SUMMARY
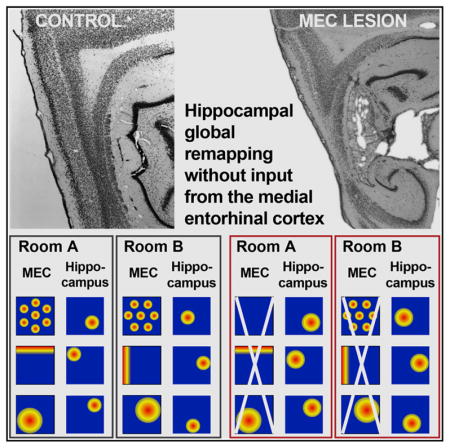
The high storage capacity of the episodic memory system relies on distinct representations for events that are separated in time and space. The spatial component of these computations includes the formation of independent maps by hippocampal place cells across environments, referred to as global re-mapping. Such remapping is thought to emerge by the switching of input patterns from specialized spatially selective cells in medial entorhinal cortex (mEC), such as grid and border cells. Although it has been shown that acute manipulations of mEC firing patterns are sufficient for inducing hippocampal remapping, it remains unknown whether specialized spatial mEC inputs are necessary for the reorganization of hippocampal spatial representations. Here, we examined remapping in rats without mEC input to the hippocampus and found that highly distinct spatial maps emerged rapidly in every individual rat. Our data suggest that hippocampal spatial computations do not depend on inputs from specialized cell types in mEC.
In Brief
Schlesiger et al. find that specialized spatial cells in mEC are not required for generating distinct hippocampal maps across environments. Inputs to the hippocampus, therefore, do not need to be specialized for spatial coding to support hippocampal spatial computations.
INTRODUCTION
The encoding of distinguishable episodic memories requires that neural representations for a multitude of places, contexts, and contents are generated with minimal overlap (Treves and Rolls, 1994). One fundamental component of this computation is the formation of distinct spatial maps by hippocampal place cells. Whereas place cells typically retain their firing locations within the same environment, minor changes to environmental features result in either rate changes without a spatial reorganization of place fields or in a partial reorganization of place field locations. In contrast, extended training across similar environments or exposure to highly distinct environments results in a reorganization of the spatial firing patterns of nearly all hippocampal cells (Muller and Kubie, 1987; Lever et al., 2002; Leutgeb et al., 2004, 2005; Alme et al., 2014). In the case when the resulting spatial representations across environments become maximally distinct, or orthogonal, this phenomenon is referred to as global remapping (Leutgeb et al., 2005).
It is widely assumed that the formation of distinct hippocampal maps depends on computations in the medial entorhinal cortex (mEC) (Buzsáki and Moser, 2013; Moser et al., 2014; Kanter et al., 2017), which sends projections to the hippocampus and contains functional cell types that are specialized in spatial coding, such as grid cells, border cells, directionally selective cells, and nongrid spatial cells (Köhler, 1985; Witter et al., 1988; Hafting et al., 2005; Sargolini et al., 2006; Solstad et al., 2008; Diehl et al., 2017; Hardcastle et al., 2017). The theory that mEC computations are part of the core mechanism for hippocampal global remapping is based on two lines of evidence. First, grid cells, border cells, and head direction cells rotate and shift their receptive fields between distinct environments and spatial nongrid cells alter their spatial firing patterns in response to differences in room configuration (Fyhn et al., 2007; Solstad et al., 2008; Diehl et al., 2017). These changes in mEC firing patterns occur along with hippocampal remapping, and it is therefore assumed that mEC provides the hippocampus with distinct spatial and directional information. Second, experimentally induced changes to mEC firing patterns have been shown to result in a spatial reorganization of hippocampal place fields (Miao et al., 2015; Rueckemann et al., 2016; Kanter et al., 2017). Taken together, these findings indicate that changes in mEC firing patterns are sufficient to cause hippocampal remapping.
The finding that mEC inputs are sufficient to induce remapping does not exclude the possibility that other inputs to hippocampus could also organize spatial maps. For example, numerous theoretical models point to the possibility that hippocampal map organization could be achieved independent of specialized mEC cells (O’Reilly and McClelland, 1994; Touretzky and Redish, 1996; Tsodyks et al., 1996; D’Albis et al., 2015; Grienberger et al., 2017). In support of the possibility that spatial firing is at least partially independent of mEC, studies that lesioned mEC or acutely inactivated mEC firing patterns found that hippocampal place fields persist (Hales et al., 2014; Schlesiger et al., 2013; Miao et al., 2015; Rueckemann et al., 2016; Kanter et al., 2017). The persistence of precise spatial firing while mEC inputs to hippocampus are diminished led to the notion that it is not the formation of spatial receptive fields but rather the selection of a particular map for each environment that is mEC dependent (Miao et al., 2015; Rueckemann et al., 2016). An implication of this view would be that firing fields emerge independent of mEC but that their arrangement is fixed when map reorganization is no longer supported by mEC inputs. To address the question whether mEC is critical for hippocampal map selection, we first performed extensive, bilateral excitotoxic lesions of the mEC, which included up to 100% of mEC superficial layers, and then examined hippocampal remapping by recording from the CA1 region in freely behaving rats in two different environments. The advantage of permanent lesions compared to reversible manipulations (Südhof, 2015 ) is that the extent of the disrupted brain regions can be well quantified and does not change over the duration of the recording experiments, which excludes the possibility that the manipulation acutely alters mEC input patterns to the hippocampus and thereby directly contributes to remapping.
