Abstract
Prolonged exposure to arsenic has been shown to increase the risk of developing a number of diseases, including cancer and type II diabetes. Arsenic is present throughout the environment in its inorganic forms, and the level of exposure varies greatly by geographical location. The current recommended maximum level of arsenic exposure by the EPA is 10 µg/L, but levels >50 – 1000 µg/L have been detected in some parts of Asia, the Middle East, and the Southwestern United States. One of the most important steps in developing treatment options for arsenic-linked pathologies is to understand the cellular pathways affected by low levels of arsenic. Here, we show that acute exposure to non-lethal, low-level arsenite, an environmentally relevant arsenical, inhibits the autophagy pathway. Furthermore, arsenite-induced autophagy inhibition initiates a transient, but moderate ER stress response. Significantly, low-level arsenite exposure does not exhibit an increase in oxidative stress. These findings indicate that compromised autophagy, and not enhanced oxidative stress occurs early during arsenite exposure, and that restoring the autophagy pathway and proper proteostasis could be a viable option for treating arsenic-linked diseases. As such, our study challenges the existing paradigm that oxidative stress is the main underlying cause of pathologies associated with environmental arsenic exposure.
Keywords: arsenic, autophagy inhibitor, ER stress, proteostasis, oxidative stress
Introduction
Arsenic is a metalloid found ubiquitously in the environment. Exposure to unsafe levels of arsenic typically occurs as a result of consuming contaminated food or drinking water, particularly over a prolonged period of time. The current World Heath Organization (WHO) and Environmental Protection Agency (EPA) recommended limit for arsenic levels in the drinking water is 10 µg/L (10 ppb); however, many countries including Bangladesh, India, Mexico, Chile, Taiwan, China, and even some parts of the United States and Canada, have reported concentrations that significantly exceed this limit, ranging anywhere from 50 to >1,000 ppb (µg/L) (Chowdhury et al., 2000; Harvey et al., 2002; Yoshida et al., 2004; Wang and Mulligan, 2006; Buschmann et al., 2008; Sorg et al., 2014; Karagas et al., 2015; Mendez et al., 2016). Furthermore, populations chronically exposed to arsenic in this range are put at an increased risk of developing a number of diseases, including skin, lung, and bladder cancer, cardiovascular, respiratory, and kidney disease, as well as type II diabetes (Tseng et al., 2003; Navas-Acien et al., 2008; Parvez et al., 2008; Maull et al., 2012; Steinmaus et al., 2013; Steinmaus et al., 2014; Cheng et al., 2017).
The carcinogenic potential of arsenic has been well established in both epidemiological studies, as well as in a host of experimental models (Morales et al., 2000; Chen et al., 2001; Eblin et al., 2007; Sun et al., 2009; Martinez et al., 2011). Arsenic is classified as a Group 1 human carcinogen by the International Agency for Research on Cancer (IARC), and currently ranks number 1 on the substance priority list put forth by the Agency for Toxic Substance and Disease Registry (ATSDR). The toxic effects associated with arsenic exposure depend on the arsenical species, as well as the concentration and time of exposure. Intracellularly, arsenic can exist in its inorganic forms, arsenate [As(V)] and arsenite [As (III)], as well as in its methylated forms, methylarsonic acid/methylarsonous acid [MMA(V/III)] or dimethylarsinic acid/dimethylarsinous acid [DMA(V/III)]. Most in vitro and in vivo studies utilize either sodium arsenite (NaAsO2) or arsenic trioxide (As2O3), producing As(III) which can be oxidized to form As(V), or further metabolized by arsenite 3-methyl transferase into MMA or DMA inside the cell (Thomas et al., 2001). As such, the pleiotropic effects attributed to arsenic toxicity are a result of the diversity of arsenical species and their subsequent effects on a variety of intracellular targets.
