterone levels were not significantly different between TSPOfl/fl and TSPOc–/– mice (n = 19-22/group), before, or C, when sampled 1 h after hCG stimulation (n 7/group). D, a modest but significant increase in testis weights was observed in TSPOc–/– mice compared with TSPOfl/fl mice (P < 0.05; n = 18/group). E, seminal vesicle weights were not significantly different between TSPOfl/fl and TSPOc–/– mice (mean SEM; n = 18/group). Panels from Morohaku et al., 2014, Endocrinology, 155 (1): 89–97.
Fig. 5.
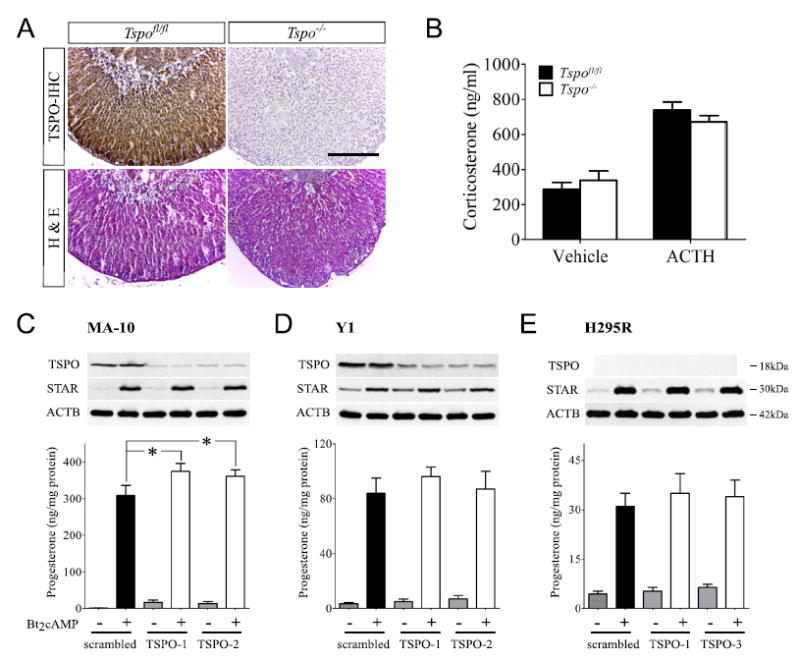
A-B: Effect of TSPO Global Knockout on Adrenal Gland Morphology and Steroid Synthesis. A, Adrenal sections from Tspofl/fl and Tspo–/– mice showing TSPO localization in adrenocortical cells with a higher density in the zona glomerulosa; no staining was observed in Tspo–/– adrenal. No difference in adrenocortical morphology was apparent between the two genotypes. B, both basal and ACTH stimulated plasma corticosterone levels were similar in Tspofl/fl and Tspo–/– mice (n = 10–14/group). C-E: TSPO Knockdown in Steroidogenic Cells Does Not Affect Steroid Hormone Biosynthesis. C and D, TSPO knockdown resulted in substantial decreases in TSPO protein in MA-10 and Y1 cells compared to controls. E, no base-line TSPO was detected in H295R cells. Bt2cAMP treatment resulted in similar increases in StAR protein expression and higher progesterone production in MA-10, Y1 and H295R cells. TSPO knockdown in MA-10 cells showed significantly higher progesterone production (p < 0.05) while progesterone production in TSPO knockdown Y1 cells was similar to the scrambled controls. H295R cells showed no TSPO expression but still produced progesterone upon Bt2cAMP treatment. Panels from Tu et al., 2014, Journal of Biological Chemistry, 289 (40): 27444-54.
In response to the findings reported from the Selvaraj laboratory, a report indicating that conditional knockout of TSPO in the adrenals and the gonads of mice resulted in a decrease in ACTH stimulated levels of corticosterone in these animals (Fan et al., 2015). Strangely, there appeared to be no correlation between the expression levels of TSPO and corticosterone synthesis in the stimulated adrenal glands from heterozygous and homozygous animals. Further, in the testes of these animals it was found that both basal and stimulated levels of testosterone were identical in the wild type, heterozygote and homozygote knockout animals, despite low levels of TSPO in the knockout. While it was shown that the loss of TSPO in the adrenal cells decreased stimulated steroid production it is extremely difficult to reconcile this loss with what is seen in the testis, in which steroidogenesis remains unimpaired. This would indicate that the regulation of steroidogenesis occurs differently in these two organs, a concept that has not been previously described to our knowledge.
Thus, some consider that the controversy as to whether or not TSPO is an absolute requirement for regulating cholesterol transport and steroidogenesis in steroidogenic cells remains. In the face of the data showing no effect on steroid production obtained from both the conditional and global TSPO knockout animals, the knockdown of TSPO in several different steroidogenic cell lines and the total deletion of TSPO in MA-10 cells, it is very difficult to understand how TSPO can be an absolute requirement for steroid synthesis. The specifics of this discussion can be found in much greater detail in several reviews that have recently been published on this topic (Selvaraj and Stocco, 2015, Selvaraj and Tu, 2016, Tu et al., 2015). Nevertheless, fundamental misinterpretations continue to sustain this debate [see commentary (Selvaraj et al., 2016)]. Despite decades of investigation (Fig. 6), it is clear that additional studies will need to be conducted to understand the precise function of TSPO and explain this gap in understanding, as without resolution, more time and resources may be spent in pursuing the wrong course of action.