RESULTS
Complete Lesions of the mEC Superficial Layers
To determine whether mEC inputs are necessary for hippocampal global remapping, we performed either N-methyl-D-aspartate (NMDA) or sham lesions of mEC and recorded neuronal activity from CA1 cells in both hemispheres of the hippocampus. In order to exclude the possibility that any retained hippocampal function was the result of spared mEC tissue, we confirmed that our lesions included the entire dorsoventral extent of the mEC, including the grid cell area located in the dorsocaudal mEC (Hafting et al., 2005; Kerr et al., 2007). Stereological quantification of the lesion extent revealed up to 100% damage in the superficial layers of the mEC (percent damage: n = 5; layer II, median: 100.0%, range: 97.9%–100.0%; layer III, median: 92.4%, range: 68.6%–100.0%). If there was minor sparing, it occurred at the most ventrolateral extent of mEC (Figure S1; see Hales et al., 2014 and Schlesiger et al., 2013 for additional photographs and detailed quantification of mEC lesions). Additional major damage was found in the dorsal and ventral parasubiculum (percent damage: medians: 78.0%–66%; ranges: 63.3%–85.8% and 31.0%–75.8%, respectively). Minor damage to lateral entorhinal cortex (lEC) along the border to mEC occurred in one of five rats, and minor damage to the ventral dentate gyrus was observed in one animal. Given that proximal and distal CA1 receive preferential input from mEC and lEC, respectively (Tamamaki and Nojyo, 1995; Witter et al., 2000; Naber et al., 2001), we additionally quantified the positions of our electrodes along the proximal-to-distal axis between CA2 and subiculum (Figures S1B and S1C). Whereas recording electrodes were distributed along the entire axis in control rats, they were preferentially confined to proximal CA1 in mEC-lesioned rats.
Hippocampal Place Fields Retained Spatial Selectivity without mEC Inputs
We examined hippocampal spatial firing by recording over 3 days in two separate rooms on each day (Figure 1). One of the two rooms was highly familiar (≥5 days of experience; referred to as room A), and the other was novel on day 1 of the recording sequence (referred to as room B). As previously reported (Schlesiger et al., 2013; Hales et al., 2014), we found that place fields remained clearly discernable in the mEC lesion group in both rooms (Figure 2A), even though place fields were larger and less informative compared to the control group (Figure S2A). We confirmed that the decrease in spatial precision was not a consequence of differences in recording quality between groups by examining standard cluster quality measures, which were similar between the mEC lesion and control group (Figure S3; L ratio, p = 0.47; isolation distance, p = 0.84; Mann-Whitney U tests). Despite the observed decrease in the spatial information scores of cells recorded in the mEC lesion group, firing rates of the active cell populations (mean firing rate ≥0.25 Hz) were similar between mEC lesion and control groups (Figure S2A). In addition, we found that the proportions of cells that were active in at least one of the rooms did not differ between the two groups (novel environment, control: 37/51 cells, mEC lesion: 51/80 cells; ≥5 days of experience, control: 48/78 cells, mEC lesion: 66/110 cells; both p values ≥0.37; chi-square tests). These results suggest that the change in spatial precision following the mEC lesion was not accompanied by a concurrent change in the average firing rates across the cell population.
Figure 1. Experimental Design.
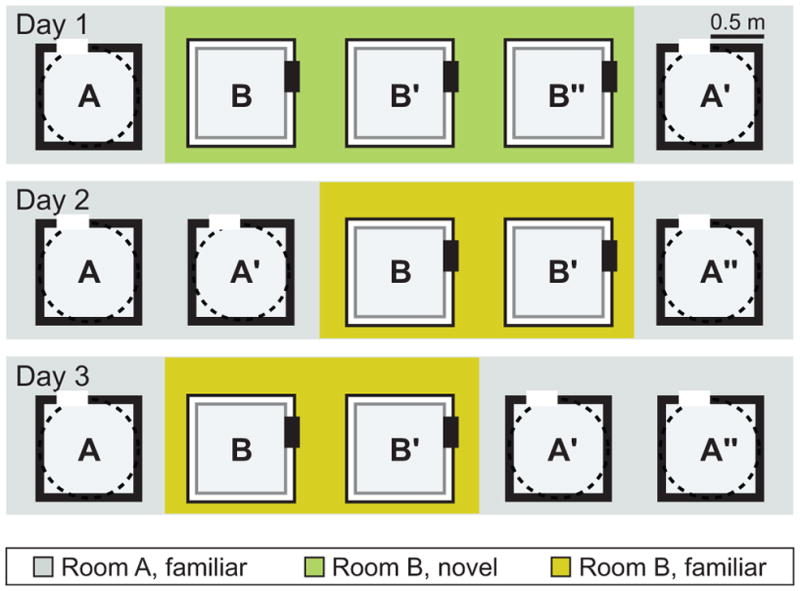
Rats were trained to forage for randomly scattered chocolate sprinkles in open field arenas. Hippocampal recordings were performed over 3 days in two separate rooms on each day. One of the rooms was highly familiar at the beginning of the recording sequence (≥5 days of experience; referred to as room A), and one was novel on day 1 (referred to as room B). The recording environment in room A was either a squared enclosure with black walls or a circular enclosure with a black wall, and the recording environment in room B was a square enclosure with white walls. Each recording day consisted of five daily 10-min sessions with intersession intervals of 5 min. On day 1, three consecutive sessions in room B were performed to examine map stability in the novel environment. On days 2 and 3, one of the recording sessions in room B was replaced with a room A recording to be able to compare map stability over two consecutive sessions in room A (e.g., A′ and A″) and in room B (B′ and B″).