Interestingly, many of the studies investigating the pathogenic effects of arsenic exposure use concentrations of arsenite in the >5 µM micromolar range [375–750 ppb As(III)], reflecting concentrations that might be observed only in the most severely affected areas. Many of the observed effects in this range, such as increased reactive oxygen species (ROS) production (Alarifi et al., 2013; Jiang et al., 2013; Kumar et al., 2014), DNA damage (Andrew et al., 2006; Hughes et al., 2011; Prakash et al., 2016), mitochondrial dysfunction (Liu et al., 2005), glutathione depletion (Li and Chen, 2016), and protein modifications (Shen et al., 2013), might not occur at lower, more relevant concentrations. Furthermore, the compound, cell type, and time of treatment matters, with As2O3 exhibiting a more consistent increase in ROS production at acute time points (Barchowsky et al., 1999; Yen et al., 2012; Lu et al., 2014), whereas chronic NaAsO2 treatment (i.e. 8–24 weeks) has been shown to increase or decrease ROS levels depending on the cell type and length of exposure (Chang et al., 2010; Padmaja Divya et al., 2015). It is important to note that As2O3 is not a common environmental contaminant, and is mainly utilized as a cancer treatment, making it not suitable for determining the cellular pathways affected by environmental exposure to more common arsenicals.
To gain a better understanding of the pathological effects associated with lower arsenic concentrations, we examined the immediate cellular stress responses to low levels of arsenic exposure [sodium arsenite in the 0.5 – 2 µM, which is equivalent to 37.5 – 150 ppb As(III)]. ROS production, autophagosome accumulation, and activation of the ER stress response, at early time points (0.5–4 h), as well as cell viability (24 to 48 h), were measured in a variety of mouse and human cell lines. Interestingly, low-level arsenite treatment up to 72 h did not result in any detectable levels of ROS as measured by electron paramagnetic resonance spectroscopy (EPR) using the 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH) spin probe. Instead, acute arsenite treatment results in inhibition of the autophagy pathway, as we reported previously (Lau et al., 2013), as well as a transient moderate ER stress response. No toxicity was observed at any concentration of arsenite up to 48 h in all four of the cell types tested. These results demonstrate that lower concentrations of arsenite significantly alter proteostasis without inducing oxidative stress, at least at early time points, challenging the existing paradigm that ROS are the main underlying cause of pathologies associated with environmental arsenic exposure.
Materials and Methods
Chemicals, antibodies, reagents, and cell culture conditions
Sodium arsenite (S7400), 2’,7’-dichlorofluorescein diacetate (H2DCFDA; D6883), MTT [3-4,5-dimethyl-2-thiazo-yl)-2,5-diphenyl-2H-tetrazolium bromide; M5655], bafilomycin A1 (B1793), and the primary antibody against LC3 (L7543) were purchased from Sigma. 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH; NOX-0.2) and Krebs-HEPES buffer (NOX-07.6.1) were purchased from Noxygen. Primary antibodies against GAPDH (sc-32233), as well as horseradish peroxidase (HRP)-conjugated secondary antibodies (goat anti-rabbit, sc-2004; goat anti-mouse, sc-2005) were from Santa Cruz. The primary antibody against SQSTM1 (89-015-843) was obtained from Abnova. The primary antibodies against EIF2S1 (9722), phosphorylated EIF2S1 (9721), and XBP1s (12782) were from Cell Signaling Technologies. The antibody against ATF6 (ab122897) was from Abcam. NIH 3T3, HeLa, and HEK 293 cell lines were purchased from the American Type Culture Collection (ATCC). NIH 3T3 and HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Corning, MT10014CV) supplemented with 10% fetal bovine serum (FBS; Atlanta Biological, S11150H), 1% L-glutamine (Gibco, 25030081), and 1% penicillin-streptomycin (15140722). HEK 293 cells were cultured in minimum essential medium (MEM; Corning, 10-010-CV) supplemented with 10% FBS, 1% L-glutamine, 0.1 mM nonessential amino acids (Hyclone, SH30238.01), 0.1 mM sodium pyruvate (Gibco, 11360070), and 0.01% gentamicin (Omega Scientific, 1350). HEMn-LP were purchased from Thermo Fisher Scientific and were cultured in medium 254 supplemented with 0.06 mM calcium chloride (Gibco, M254CF500), 1X human melanocyte growth supplement (HMGS; Thermo Fisher Scientific, S0025), and 1X gentamicin/amphotericin (Thermo Fisher Scientific, R01510). All cells were incubated at 37 °C in a humidified incubator with 5% CO2.