Fig 6.
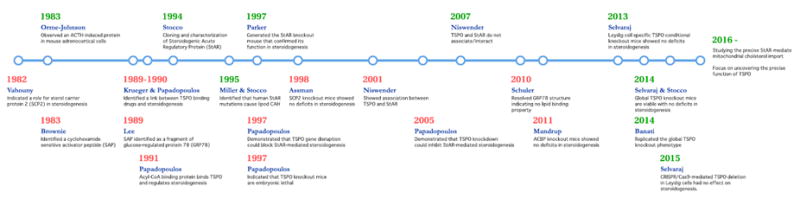
Timeline representing key studies focused on the mechanism of mitochondrial cholesterol import for steroidogenesis and their outcomes. References in chronology: (Chanderbhan et al., 1982), (Krueger and Orme-Johnson, 1983), (Pedersen and Brownie, 1983), (Mukhin et al., 1989), (Krueger and Papadopoulos, 1990), (Li et al., 1989), (Papadopoulos et al., 1991a, b, c), (Clark et al., 1994), (Lin et al., 1995), (Caron et al., 1997), (Papadopoulos et al., 1997a, b), (Seedorf et al., 1998), (West et al., 2001), (Hauet et al., 2005), (Bogan et al., 2007), (Wisniewska et al., 2010), (Neess et al., 2011), (Morohaku et al., 2014), (Tu et al., 2014), (Banati et al., 2014), (Tu et al., 2015).
Acknowledgments
Funding
This work was funded by the National Institutes of Health Grant # HD17481 (DMS), Grant B1-0028 from the Robert A. Welch Foundation (DMS) and startup funds from Cornell University (VS).
References
- Aghazadeh Y, Zirkin BR, Papadopoulos V. Pharmacological regulation of the cholesterol transport machinery in steroidogenic cells of the testis. Vitam Horm. 2015;98:189–227. doi: 10.1016/bs.vh.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Alberta JA, Epstein LF, Pon LA, Orme-Johnson NR. Mitochondrial localization of a phosphoprotein that rapidly accumulates in adrenal cortex cells exposed to adrenocorticotropic hormone or to cAMP. J Biol Chem. 1989;264:2368–2372. [PubMed] [Google Scholar]
- Anuka E, Gal M, Stocco DM, Orly J. Expression and roles of steroidogenic acute regulatory (StAR) protein in ‘non-classical’, extra-adrenal and extra-gonadal cells and tissues. Mol Cell Endocrinol. 2013;371:47–61. doi: 10.1016/j.mce.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Arakane F, Sugawara T, Nishino H, Liu Z, Holt JA, Pain D, Stocco DM, Miller WL, Strauss JF., 3rd Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: implications for the mechanism of StAR action. Proc Natl Acad Sci U S A. 1996;93:13731–13736. doi: 10.1073/pnas.93.24.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arensburg J, Payne AH, Orly J. Expression of steroidogenic genes in maternal and extraembryonic cells during early pregnancy in mice. Endocrinology. 1999;140:5220–5232. doi: 10.1210/endo.140.11.7144. [DOI] [PubMed] [Google Scholar]
- Arthur JR, Boyd GS. The effect of inhibitors of protein synthesis on cholesterol side-chain cleavage in the mitochondria of luteinized rat ovaries. Eur J Biochem. 1974;49:117–127. doi: 10.1111/j.1432-1033.1974.tb03817.x. [DOI] [PubMed] [Google Scholar]
- Baker BY, Yaworsky DC, Miller WL. A pH-dependent molten globule transition is required for activity of the steroidogenic acute regulatory protein, StAR. J Biol Chem. 2005;280:41753–41760. doi: 10.1074/jbc.M510241200. [DOI] [PubMed] [Google Scholar]
- Balasubramanian K, Lavoie HA, Garmey JC, Stocco DM, Veldhuis JD. Regulation of porcine granulosa cell steroidogenic acute regulatory protein (StAR) by insulin-like growth factor I: synergism with follicle-stimulating hormone or protein kinase A agonist. Endocrinology. 1997;138:433–439. doi: 10.1210/endo.138.1.4894. [DOI] [PubMed] [Google Scholar]
- Banati RB, Middleton RJ, Chan R, Hatty CR, Kam WW, Quin C, Graeber MB, Parmar A, Zahra D, Callaghan P, Fok S, Howell NR, Gregoire M, Szabo A, Pham T, Davis E, Liu GJ. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat Commun. 2014;5:5452. doi: 10.1038/ncomms6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao B, Calder MD, Xie S, Smith MF, Salfen BE, Youngquist RS, Garverick HA. Expression of steroidogenic acute regulatory protein messenger ribonucleic acid is limited to theca of healthy bovine follicles collected during recruitment, selection, and dominance of follicles of the first follicular wave. Biol Reprod. 1998;59:953–959. doi: 10.1095/biolreprod59.4.953. [DOI] [PubMed] [Google Scholar]
- Black SM, Harikrishna JA, Szklarz GD, Miller WL. The mitochondrial environment is required for activity of the cholesterol side-chain cleavage enzyme, cytochrome P450scc. Proc Natl Acad Sci U S A. 1994;91:7247–7251. doi: 10.1073/pnas.91.15.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan RL, Davis TL, Niswender GD. Peripheral-type benzodiazepine receptor (PBR) aggregation and absence of steroidogenic acute regulatory protein (StAR)/PBR association in the mitochondrial membrane as determined by bioluminescence resonance energy transfer (BRET) J steroid Biochem Mol Biol. 2007;104:61–67. doi: 10.1016/j.jsbmb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Bose HS, Whittal RM, Baldwin MA, Miller WL. The active form of the steroidogenic acute regulatory protein, StAR, appears to be a molten globule. Proc Natl Acad Sci U S A. 1999;96:7250–7255. doi: 10.1073/pnas.96.13.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose HS, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]