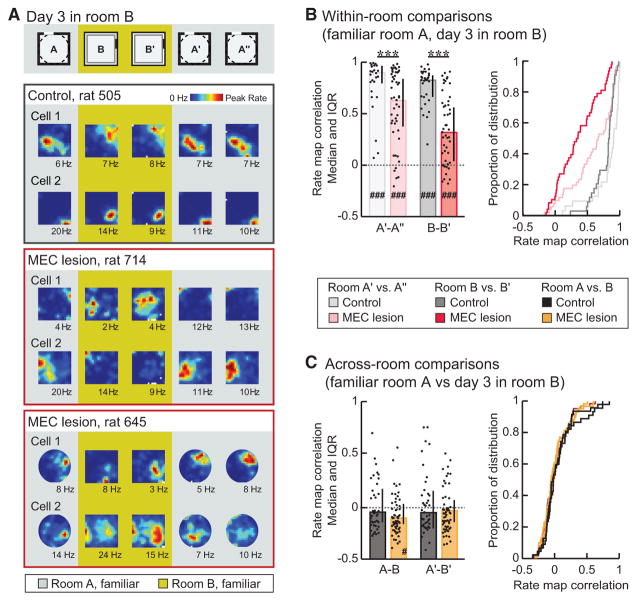
Figure 2. Map Stability between Repeated Sessions in the Same Room Was Reduced, but Remapping across Rooms Was Not Disrupted by mEC Lesions.
(A) The rate map correlation between two consecutive sessions within and across rooms was analyzed in familiar rooms (≥5 and ≥1 day of experience in room A and B, respectively). Hippocampal rate maps from representative simultaneously recorded example cells in control rat 505 and mEC-lesioned rats 714 and 645. The color scale is from 0 Hz (blue) to peak rate (red, indicated to the right of each session in Hz).
(B) Spatial correlations between pairs of consecutive sessions within the same room. Medians and inter-quartile range (IQR) (left panel) and cumulative distribution functions (right panel) are shown. Within-room map similarity was decreased in mEC-lesioned compared to control rats (p values < 0.001; MWU tests) but nonetheless remained above chance (i.e., the median of the shuffled distribution) for all comparisons (p values < 0.001; sign tests).
(C) Across-room map similarity was as low in the mEC lesion as in the control group (p values ≥ 0.24; MWU tests) and did not differ from chance for any comparison between rooms (all p values ≥ 0.35; sign tests) except for the first pair of sessions in the mEC lesion group (A–B), which showed a rate map correlation lower than chance (p = 0.0086). Error bars represent IQR, and black dots are values for individual cells. Dotted line indicates chance level. Holm-Bonferroni correction procedure was applied for multiple comparisons. mEC lesion versus control group, ***p ≤ 0.001; mEC lesion or control group versus shuffled distribution, #p ≤ 0.05 and ###p ≤ 0.001.
See Figure S1 for histology, Figure S2 for quantification of firing patterns within sessions, and Figure S3 for quantification of cluster stability.
Hippocampal Place Field Stability Was Partially Retained without Inputs from the mEC
We next examined whether place field locations were stable between sessions within the same room (Figures 2A and 2B) by computing the spatial correlations between pairs of rate maps from two consecutive recording sessions. As expected, control rats showed high map similarity in the highly familiar room (room A; ≥5 days of experience) as well as in the recently familiarized room (room B; 1 or 2 days of experience; median, 0.89 and 0.82, respectively; p = 0.65; χ2 = 0.20; Friedman test). Rats in the mEC lesion group showed higher map stability in the highly familiar room than in the recently familiarized room (median, 0.65 and 0.33, respectively; p = 0.0065; χ2 = 7.40; Friedman test) and lower map stability than controls in both conditions (both p values < 0.001; Mann-Whitney U tests). However, the remaining degree of stability was higherthan whatwould be expected bychancefor all comparisons, even when there was an intervening session in a different room (all p values < 0.001; sign tests with Holm-Bonferroni correction). There was therefore sufficient hippocampal map stability in our experimental conditions to test the contribution of the mEC to remapping across rooms.
To further examine the time course of map stabilization, we analyzed map stability within the first and within the second daily session in each environment (first versus second 5 min of a session; Figure S2B). Similar to the results obtained across sessions (Figure 2B), we found that within-session stability was lower in mEC-lesioned than in control rats in all sessions and environments (p values ≤ 0.0036; Mann-Whitney U tests). However, whereas the spatial firing patterns of control rats showed high stability within both the first and second session of a day, mEC-lesioned rats showed lower within-session stability during the first session compared to the second session irrespective of the familiarity of the environment (all p values ≤0.034; Wilcoxon signed-rank tests). Taken together, these results indicate that there was a short-term increase in stability in the MEC-lesioned group toward control levels within a recording day but that stabilized maps did not persist over longer time periods, such that each recording day in a familiar room started with stability levels that were as low as in a novel environment.
Global Remapping in Hippocampal CA1 Ensembles Did Not Require mEC Input
By next comparing pairs of rate maps between consecutive recording sessions in two different rooms, we observed that spatial maps reorganized to the same extent in the mEC lesion group as in the control group (Figure 2C; both p values ≥ 0.24; Mann-Whitney U tests). In both groups, the spatial correlation across rooms was similar to what would be expected by chance (all p values ≥0.35; sign tests) or was below chance (first pair of sessions in the mEC lesion group; p = 0.0086), confirming the formation of independent spatial maps for different spatial environments in both groups. In addition, map similarity for recordings across rooms was lower than for repeated recordings within the same room in both the mEC lesion and control group (both p values < 0.001; χ2 ≥ 46.14; Friedman tests). Therefore, global remapping of CA1 ensembles between two familiar environments was intact after mEC lesions.