Transfection and live cell fluorescent imaging
For imaging, 7.5×104 NIH 3T3 cells were seeded in quad chamber glass bottom 35 mm dishes. Cells were transfected with 1 µg RFP-GFP-LC3 using Lipofectamine 3000 (Thermo Fisher Scientific, L3000075) according to the manufacturer’s instructions. 24 h later, cells were either left untreated, treated with sodium arsenite (0.25, 0.5, 1, or 2 µM) or bafilomycin (0.1 µM), or starved with Hank’s balanced salt solution (HBSS, 1X, Hyclone, SH30268.01) for the indicated time points. Prior to imaging, cells were gently washed with 1X phosphate buffered saline (PBS) and DMEM without phenol red was added. Images were taken with a Zeiss Observer.Z1 microscope using the Slidebook 4.2.0.11 software (Intelligent Imaging Innovations, Inc.).
Immunoblot analysis
To detect protein expression, 1.5×105 cells were seeded in 12-well plates, and 24 h later were either left untreated, treated with sodium arsenite or bafilomycin, or starved in HBSS for the indicated time points. Cells were washed in 1X PBS, harvested in 1X Laemmli or 1X NuPAGE LDS Sample Buffer with 1X NuPAGE Reducing Agent (Thermo Fisher Scientific, NP0007), and boiled for 5 min. Cell lysates were resolved by SDS-polyacrylamide gel electrophoresis and subjected to immunoblot analysis with the indicated antibodies.
Cell toxicity assay
To measure cell toxicity, 1×104 cells were seeded in 96-well plates, and 24 h later were either left untreated or treated with the indicated concentrations of sodium arsenite for 24 and 48 h. 20 µL of MTT (2 mg/mL) were added to each well and the plates were incubated for 2 h at 37 °C. Medium was removed and formazan salts were diluted by adding 100 µL of isopropanol/HCl. The plates were shaken at room temperature for 10 min and absorbance was measured at 570 nm using a BioTek plate reader. Cells were seeded in quadruplicate.
Reactive oxygen species measurements
To measure the generation of reactive oxygen species using EPR, 7.5×104 cells were seeded in 24-well plates, and 24 h later were either left untreated, or treated with sodium arsenite (0.5, 1, 2 or 10 µM), or with 250 – 500 µM hydrogen peroxide (H2O2), or 25–50 µM FCCP for the indicated time points. Following treatment, cells were incubated in 20 mM Krebs-HEPES buffer (ph 7.4) containing 200 µM CMH for 30 min. Buffer was then collected, and changes in CMH oxidation were measured for 15 min using the e-scanM Multipurpose Bench-top EPR system (Noxygen Science and Transfer Diagnostics GmbH). Cells were lysed in RIPA buffer (50mM Tris (pH 7.8), 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, and 0.1% SDS) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and a protease inhibitor cocktail, and total protein was quantified using the Pierce BCA protein assay (ThermoFisher). CMH signal was determined as nM/min/mg of protein, and all treatment groups were normalized to control.
To determine ROS production using DCF fluorescence, 3×105 cells were seeded in 35 mm dishes and 24 h later were either left untreated or treated with sodium arsenite (0.25, 0.5, 1, or 2 µM) for 4 h, or with 100 µM hydrogen peroxide (H2O2) for 1 h. Media was removed and replaced by fresh media containing 10 µg/mL H2DCFDA and cells were incubated at 37 °C for 1 h. Next, cells were washed, trypsinized, washed again, and resuspended in PBS. Fluorescence was analyzed by flow cytometry with a FACSCanto II (BD Biosciences) at the University of Arizona Flow Cytometry Core.
Statistics
Results were expressed as mean ± SEM of three independent replicates. Two-way analysis of variance (ANOVA) followed by Fisher’s post hoc test was performed for cell viability using GraphPad Prism 7.