- Bose M, Whittal RM, Miller WL, Bose HS. Steroidogenic activity of StAR requires contact with mitochondrial VDAC1 and phosphate carrier protein. J Biol Chem. 2008;283:8837–8845. doi: 10.1074/jbc.M709221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann HB, Hales KH, Li X, Liu Z, Stocco DM, Hales DB. Acute in vivo inhibition of testosterone by endotoxin parallels loss of steroidogenic acute regulatory (StAR) protein in Leydig cells. Endocrinology. 1996;137:4522–4525. doi: 10.1210/endo.137.10.8828518. [DOI] [PubMed] [Google Scholar]
- Boujrad N, Hudson JR, Jr, Papadopoulos V. Inhibition of hormone-stimulated steroidogenesis in cultured Leydig tumor cells by a cholesterol-linked phosphorothioate oligodeoxynucleotide antisense to diazepam-binding inhibitor. Proc Natl Acad Sci U S A. 1993;90:5728–5731. doi: 10.1073/pnas.90.12.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand C, Cherradi N, Defaye G, Chinn A, Chambaz EM, Feige JJ, Bailly S. Transforming growth factor beta1 decreases cholesterol supply to mitochondria via repression of steroidogenic acute regulatory protein expression. J Biol Chem. 1998;273:6410–6416. doi: 10.1074/jbc.273.11.6410. [DOI] [PubMed] [Google Scholar]
- Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci U S A. 1997;94:11540–11545. doi: 10.1073/pnas.94.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanderbhan R, Noland BJ, Scallen TJ, Vahouny GV. Sterol carrier protein2. Delivery of cholesterol from adrenal lipid droplets to mitochondria for pregnenolone synthesis. J Biol Chem. 1982;257:8928–8934. [PubMed] [Google Scholar]
- Chen YJ, Feng Q, Liu YX. Expression of the steroidogenic acute regulatory protein and luteinizing hormone receptor and their regulation by tumor necrosis factor alpha in rat corpora lutea. Biol Reprod. 1999;60:419–427. doi: 10.1095/biolreprod60.2.419. [DOI] [PubMed] [Google Scholar]
- Cherradi N, Brandenburger Y, Rossier MF, Vallotton MB, Stocco DM, Capponi AM. Atrial natriuretic peptide inhibits calcium-induced steroidogenic acute regulatory protein gene transcription in adrenal glomerulosa cells. Mol Endocrinol. 1998;12:962–972. doi: 10.1210/mend.12.7.0132. [DOI] [PubMed] [Google Scholar]
- Christensen K, Bose HS, Harris FM, Miller WL, Bell JD. Binding of steroidogenic acute regulatory protein to synthetic membranes suggests an active molten globule. J Biol Chem. 2001;276:17044–17051. doi: 10.1074/jbc.M100903200. [DOI] [PubMed] [Google Scholar]
- Chung PH, Sandhoff TW, McLean MP. Hormone and prostaglandin F2 alpha regulation of messenger ribonucleic acid encoding steroidogenic acute regulatory protein in human corpora lutea. Endocrine. 1998;8:153–160. doi: 10.1385/ENDO:8:2:153. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- Clark BJ, Soo SC, Caron KM, Ikeda Y, Parker KL, Stocco DM. Hormonal and developmental regulation of the steroidogenic acute regulatory protein. Mol Endocrinol. 1995;9:1346–1355. doi: 10.1210/mend.9.10.8544843. [DOI] [PubMed] [Google Scholar]
- Davis WW, Garren LD. On the mechanism of action of adrenocorticotropic hormone. The inhibitory site of cycloheximide in the pathway of steroid biosynthesis. J Biol Chem. 1968;243:5153–5157. [PubMed] [Google Scholar]
- Epstein LF, Orme-Johnson NR. Regulation of steroid hormone biosynthesis. Identification of precursors of a phosphoprotein targeted to the mitochondrion in stimulated rat adrenal cortex cells. J Biol Chem. 1991a;266:19739–19745. [PubMed] [Google Scholar]
- Epstein LF, Orme-Johnson NR. Acute action of luteinizing hormone on mouse Leydig cells: accumulation of mitochondrial phosphoproteins and stimulation of testosterone synthesis. Mol Cell Endocrinol. 1991b;81:113–126. doi: 10.1016/0303-7207(91)90210-j. [DOI] [PubMed] [Google Scholar]
- Fan J, Campioli E, Midzak A, Culty M, Papadopoulos V. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc Natl Acad Sci U S A. 2015;112:7261–7266. doi: 10.1073/pnas.1502670112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkash Y, Timberg R, Orly J. Preparation of antiserum to rat cytochrome P-450 cholesterol side chain cleavage, and its use for ultrastructural localization of the immunoreactive enzyme by protein A-gold technique. Endocrinology. 1986;118:1353–1365. doi: 10.1210/endo-118-4-1353. [DOI] [PubMed] [Google Scholar]
- Ferguson JJ., Jr Puromycin and adrenal responsiveness to adrenocorticotropic hormone. Biochim Biophys Acta. 1962;57:616–617. [Google Scholar]
- Ferguson JJ., Jr Protein synthesis and adrenocorticotropin responsiveness. J Biol Chem. 1963;238:2754–2759. [PubMed] [Google Scholar]