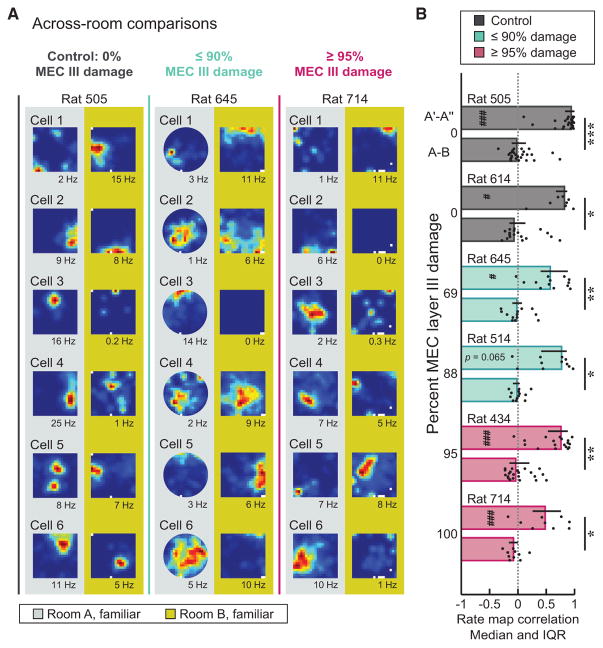
As not all of our lesions resulted in 100% elimination of the superficial layers, we also examined remapping of CA1 ensembles within individual rats (Figures 3A and 3B). The degree of remapping was similar across all rats, and individual rats in the mEC lesion and control groups remapped to the same extent (p = 0.82; χ2 = 2.20; Kruskal-Wallis test). Moreover, in each individual control and mEC-lesioned rat, across-room map similarity did not differ from chance (all p values ≥ 0.27; sign tests) and was lower than the corresponding within-room comparison (all p values ≤ 0.047; Mann-Whitney U tests with Holm-Bonferroni correction). Of note, we confirmed that a rat with 100% bilateral damage to both mEC layer II and III retained global remapping comparable to controls (see rat 714 in Figures 2A, 3A, 3B, and S1), demonstrating that remapping in mEC-lesioned rats was not driven by spared mEC tissue.
Figure 3. Global Remapping Was Intact in All mEC-Lesioned Rats, including Those with the Most Extensive Lesions.
(A) Rate maps from representative example cells recorded in consecutive sessions across two different rooms are shown for rats with varying extent of damage to mEC layer III. Note that the amount of mEC layer II damage was ~100% in all mEC-lesioned rats. The color scale is from 0 Hz (blue) to peak rate (red, indicated to the right of each session in Hz).
(B) Remapping was intact in every individual rat. Within-room map similarity was higher than across-room map similarity for each rat in the control and mEC lesion group (all p values ≤0.047; MWU tests). Moreover, each rat showed across-room map similarity that was not different from chance (median of shuffled distribution; all p values ≥ 0.27; sign tests), whereas the within-room map similarity was higher than chance (all p values ≤ 0.016; sign tests) for all rats except for mEC-lesioned rat 714 (p = 0.065; sign test). Error bars represent IQR, and black dots are values for individual cells. Dotted line indicates chance level. Holm-Bonferroni correction procedure was applied for multiple comparisons. Within-room versus across-room comparison, *p ≤ 0.05, **p ≤ 0.005, and ***p ≤ 0.001; each rat versus shuffled distribution, #p ≤0.05 and ###p ≤0.001.
In control rats, place field locations can be influenced by distal as well as proximal cues, such as the room geometry or a polarizing cue card (Fenton et al., 2000; Lee et al., 2004). In our experimental design, the orientation of the cue card was rotated across rooms (Figure 1) by either 90 or 270 degrees. This raises the possibility that the low map similarity observed in mEC-lesioned rats was not the result of a random redistribution of place field locations but instead emerged because the same spatial map was oriented to the new cue card. To test this possibility, we recalculated the correlations between pairs of rate maps obtained in different rooms after analytically rotating the map in the novel room according to the cue orientation (Figure S4A). We observed that the similarity between pairs of maps was not different from chance and did not differ between mEC-lesioned and control rats (Figure S4B). To account for the possibility that a constant map could be reoriented by any of the box walls or other shared properties between the two rooms, we also calculated the correlations between pairs of maps after rotating one of the maps in 90-degree steps (Figure S4A) and subsequently selecting the highest pairwise correlation for each cell. Using this method, we found that the scores for the mEC lesion and control group were not different from each other (both p values ≥ 0.65; Mann-Whitney U tests) and not different from chance (Figure S4B; all p values ≥ 0.37; sign tests). For both types of rotation analyses, corresponding results were also obtained when examining each rat individually (Figures S4C).