Results
Low concentrations of arsenite do not increase ROS production
To test if low concentrations of sodium arsenite generate ROS, four cell lines (mouse embryonic fibroblast, NIH 3T3; human cervical carcinoma, HeLa; human embryonic kidney, HEK 293; and primary human neonatal epidermal melanocytes, low pigmented, HEMn-LP) were left untreated, or treated with low-level sodium arsenite (0.5, 1, or 2 µM), a high concentration (10 µM) of sodium arsenite, as well 250–500 µM hydrogen peroxide (H2O2) or 25–50 µM (Carbonyl cyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP)) as positive controls, and ROS production was measured at 2 and 4 h using electron paramagnetic resonance spectroscopy (EPR), utilizing the 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH) spin probe. Oxidation of CMH results in a signal increase that can be measured over time to indicate increased production of ROS. While H2O2 resulted in significant oxidation of the CMH probe in NIH 3T3, HEK 293, and HEMn-LP cells at 4 h, as well as HEMn-LP cells at 2 h, indicating increased endogenous ROS production, arsenite-treated cell ROS levels were comparable to control (Figure 1A). Interestingly, HeLa cells were resistant to H2O2 treatment at 2 and 4 h; however, exposure to 25 and 50 µM FCCP, a mitochondrial uncoupler, for 2 h resulted in a significant increase in ROS levels. Importantly, treatment with arsenite did not generate any ROS in this cell line at either time point (Figure 1A). To determine if longer-term exposure to arsenite increased ROS, NIH 3T3 cells were treated with 2 µM sodium arsenite for 24, 48, or 72 h, and oxidation of CMH was measured. Similar to the 2 and 4 h results, arsenite did not result in increased oxidation of CMH compared to control, indicating that exposure to low-level sodium arsenite up to 72 h does not enhance ROS formation (Figure 1C).
Figure 1. ROS are not generated by low-level sodium arsenite.
Changes in ROS production were measured via EPR using the CMH spin probe (A), or DCF fluorescence and flow cytometry (B) in NIH 3T3, HeLa, HEK 293, or HEMn-LP cells treated with the indicated concentrations of sodium arsenite for 2 or 4 h. Treatment with 100–500 µM hydrogen peroxide (H2O2) or 25–50 µM FCCP for the indicated times were used as positive controls. (C) NIH 3T3 cells were left untreated, or treated with 2 µM sodium arsenite for 24, 48, or 72 h. Treatment with 250 µM hydrogen peroxide (H2O2) for 4 h was used as a positive control. Data = mean ± SEM, n = 3. *p<.05 compared to control. Student’s t-test.
These results were further confirmed using 2',7'-dichlorofluorescein (DCF) fluorescence and flow cytometry (Fig. 1B). Oxidation of the non-fluorescent 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA) into its fluorescent derivative DCF results in a fluorescence intensity peak shift that can be measured and quantified by flow cytometry. Cells were left untreated, or treated with sodium arsenite (0.25, 0.5, 1, or 2 µM) for 4 h, or with 100 µM hydrogen peroxide (H2O2) as a positive control, and DCF signal was determined using flow cytometry. While treatment with H2O2 resulted in the expected peak shift in NIH 3T3, HEK 293, and HEMn-LP cells, HeLa cells did not show any shift, similar to the EPR results (Fig. 1A–B). On the other hand, none of the tested concentrations of sodium arsenite cause a significant fluorescence intensity peak shift in all four cell lines tested, confirming that low levels of sodium arsenite do not generate ROS at acute time points.
Low-level sodium arsenite blocks autophagy in multiple cell types
Our lab has previously reported that low levels of sodium arsenite inhibit late stage autophagy flux in BEAS-2B cells, human bronchial epithelial (HBE) cells, as well as the mouse fibroblast NIH 3T3 cell line (Lau et al., 2013). Due to a lack of ROS production at lower concentrations of arsenic, we believe the pathogenesis associated with early exposure to low concentrations of arsenic can instead be attributed to autophagic dysfunction and altered proteostasis. Thus, to determine if sodium arsenite blocks autophagy at concentrations that do not increase ROS production, NIH 3T3, HeLa, HEK 293, and HEMn-LP cells were treated with sodium arsenite (0, 0.25, 0.5, 1, or 2 µM) for 0.5, 1, 2 or 4 h, using 0.1 µM bafilomycin (BAF; autophagy inhibitor) and starvation (HBSS (St); autophagy activator) as controls, and the protein levels of SQSTM1, MAP1LC3A/B-I, and MAP1LC3A/B-II were analyzed by immunoblot analysis (Figure 2). Sodium arsenite caused a concentration- and time-dependent increase in SQSTM1 and MAP1LC3A/B-II protein levels, similar to what was observed for BAF, across the four cell lines (Figure 2A–D). While the level of autophagy inhibition varied among the different cell types, as well as across the arsenite concentrations and time points, arsenite consistently resulted in autophagy inhibition.
Figure 2. Sodium arsenite inhibits autophagy in multiple cell types. (A–D).