- Fleury A, Ducharme L, LeHoux JG. In vivo effects of adrenocorticotrophin on the expression of the hamster steroidogenic acute regulatory protein. J Mol Endocrinol. 1998;21:131–139. doi: 10.1677/jme.0.0210131. [DOI] [PubMed] [Google Scholar]
- Garnier M, Boujrad N, Oke BO, Brown AS, Riond J, Ferrara P, Shoyab M, Suarez-Quian CA, Papadopoulos V. Diazepam binding inhibitor is a paracrine/autocrine regulator of Leydig cell proliferation and steroidogenesis: action via peripheral-type benzodiazepine receptor and independent mechanisms. Endocrinology. 1993;132:444–458. doi: 10.1210/endo.132.1.8380386. [DOI] [PubMed] [Google Scholar]
- Garnier M, Boujrad N, Ogwuegbu SO, Hudson JR, Jr, Papadopoulos V. The polypeptide diazepam-binding inhibitor and a higher affinity mitochondrial peripheral-type benzodiazepine receptor sustain constitutive steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem. 1994;269:22105–22112. [PubMed] [Google Scholar]
- Garren LD, Ney RL, Davis WW. Studies on the role of protein synthesis in the regulation of corticosterone production by adrenocorticotropic hormone in vivo. Proc Natl Acad Sci U S A. 1965;53:1443–1450. doi: 10.1073/pnas.53.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garren LD, Davis WW, Crocco RM, Ney RL. Puromycin analogs: action of adrenocorticotropic hormone and the role of glycogen. Science. 1966;152:1386–1388. doi: 10.1126/science.152.3727.1386. [DOI] [PubMed] [Google Scholar]
- Garren LD. The mechanism of action of adrenocorticotropic hormone. Vitam Horm. 1968;26:119–145. doi: 10.1016/s0083-6729(08)60753-0. [DOI] [PubMed] [Google Scholar]
- Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisinger G, Weizman A. Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev. 1999;51:629–650. [PubMed] [Google Scholar]
- Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, Li W, Hales DB, Miller WL, Culty M, Papadopoulos V. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into leydig cell mitochondria. Mol Endocrinol. 2005;19:540–554. doi: 10.1210/me.2004-0307. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Haworth JD, Rollyson MK, Silva PJ, Sawyer HR, Niswender GD. Effect of dose of prostaglandin F(2alpha) on steroidogenic components and oligonucleosomes in ovine luteal tissue. Biol Reprod. 2000;62:1047–1051. doi: 10.1095/biolreprod62.4.1047. [DOI] [PubMed] [Google Scholar]
- Kanzaki M, Morris PL. Growth hormone regulates steroidogenic acute regulatory protein expression and steroidogenesis in Leydig cell progenitors. Endocrinology. 1999;140:1681–1686. doi: 10.1210/endo.140.4.6661. [DOI] [PubMed] [Google Scholar]
- Kerban A, Boerboom D, Sirois J. Human chorionic gonadotropin induces an inverse regulation of steroidogenic acute regulatory protein messenger ribonucleic acid in theca interna and granulosa cells of equine preovulatory follicles. Endocrinology. 1999;140:667–674. doi: 10.1210/endo.140.2.6499. [DOI] [PubMed] [Google Scholar]
- Kiriakidou M, McAllister JM, Sugawara T, Strauss JF., 3rd Expression of steroidogenic acute regulatory protein (StAR) in the human ovary. J Clin Endocrinol Metab. 1996;81:4122–4128. doi: 10.1210/jcem.81.11.8923870. [DOI] [PubMed] [Google Scholar]
- Krueger KE, Papadopoulos V. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J Biol Chem. 1990;265:15015–15022. [PubMed] [Google Scholar]
- Krueger RJ, Orme-Johnson NR. Acute adrenocorticotropic hormone stimulation of adrenal corticosteroidogenesis. Discovery of a rapidly induced protein. J Biol Chem. 1983;258:10159–10167. [PubMed] [Google Scholar]
- LaVoie HA, Garmey JC, Veldhuis JD. Mechanisms of insulin-like growth factor I augmentation of follicle-stimulating hormone-induced porcine steroidogenic acute regulatory protein gene promoter activity in granulosa cells. Endocrinology. 1999;140:146–153. doi: 10.1210/endo.140.1.6407. [DOI] [PubMed] [Google Scholar]
- Leers-Sucheta S, Stocco DM, Azhar S. Down-regulation of steroidogenic acute regulatory (StAR) protein in rat Leydig cells: implications for regulation of testosterone production during aging. Mech Ageing Dev. 1999;107:197–203. doi: 10.1016/s0047-6374(98)00149-3. [DOI] [PubMed] [Google Scholar]
- Lehoux JG, Hales DB, Fleury A, Briere N, Martel D, Ducharme L. The in vivo effects of adrenocorticotropin and sodium restriction on the formation of the different species of steroidogenic acute regulatory protein in rat adrenal. Endocrinology. 1999;140:5154–5164. doi: 10.1210/endo.140.11.7101. [DOI] [PubMed] [Google Scholar]