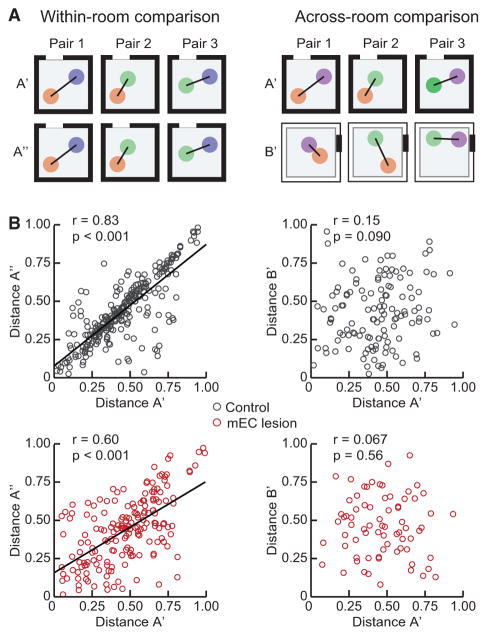
After confirming that maps did not simply rotate across rooms, we then more generally examined the possibility that map organization could have been retained across rooms. To this end, we calculated the distances between place field peaks of simultaneously recorded neurons (Figures 4A and 4B). We first established a baseline over repeated sessions in the same room and found that distances were retained across sessions in mEC-lesioned and control rats (p values < 0.001; r values ≥ 0.60; Pearson product-moment correlations), as would be expected if map geometry was corresponding. In contrast, distances between place fields in one room were uncorrelated to the distances in the second room for both groups (p values ≥ 0.090; r values ≥ 0.15; Pearson product-moment correlations), which indicates that there were no preserved spatial relations.
Figure 4. Place Field Distances Were Reorganized across Rooms in Both the mEC Lesion and the Control Group.
The distances between place field peaks of pairs of simultaneously recorded cells were compared between two sessions within the same room (left) and across two different rooms (right).
(A) Schematic of expected distances between the place fields of three simultaneously recorded cells. Over consecutive recording sessions within the same room (A′ and A″), place field distances are expected to be similar. In contrast, reorganization is expected to result in unrelated distances across two sessions in different rooms (A′ and B′).
(B) Over repeated sessions within the same room, place field peak distances were highly correlated in both control and mEC-lesioned rats (both p values < 0.001; both r values ≥ 0.60; Pearson product-moment correlations). Across rooms, distances were uncorrelated for both groups (p values ≥ 0.090; r values ≥ 0.15; Pearson product-moment correlations).
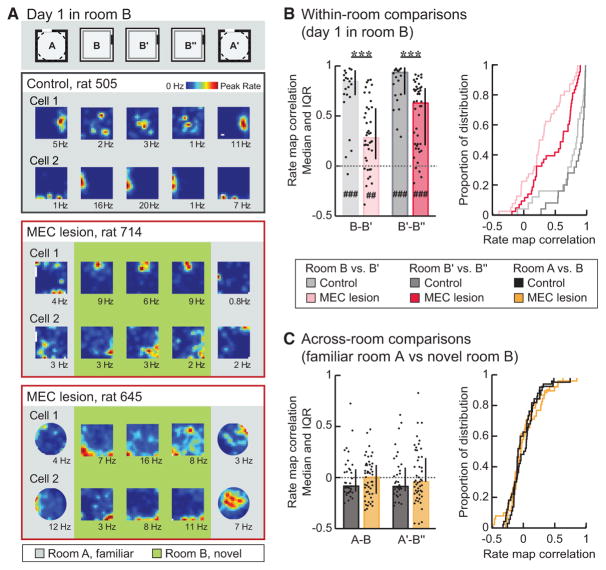
Finally, we tested whether experience was required for distinct hippocampal representations to emerge after mEC lesions (Figure 5) by examining the place fields on day 1 of the recording series, when room B was novel to the rats (Figure 1). We first confirmed that stable maps were formed and retained in the novel room. We found that, for both control and mEC-lesioned rats, within-room map similarity was already above chance for the first pair of sessions in the novel room (Figures 5A and 5B; both p values ≤ 0.0020; sign tests). We then examined remapping between the familiar and novel rooms (Figure 5C) and found no difference between control and mEC-lesioned rats (both p values = 1.00; Mann-Whitney U tests). For both the control and mEC lesion group, the amount of correlation between maps for the different rooms did not exceed what would be expected by chance (all p values ≥0.13; sign tests). Moreover, the map similarity between consecutive sessions in different rooms was lower than the map similarity for consecutive recording sessions in the same room for both the mEC lesion and the control group (both p values ≤ 0.047; both X2 values ≥ 1.57; Friedman tests). In the mEC lesion and control group, remapping thus occurred rapidly upon exposure to a novel environment.
Figure 5. Distinct Hippocampal Maps Emerged Rapidly upon the First Exposure to a Novel Environment in Both mEC-Lesioned and Control Rats.
Rate map correlations between consecutive sessions within and across rooms were analyzed on day 1 of the experiment, when room B was novel. (A) Hippocampal rate maps from representative CA1 cells in control rat 505 and mEC-lesioned rats 714 and 645. The color scale is from 0 Hz (blue) to peak rate (red).
(B) Rate map correlations for pairs of consecutive sessions recorded within the same room. Medians and IQR (left panel) and cumulative distribution functions (right panel) are shown. Within-room map similarity in the mEC lesion group was lower than in the control group (p values < 0.001; MWU tests) but higher than chance (i.e., median of the shuffled distribution), even for the first pair of sessions in the novel room (all p values ≤ 0.0034; sign tests). The rate map correlation was higher for the second pair of sessions within the novel room (B′–B″) compared to the first pair of sessions in the novel room (B–B′) for both the control and mEC lesion group (p values ≤ 0.030; Wilcoxon signed-rank tests).
(C) Across-room map similarity was as low in the mEC lesion as in the control group (both p values ≥ 1.00; MWU tests) and did not differ from chance in either group (all p values ≥ 0.13; sign tests).
Error bars represent IQR, and black dots are values for individual cells. Dotted line indicates chance level. Holm-Bonferroni correction procedure was applied for multiple comparisons. MEC lesion versus control group, ***p ≤0.001; mEC lesion or control group versus shuffled distribution, ##p ≤ 0.005 and ###p ≤ 0.001. See Figure S4 for analysis of across-room map similarity that allows for box rotation.
DISCUSSION
It has been proposed that inputs from specialized cell types in the mEC are essential for the reorganization of hippocampal maps between different spatial environments (Monaco et al., 2011; Buzsáki and Moser, 2013; Kammerer and Leibold, 2014; Rowland and Moser, 2014; Miao et al., 2015). Whereas it has been shown that manipulations of mEC can result in hippocampal remapping, the previous studies did not ask whether mEC inputs are necessary for reorganizing hippocampal maps or whether the same function could also be performed without the highly specialized spatial and directional cell types that are found selectively in mEC. We addressed this question by recording hippocampal firing patterns in two different rooms in the absence of mEC input and found that highly distinct spatial maps emerged rapidly after exposure to a novel environment. The amount of rate map correlation between rooms was similar to what would be expected by chance and lower than during repeated recordings within the same room. Importantly, hippocampal global remapping was intact in each mEC-lesioned rat, even in individuals that had no detectable sparing of the superficial layers, which renders it unlikely that spared mEC input accounted for the formation of distinct hippocampal maps. Whereas mEC lesions did not preclude map formation in novel environments, the within-session stability of hippocampal place cells was decreased irrespective of room familiarity (Figure S2B). Therefore, our results indicate that map formation and map stabilization depend on separate entorhino-hippocampal circuits.
Since the discovery of remapping, it has been proposed that differences in the firing patterns of entorhinal cells are forwarded to the hippocampus, such that separate hippocampal maps emerge from the readout of this information (Muller and Kubie, 1987). Following the finding that hippocampal global remapping is accompanied by a coordinated shift in medial entorhinal grid cell firing patterns (Fyhn et al., 2007), this hypothesis was modified to suggest that the spatial reorganization of grid cells generates a redistribution of firing locations in the hippocampus (Monaco et al., 2011; Buzsáki and Moser, 2013; Kammerer and Leibold, 2014; Rowland and Moser, 2014; Miao et al., 2015). However, recent studies demonstrate that grid cells are not required for hippocampal global remapping (Brandon et al., 2014), raising the possibility that mEC cell types other than grid cells could provide distinct spatial information to the hippocampus. For example, it was recently shown that nongrid spatial cells remap in response to changes in environmental context (Diehl et al., 2017). Head direction cells and border cells are also known to distinguish environments with a coordinated rotation in their firing patterns and could thus be an additional contributor to hippocampal global remapping (Solstad et al., 2008). In addition to these correlative findings, acute manipulations of the mEC were shown to result in various degrees of hippocampal re-mapping (Miao et al., 2015; Rueckemann et al., 2016; Kanter et al., 2017). Taken together, these findings suggest that altered mEC inputs are sufficient to result in the formation of distinct spatial maps in the hippocampus.
Whereas previous studies that manipulated mEC firing patterns suggested that the mEC contributes to hippocampal re-mapping in the intact brain (Miao et al., 2015; Rueckemann et al., 2016; Kanter et al., 2017), they did not address whether the reorganization of hippocampal spatial maps requires highly specialized spatial and directional input from the mEC or whether spatial reorganization can also be achieved exclusively by the neuronal processing of less well-defined spatial inputs, for example, from the lEC (Hargreaves et al., 2005; Tsao et al., 2013). By performing extensive, bilateral mEC lesions that included up to 100% of the cells in the superficial layers, we tested whether mEC spatial inputs are necessary for the generation of new and distinct hippocampal maps. The lesion approach has the advantage that inputs from an entire brain region can be permanently eliminated before the experiment is performed. In contrast, optogenetic and chemogenetic inactivation techniques are known to not completely silence a target region and to allow for a compensatory increase of firing rates in a small population of cells. Such complex responses in the area that is targeted for inactivation would therefore not only reduce neuronal activity in mEC but also acutely alter the activity patterns during the recording experiment (Rueckemann et al., 2016; Miao et al., 2015; Kanter et al., 2017). Such acute manipulations can thus not answer the question whether mEC inputs are necessary for hippocampal remapping. However, our observation that global remapping is at control levels without sparing of the superficial layers allows for the conclusion that mEC is not required for hippocampal global remapping. These results are even more striking as recordings in mEC-lesioned rats were mostly obtained from proximal CA1, which would normally receive mEC input (Figures S1B and S1C). Anatomical adaptations, such as sprouting, or physiological adaptations, such as heightened responsiveness to CA3 inputs, may increase the contribution of the remaining entorhinal inputs to CA1, including inputs that reach CA1 indirectly through CA3 and dentate gyrus (DG). Although neuronal firing patterns in lEC are substantially less spatial than in mEC (Hargreaves et al., 2005), they nonetheless change across distinct contexts (Tsao et al., 2013; Keene et al., 2016). Our results thus raise the possibility that lEC contributions are not limited to non-spatial modifications of the hippocampal maps, as has been concluded from a previous study with partial lEC lesions (Lu et al., 2013), but are also sufficient to reorganize spatial maps.