- Lejeune H, Sanchez P, Chuzel F, Langlois D, Saez JM. Time-course effects of human recombinant luteinizing hormone on porcine Leydig cell specific differentiated functions. Mol Cell Endocrinol. 1998;144:59–69. doi: 10.1016/s0303-7207(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Li H, Yao Z, Degenhardt B, Teper G, Papadopoulos V. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc Natl Acad Sci U S A. 2001;98:1267–1272. doi: 10.1073/pnas.031461598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XA, Warren DW, Gregoire J, Pedersen RC, Lee AS. The rat 78,000 dalton glucose-regulated protein (GRP78) as a precursor for the rat steroidogenesis-activator polypeptide (SAP): the SAP coding sequence is homologous with the terminal end of GRP78. Mol Endocrinol. 1989;3:1944–1952. doi: 10.1210/mend-3-12-1944. [DOI] [PubMed] [Google Scholar]
- Lin D, Sugawara T, Strauss JF, 3rd, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- Lin T, Wang D, Hu J, Stocco DM. Upregulation of human chorionic gonadotrophin-induced steroidogenic acute regulatory protein by insulin-like growth factor-I in rat Leydig cells. Endocrine. 1998;8:73–78. doi: 10.1385/ENDO:8:1:73. [DOI] [PubMed] [Google Scholar]
- Liu J, Heikkila P, Kahri AI, Voutilainen R. Expression of the steroidogenic acute regulatory protein mRNA in adrenal tumors and cultured adrenal cells. J Endocrinol. 1996;150:43–50. doi: 10.1677/joe.0.1500043. [DOI] [PubMed] [Google Scholar]
- Luo L, Chen H, Stocco DM, Zirkin BR. Leydig cell protein synthesis and steroidogenesis in response to acute stimulation by luteinizing hormone in rats. Biol Reprod. 1998;59:263–270. doi: 10.1095/biolreprod59.2.263. [DOI] [PubMed] [Google Scholar]
- Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamluk R, Greber Y, Meidan R. Hormonal regulation of messenger ribonucleic acid expression for steroidogenic factor-1, steroidogenic acute regulatory protein, and cytochrome P450 side-chain cleavage in bovine luteal cells. Biol Reprod. 1999;60:628–634. doi: 10.1095/biolreprod60.3.628. [DOI] [PubMed] [Google Scholar]
- Mauduit C, Gasnier F, Rey C, Chauvin MA, Stocco DM, Louisot P, Benahmed M. Tumor necrosis factor-alpha inhibits leydig cell steroidogenesis through a decrease in steroidogenic acute regulatory protein expression. Endocrinology. 1998;139:2863–2868. doi: 10.1210/endo.139.6.6077. [DOI] [PubMed] [Google Scholar]
- Midzak A, Zirkin B, Papadopoulos V. Translocator protein: pharmacology and steroidogenesis. Biochem Soc Trans. 2015;43:572–578. doi: 10.1042/BST20150061. [DOI] [PubMed] [Google Scholar]
- Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- Miller WL. Early steps in androgen biosynthesis: from cholesterol to DHEA. Baillieres Clin Endocrinol Metab. 1998;12:67–81. doi: 10.1016/s0950-351x(98)80461-8. [DOI] [PubMed] [Google Scholar]
- Miller WL. Disorders in the initial steps of steroid hormone synthesis. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.03.009. http://dx.doi.org/10.1016/j.jsbmb.2016.03.009 [Epub ahead of print] [DOI] [PubMed]
- Morohaku K, Pelton SH, Daugherty DJ, Butler WR, Deng W, Selvaraj V. Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology. 2014;155:89–97. doi: 10.1210/en.2013-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhin AG, Papadopoulos V, Costa E, Krueger KE. Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proc Natl Acad Sci U S A. 1989;86:9813–9816. doi: 10.1073/pnas.86.24.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neess D, Bloksgaard M, Bek S, Marcher AB, Elle IC, Helledie T, Due M, Pagmantidis V, Finsen B, Wilbertz J, Kruhoffer M, Faergeman N, Mandrup S. Disruption of the acyl-CoA-binding protein gene delays hepatic adaptation to metabolic changes at weaning. J Biol Chem. 2011;286:3460–3472. doi: 10.1074/jbc.M110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol MR, Wang H, Ivell R, Morley SD, Walker SW, Mason JI. The expression of steroidogenic acute regulatory protein (StAR) in bovine adrenocortical cells. Endocr Res. 1998;24:565–569. doi: 10.3109/07435809809032646. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Sasano H, Omura M, Suematsu S. Regulation of expression of the steroidogenic acute regulatory (StAR) protein by ACTH in bovine adrenal fasciculata cells. Biochem Biophys Res Commun. 1996;223:12–18. doi: 10.1006/bbrc.1996.0838. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Yanagibashi K, Yonezawa Y, Ishiwatari S, Matsuba M. A possible role of “steroidogenic factor” in the corticoidogenic response to ACTH; effect of ACTH, cycloheximide and aminoglutethimide on the content of cholesterol in the outer and inner mitochondrial membrane of rat adrenal cortex. Endocrinol Jpn. 1983;30:335–338. doi: 10.1507/endocrj1954.30.335. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Mukhin AG, Costa E, Krueger KE. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J Biol Chem. 1990;265:3772–3779. [PubMed] [Google Scholar]