In addition to a possible contribution from lEC, it can also be speculated that hippocampal remapping could be induced more indirectly by the medial prefrontal cortex (mPFC). Cells in the mPFC strongly differentiate distinct spatial environments with firing rate changes and are additionally modulated by other task contingencies, such as the receipt of reward (Miyazaki et al., 2004; Hyman et al., 2005, 2012; Ito et al., 2015). Whereas a recent study demonstrated that medial prefrontal projections drive hippocampal rate remapping via the nucleus reunions of the thalamus (NR) (Ito et al., 2015), it is possible that the mPFC-NR pathway is also involved in the generation of global re-mapping. Future studies are needed to determine whether any of the inputs to hippocampus can generate distinct spatial maps as long as they provide sufficiently distinct patterns across environments. Irrespective of the nature of the alternative inputs to hippocampus that result in remapping in mEC-lesioned rats, our findings demonstrate that distinct hippocampal maps emerge without input from specialized cell types in the mEC and that weakly spatially selective inputs in combination with intrahippocampal processing are sufficient to result in a major spatial reorganization of the hippocampal map.
EXPERIMENTAL PROCEDURES
Further details and an outline of resources used in this work can be found in Supplemental Experimental Procedures.
Animals
Seven male adult Long-Evans rats were used for recordings from the hippocampal CA1 cell layer. Five received mEC lesions, and two received sham lesions. The mEC lesion extent was quantified in NeuN-stained sections. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of California, San Diego.
Behavioral Procedures
Rats randomly foraged in up to 4 different environments with each environment placed in a different room. Over 3 consecutive days, a series of 10-min recording sessions was performed in two different rooms on each day (Figure 1). One of the two rooms was familiar to the rats, whereas the second room was novel to the rats at the beginning of the recording sequence.
Data Analysis
All data analysis was performed by importing position and spike data into MATLAB and by further processing the data with custom-written scripts and functions. Spike sorting was performed manually using the graphical cluster-cutting software (MClust, D. Redish), which we modified in order to reliably track clusters across sessions. Spatial firing rate distributions were constructed for 5 cm by 5 cm bins and by smoothing with a Gaussian filter with a SD of approximately 1 bin. The spatial information was calculated for the rate map of each session, and spatial similarity between rate maps was compared across sessions using Pearson’s correlation. In addition, the distances between place field peaks were calculated for each pair of simultaneously recorded cells when each cell in the pair had at least one field.
Statistical Analysis
All statistical tests were two sided with α = 0.05. Proportions were compared with chi-square tests. For all remaining statistical analysis, Kolmogorov-Smirnov tests were first performed to test for normality. Because all distributions were non-normal, Mann-Whitney U (MWU) tests and Kruskal-Wallis tests were used for between-group comparisons and Wilcoxon signed-rank tests and Friedman tests for within-group comparisons. Sign tests were used to test the samples against chance. Multiple comparisons were corrected with the Holm-Bonferroni procedure, and Tukey-Kramer tests were used for post hoc analysis.
DATA AND SOFTWARE AVAILABILITY
MClust software is freely available from A.D. Redish at http://redishlab.neuroscience.umn.edu/MClust/MClust.html. Reasonable requests for data and for software will be fulfilled by the lead contact.
Supplementary Material
Highlights.
Hippocampal maps without mEC inputs are sufficiently stable to test for remapping
Hippocampal global remapping is intact without mEC inputs
Rapid generation of distinct hippocampal maps does not require mEC inputs
Maps that form without mEC inputs in novel rooms stabilize within minutes
Acknowledgments
The authors thank Mandy Wong for technical assistance. This work was supported by a Boehringer Ingelheim Fonds PhD fellowship, Walter F. Heiligenberg Professorship, and NIH grants R01 NS086947, R01 NS084324, and R01 MH100349.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.02.082.
DECLARATION OF INTERESTS
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Conceptualization, M.I.S., S.L., and J.K.L.; Methodology, M.I.S., S.L., and J.K.L.; Investigation, M.I.S., B.L.B., and J.B.H.; Formal Analysis, M.I.S.; Writing, M.I.S., S.L., and J.K.L.; Funding Acquisition, M.I.S., S.L., and J.K.L.
References
- Alme CB, Miao C, Jezek K, Treves A, Moser EI, Moser MB. Place cells in the hippocampus: eleven maps for eleven rooms. Proc Natl Acad Sci USA. 2014;111:18428–18435. doi: 10.1073/pnas.1421056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MP, Koenig J, Leutgeb JK, Leutgeb S. New and distinct hippocampal place codes are generated in a new environment during septal inactivation. Neuron. 2014;82:789–796. doi: 10.1016/j.neuron.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Albis T, Jaramillo J, Sprekeler H, Kempter R. Inheritance ofhippocampal place fieldsthrough hebbian learning: effects of theta modulation and phase precession on structure formation. Neural Comput. 2015;27:1624–1672. doi: 10.1162/NECO_a_00752. [DOI] [PubMed] [Google Scholar]
- Diehl GW, Hon OJ, Leutgeb S, Leutgeb JK. Grid and nongrid cells in medial entorhinal cortex represent spatial location and environmental features with complementary coding schemes. Neuron. 2017;94:83–92.e6. doi: 10.1016/j.neuron.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Csizmadia G, Muller RU. Conjoint control of hippocampal place cell firing by two visual stimuli. Ii. A vector-field theory that predicts modifications of the representation of the environment. J Gen Physiol. 2000;116:211–221. doi: 10.1085/jgp.116.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- Grienberger C, Milstein AD, Bittner KC, Romani S, Magee JC. Inhibitory suppression of heterogeneously tuned excitation enhances spatial coding in CA1 place cells. Nat Neurosci. 2017;20:417–426. doi: 10.1038/nn.4486. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hales JB, Schlesiger MI, Leutgeb JK, Squire LR, Leutgeb S, Clark RE. Medial entorhinal cortex lesions only partially disrupt hippocampal place cells and hippocampus-dependent place memory. Cell Rep. 2014;9:893–901. doi: 10.1016/j.celrep.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle K, Ganguli S, Giocomo LM. Cell types for our sense of location: where we are and where we are going. Nat Neurosci. 2017;20:1474–1482. doi: 10.1038/nn.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial pre-frontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci USA. 2012;109:5086–5091. doi: 10.1073/pnas.1114415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Zhang SJ, Witter MP, Moser EI, Moser MB. A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature. 2015;522:50–55. doi: 10.1038/nature14396. [DOI] [PubMed] [Google Scholar]
- Kammerer A, Leibold C. Hippocampal remapping is constrained by sparseness rather than capacity. PLoS Comput Biol. 2014;10:e1003986. doi: 10.1371/journal.pcbi.1003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter BR, Lykken CM, Avesar D, Weible A, Dickinson J, Dunn B, Borgesius NZ, Roudi Y, Kentros CG. A novel mechanism for the grid-to-place cell transformation revealed by transgenic depolarization of medial entorhinal cortex layer II. Neuron. 2017;93:1480–1492.e6. doi: 10.1016/j.neuron.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bladon J, McKenzie S, Liu CD, O’Keefe J, Eichenbaum H. Complementary functional organization of neuronal activity patterns in the perirhinal, lateral entorhinal, and medial entorhinal cortices. J Neurosci. 2016;36:3660–3675. doi: 10.1523/JNEUROSCI.4368-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17:697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Köhler C. Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. J Comp Neurol. 1985;236:504–522. doi: 10.1002/cne.902360407. [DOI] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Lever C, Wills T, Cacucci F, Burgess N, O’Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416:90–94. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- Lu L, Leutgeb JK, Tsao A, Henriksen EJ, Leutgeb S, Barnes CA, Witter MP, Moser MB, Moser EI. Impaired hippocampal rate coding after lesions of the lateral entorhinal cortex. Nat Neurosci. 2013;16:1085–1093. doi: 10.1038/nn.3462. [DOI] [PubMed] [Google Scholar]
- Miao C, Cao Q, Ito HT, Yamahachi H, Witter MP, Moser MB, Moser EI. Hippocampal remapping after partial inactivation of the medial entorhinal cortex. Neuron. 2015;88:590–603. doi: 10.1016/j.neuron.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Miyazaki KW, Matsumoto G. Different representation of forthcoming reward in nucleus accumbens and medial prefrontal cortex. Neuroreport. 2004;15:721–726. doi: 10.1097/00001756-200403220-00030. [DOI] [PubMed] [Google Scholar]
- Monaco JD, Knierim JJ, Zhang K. Sensory feedback, error correction, and remapping in a multiple oscillator model of place-cell activity. Front Comput Neurosci. 2011;5:39. doi: 10.3389/fncom.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Roudi Y, Witter MP, Kentros C, Bonhoeffer T, Moser MB. Grid cells and cortical representation. Nat Rev Neurosci. 2014;15:466–481. doi: 10.1038/nrn3766. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber PA, Lopes da Silva FH, Witter MP. Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus. 2001;11:99–104. doi: 10.1002/hipo.1028. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Rowland DC, Moser MB. From cortical modules to memories. Curr Opin Neurobiol. 2014;24:22–27. doi: 10.1016/j.conb.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Rueckemann JW, DiMauro AJ, Rangel LM, Han X, Boyden ES, Eichenbaum H. Transient optogenetic inactivation of the medial entorhinal cortex biases the active population of hippocampal neurons. Hippocampus. 2016;26:246–260. doi: 10.1002/hipo.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Schlesiger MI, Cressey JC, Boublil B, Koenig J, Melvin NR, Leutgeb JK, Leutgeb S. Hippocampal activation during the recall of remote spatial memories in radial maze tasks. Neurobiol Learn Mem. 2013;106:324–333. doi: 10.1016/j.nlm.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Südhof TC. Reproducibility: experimental mismatch in neural circuits. Nature. 2015;528:338–339. doi: 10.1038/nature16323. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nojyo Y. Preservation of topography in the connections between the subiculum, field CA1, and the entorhinal cortex in rats. J Comp Neurol. 1995;353:379–390. doi: 10.1002/cne.903530306. [DOI] [PubMed] [Google Scholar]
- Touretzky DS, Redish AD. Theory of rodent navigation based on interacting representations of space. Hippocampus. 1996;6:247–270. doi: 10.1002/(SICI)1098-1063(1996)6:3<247::AID-HIPO4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Tsao A, Moser MB, Moser EI. Traces of experience in the lateral entorhinal cortex. Curr Biol. 2013;23:399–405. doi: 10.1016/j.cub.2013.01.036. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Skaggs WE, Sejnowski TJ, McNaughton BL. Population dynamics and theta rhythm phase precession of hippocampal place cell firing: a spiking neuron model. Hippocampus. 1996;6:271–280. doi: 10.1002/(SICI)1098-1063(1996)6:3<271::AID-HIPO5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Witter MP, Holtrop R, Van de Loosdrecht AA. Direct projections from the periallocortical subicular complex to the fascia dentata in the rat. An anatomical tracing study using Phaseolus vulgaris leucoagglutinin. Neurosci. Res. Commun. 1988;2:61–68. [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.