- Papadopoulos V, Berkovich A, Krueger KE. The role of diazepam binding inhibitor and its processing products at mitochondrial benzodiazepine receptors: regulation of steroid biosynthesis. Neuropharmacology. 1991a;30:1417–1423. doi: 10.1016/s0028-3908(11)80011-0. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Berkovich A, Krueger KE, Costa E, Guidotti A. Diazepam binding inhibitor and its processing products stimulate mitochondrial steroid biosynthesis via an interaction with mitochondrial benzodiazepine receptors. Endocrinology. 1991b;129:1481–1488. doi: 10.1210/endo-129-3-1481. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Nowzari FB, Krueger KE. Hormone-stimulated steroidogenesis is coupled to mitochondrial benzodiazepine receptors. Tropic hormone action on steroid biosynthesis is inhibited by flunitrazepam. J Biol Chem. 1991c;266:3682–3687. [PubMed] [Google Scholar]
- Papadopoulos V, Guarneri P, Kreuger KE, Guidotti A, Costa E. Pregnenolone biosynthesis in C6-2B glioma cell mitochondria: regulation by a mitochondrial diazepam binding inhibitor receptor. Proc Natl Acad Sci U S A. 1992;89:5113–5117. doi: 10.1073/pnas.89.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, Hardwick M, Li H, Vidic B, Brown AS, Reversa JL, Bernassau JM, Drieu K. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997a;62:21–28. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem. 1997b;272:32129–32135. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Miller WL. Role of mitochondria in steroidogenesis. Best Pract Res Clin Endocrinol Metab. 2012;26:771–790. doi: 10.1016/j.beem.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Aghazadeh Y, Fan J, Campioli E, Zirkin B, Midzak A. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol Cell Endocrinol. 2015;408:90–98. doi: 10.1016/j.mce.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen RC, Brownie AC. Cholesterol side-chain cleavage in the rat adrenal cortex: isolation of a cycloheximide-sensitive activator peptide. Proc Natl Acad Sci U S A. 1983;80:1882–1886. doi: 10.1073/pnas.80.7.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen RC, Brownie AC. Steroidogenesis-activator polypeptide isolated from a rat Leydig cell tumor. Science. 1987;236:188–190. doi: 10.1126/science.3563495. [DOI] [PubMed] [Google Scholar]
- Pescador N, Soumano K, Stocco DM, Price CA, Murphy BD. Steroidogenic acute regulatory protein in bovine corpora lutea. Biol Reprod. 1996;55:485–491. doi: 10.1095/biolreprod55.2.485. [DOI] [PubMed] [Google Scholar]
- Pescador N, Houde A, Stocco DM, Murphy BD. Follicle-stimulating hormone and intracellular second messengers regulate steroidogenic acute regulatory protein messenger ribonucleic acid in luteinized porcine granulosa cells. Biol Reprod. 1997;57:660–668. doi: 10.1095/biolreprod57.3.660. [DOI] [PubMed] [Google Scholar]
- Pescador N, Stocco DM, Murphy BD. Growth factor modulation of steroidogenic acute regulatory protein and luteinization in the pig ovary. Biol Reprod. 1999;60:1453–1461. doi: 10.1095/biolreprod60.6.1453. [DOI] [PubMed] [Google Scholar]
- Peters B, Clausmeyer S, Obermuller N, Woyth A, Kranzlin B, Gretz N, Peters J. Specific regulation of StAR expression in the rat adrenal zona glomerulosa. An in situ hybridization study. J Histochem Cytochem. 1998;46:1215–1221. doi: 10.1177/002215549804601101. [DOI] [PubMed] [Google Scholar]
- Pilon N, Daneau I, Brisson C, Ethier JF, Lussier JG, Silversides DW. Porcine and bovine steroidogenic acute regulatory protein (StAR) gene expression during gestation. Endocrinology. 1997;138:1085–1091. doi: 10.1210/endo.138.3.5003. [DOI] [PubMed] [Google Scholar]
- Pollack SE, Furth EE, Kallen CB, Arakane F, Kiriakidou M, Kozarsky KF, Strauss JF., 3rd Localization of the steroidogenic acute regulatory protein in human tissues. J Clin Endocrinol Metab. 1997;82:4243–4251. doi: 10.1210/jcem.82.12.4445. [DOI] [PubMed] [Google Scholar]
- Pon LA, Epstein LF, Orme-Johnson NR. Acute cAMP stimulation in Leydig cells: rapid accumulation of a protein similar to that detected in adrenal cortex and corpus luteum. Endocr Res. 1986a;12:429–446. doi: 10.3109/07435808609035449. [DOI] [PubMed] [Google Scholar]
- Pon LA, Hartigan JA, Orme-Johnson NR. Acute ACTH regulation of adrenal corticosteroid biosynthesis. Rapid accumulation of a phosphoprotein. J Biol Chem. 1986b;261:13309–13316. [PubMed] [Google Scholar]
- Pon LA, Orme-Johnson NR. Acute stimulation of corpus luteum cells by gonadotrophin or adenosine 3’,5’-monophosphate causes accumulation of a phosphoprotein concurrent with acceleration of steroid synthesis. Endocrinology. 1988;123:1942–1948. doi: 10.1210/endo-123-4-1942. [DOI] [PubMed] [Google Scholar]
- Prasad M, Kaur J, Pawlak KJ, Bose M, Whittal RM, Bose HS. Mitochondria-associated endoplasmic reticulum membrane MAM regulates steroidogenic activity via steroidogenic acute regulatory protein (StAR)-voltage-dependent anion channel 2 (VDAC2) interaction. J Biol Chem. 2015;290:2604–2616. doi: 10.1074/jbc.M114.605808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle CT, Crivello JF, Jefcoate CR. Regulation of intramitochondrial cholesterol transfer to side-chain cleavage cytochrome P-450 in rat adrenal gland. Proc Natl Acad Sci U S A. 1983;80:702–706. doi: 10.1073/pnas.80.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaksha M, Kaur J, Bose M, Whittal RM, Bose HS. Cholesterol-mediated conformational changes in the steroidogenic acute regulatory protein are essential for steroidogenesis. Biochemistry. 2013;52:7242–7253. doi: 10.1021/bi401125v. [DOI] [PubMed] [Google Scholar]
- Rajapaksha M, Kaur J, Prasad M, Pawlak KJ, Marshall B, Perry EW, Whittal RM, Bose HS. An outer mitochondrial translocase, Tom22, is crucial for inner mitochondrial steroidogenic regulation in adrenal and gonadal tissues. Mol Cell Biol. 2016;36:1032–1047. doi: 10.1128/MCB.01107-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rone MB, Midzak AS, Issop L, Rammouz G, Jagannathan S, Fan J, Ye X, Blonder J, Veenstra T, Papadopoulos V. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol Endocrinol. 2012;26:1868–1882. doi: 10.1210/me.2012-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology. 1998;139:303–315. doi: 10.1210/endo.139.1.5694. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Seedorf U, Raabe M, Ellinghaus P, Kannenberg F, Fobker M, Engel T, Denis S, Wouters F, Wirtz KW, Wanders RJ, Maeda N, Assmann G. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes Dev. 1998;12:1189–1201. doi: 10.1101/gad.12.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar N, Garmey JC, Veldhuis JD. Mechanisms underlying the steroidogenic synergy of insulin and luteinizing hormone in porcine granulosa cells: joint amplification of pivotal sterol-regulatory genes encoding the low-density lipoprotein (LDL) receptor, steroidogenic acute regulatory (stAR) protein and cytochrome P450 side-chain cleavage (P450scc) enzyme. Mol Cell Endocrinol. 2000;159:25–35. doi: 10.1016/s0303-7207(99)00203-8. [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Stocco DM. The changing landscape in translocator protein (TSPO) function. Trends Endocrinol Metab. 2015;26:341–348. doi: 10.1016/j.tem.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V, Tu LN. Current status and future perspectives: TSPO in steroid neuroendocrinology. J Endocrinol. 2016 doi: 10.1530/JOE-16-0241. http://dx.doi.org/10.1530/JOE-16-0241. [DOI] [PubMed]
- Selvaraj V, Tu LN, Stocco DM. Crucial role reported for TSPO in viability and steroidogenesis is a misconception. Front Endocrinol. 2016;7 doi: 10.3389/fendo.2016.00091. http://dx.doi.org/10.3389/fendo/2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E, Lauber M, Demeter M, Means G, Mahendroo M, Kilgore M, Mendelson C, Waterman M. Regulation of expression of the genes encoding steroidogenic enzymes in the ovary. J Steroid Biochem Mol Biol. 1992;41:409–413. doi: 10.1016/0960-0760(92)90366-q. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Boyd GS. The cholesterol side-chain cleavage system of the adrenal cortex: a mixed-function oxidase. Biochem Biophys Res Commun. 1966;24:10–17. doi: 10.1016/0006-291x(66)90402-5. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Waterman MR. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu Rev Physiol. 1988;50:427–440. doi: 10.1146/annurev.ph.50.030188.002235. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. The requirement of phosphorylation on a threonine residue in the acute regulation of steroidogenesis in MA-10 mouse Leydig cells. J Steroid Biochem Mol Biol. 1993;46(3):337–347. doi: 10.1016/0960-0760(93)90223-j. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Kilgore MW. Induction of mitochondrial proteins in MA-10 Leydig tumour cells with human choriogonadotropin. Biochem J. 1988;249:95–103. doi: 10.1042/bj2490095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM, Chaudhary LR. Evidence for the functional coupling of cyclic AMP in MA-10 mouse Leydig tumour cells. Cell Signal. 1990;2:161–170. doi: 10.1016/0898-6568(90)90019-7. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Chen W. Presence of identical mitochondrial proteins in unstimulated constitutive steroid-producing R2C rat Leydig tumor and stimulated nonconstitutive steroid-producing MA-10 mouse Leydig tumor cells. Endocrinology. 1991;128:1918–1926. doi: 10.1210/endo-128-4-1918. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Sodeman TC. The 30-kDa mitochondrial proteins induced by hormone stimulation in MA-10 mouse Leydig tumor cells are processed from larger precursors. J Biol Chem. 1991;266:19731–19738. [PubMed] [Google Scholar]
- Stocco DM. Further evidence that the mitochondrial proteins induced by hormone stimulation in MA-10 mouse Leydig tumor cells are involved in the acute regulation of steroidogenesis. J Steroid Biochem Mol Biol. 1992;43:319–333. doi: 10.1016/0960-0760(92)90167-h. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Ascoli M. The use of genetic manipulation of MA-10 Leydig tumor cells to demonstrate the role of mitochondrial proteins in the acute regulation of steroidogenesis. Endocrinology. 1993;132:959–967. doi: 10.1210/endo.132.3.8382603. [DOI] [PubMed] [Google Scholar]
- Stocco DM, King S, Clark BJ. Differential effects of dimethylsulfoxide on steroidogenesis in mouse MA-10 and rat R2C Leydig tumor cells. Endocrinology. 1995;136:2993–2999. doi: 10.1210/endo.136.7.7789324. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- Stone D, Hechter O. Studies on ACTH action in perfused bovine adrenals: the site of action of ACTH in corticosteroidogenesis. Arch Biochem Biophys. 1954;51:457–469. doi: 10.1016/0003-9861(54)90501-9. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Lin D, Holt JA, Martin KO, Javitt NB, Miller WL, Strauss JF., 3rd Structure of the human steroidogenic acute regulatory protein (StAR) gene: StAR stimulates mitochondrial cholesterol 27-hydroxylase activity. Biochemistry. 1995;34:12506–12512. doi: 10.1021/bi00039a004. [DOI] [PubMed] [Google Scholar]
- Thompson WE, Powell J, Thomas KH, Whittaker JA. Immunolocalization and expression of the steroidogenic acute regulatory protein during the transitional stages of rat follicular differentiation. J Histochem Cytochem. 1999;47:769–776. doi: 10.1177/002215549904700606. [DOI] [PubMed] [Google Scholar]
- Townson DH, Wang XJ, Keyes PL, Kostyo JL, Stocco DM. Expression of the steroidogenic acute regulatory protein in the corpus luteum of the rabbit: dependence upon the luteotropic hormone, estradiol-17 beta. Biol Reprod. 1996;55:868–874. doi: 10.1095/biolreprod55.4.868. [DOI] [PubMed] [Google Scholar]
- Tsujishita Y, Hurley JH. Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol. 2000;7:408–414. doi: 10.1038/75192. [DOI] [PubMed] [Google Scholar]
- Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, Selvaraj V. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J Biol Chem. 2014;289:27444–27454. doi: 10.1074/jbc.M114.578286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LN, Zhao AH, Stocco DM, Selvaraj V. PK11195 effect on steroidogenesis is not mediated through the translocator protein (TSPO) Endocrinology. 2015;156:1033–1039. doi: 10.1210/en.2014-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahouny GV, Chanderbhan R, Kharroubi A, Noland BJ, Pastuszyn A, Scallen TJ. Sterol carrier and lipid transfer proteins. Adv Lipid Res. 1987;22:83–113. doi: 10.1016/b978-0-12-024922-0.50007-2. [DOI] [PubMed] [Google Scholar]
- Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu Z, Eimerl S, Timberg R, Weiss AM, Orly J, Stocco DM. Effect of truncated forms of the steroidogenic acute regulatory protein on intramitochondrial cholesterol transfer. Endocrinology. 1998;139:3903–3912. doi: 10.1210/endo.139.9.6204. [DOI] [PubMed] [Google Scholar]
- Waterman MR, Simpson ER. Regulation of the biosynthesis of cytochromes P-450 involved in steroid hormone synthesis. Mol Cell Endocrinol. 1985;39:81–89. doi: 10.1016/0303-7207(85)90123-6. [DOI] [PubMed] [Google Scholar]
- West LA, Horvat RD, Roess DA, Barisas BG, Juengel JL, Niswender GD. Steroidogenic acute regulatory protein and peripheral-type benzodiazepine receptor associate at the mitochondrial membrane. Endocrinology. 2001;142:502–505. doi: 10.1210/endo.142.1.8052. [DOI] [PubMed] [Google Scholar]
- Wey S, Luo B, Tseng CC, Ni M, Zhou H, Fu Y, Bhojwani D, Carroll WL, Lee AS. Inducible knockout of GRP78/BiP in the hematopoietic system suppresses Pten-null leukemogenesis and AKT oncogenic signaling. Blood. 2012;119:817–825. doi: 10.1182/blood-2011-06-357384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska M, Karlberg T, Lehtio L, Johansson I, Kotenyova T, Moche M, Schuler H. Crystal structures of the ATPase domains of four human Hsp70 isoforms: HSPA1L/Hsp70-hom, HSPA2/Hsp70-2, HSPA6/Hsp70B’, and HSPA5/BiP/GRP78. PLoS One. 2010;5:e8625. doi: 10.1371/journal.pone.0008625. [DOI] [PMC free article] [PubMed] [Google Scholar]