Immunoblot analysis of changes in MAP1LC3A/B-I, MAP1LC3A/B-II, and SQSTM1 protein levels following treatment with the indicated concentrations of sodium arsenite in NIH 3T3 (A), HeLa (B), HEK 293 (C), or HEMn-LP (D) cells at 2 and 4 h. Starvation (HBSS), or 0.1 µM BAF for 2 or 4 h were used as controls. GAPDH was used as an internal loading control.
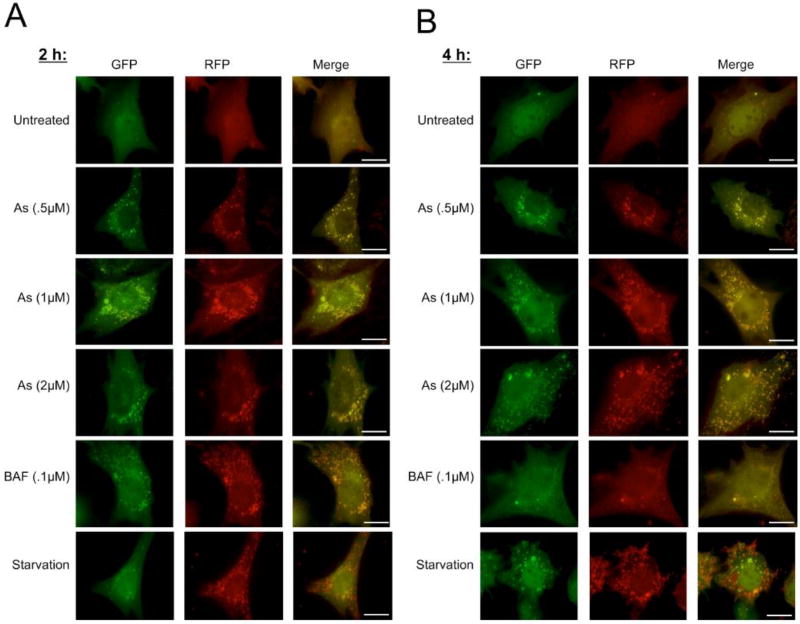
Another useful tool to ensure arsenite is inhibiting autophagic flux is the RFP-GFP-LC3 tandem fluorescent protein, which allows for the monitoring of the progression from autophagosome to autolysosome in live cells (Kimura et al., 2007). When autophagy is activated, LC3 localizes to the autophagosome membrane, where yellow puncta are observed due to the overlap of the RFP and GFP signals. Once fusion with the lysosome to form the autolysosome occurs, the acidic environment quenches the GFP signal, and only a red signal is observed, indicating formation of the autolysosome and completion of autophagy. To ensure that the observed increase in SQSTM1 and MAP1LC3A/B-II were a result of decreased autophagic flux, NIH 3T3 cells were transfected with RFP-GFP-LC3 for 24 h, and then left untreated (0 µM), or treated with sodium arsenite (0.25, 0.5, 1, or 2 µM) for 2 or 4 h prior to imaging. BAF (0.1 µM) and St were again used as positive controls for autophagy inhibition and activation, respectively. Autophagy levels in untreated cells were low, since few small puncta were observed, and autophagy flux was not compromised, since both yellow (autophagosomes) and red (autolysosomes) puncta were present (Figure 3). Sodium arsenite treatment increased the number and size of yellow puncta compared to the untreated group, and very few autolysosomes (red puncta) were observed at any time point or concentration. BAF treated cells also displayed yellow puncta, but these were smaller, and a few red puncta were also observed. In contrast, starved cells had predominantly red puncta, consistent with autophagy activation (Figure 3). These results indicate that sodium arsenite inhibits the late stage of autophagy rapidly, and at low concentrations.
Figure 3. RFP-GFP-LC3 imaging of sodium arsenite-mediated autophagy inhibition.
(A – B) NIH 3T3 cells were transfected with the RFP-GFP-LC3 construct for 24 h, then left untreated or treated with the indicated concentrations of sodium arsenite, BAF, or HBSS (St), for 2 h (A) or 4 h (B). Yellow puncta = autophagosomes, red puncta = autolysosomes. Scale bar = 10 µm.
Arsenite-induced autophagy inhibition causes a transient increase in ER stress
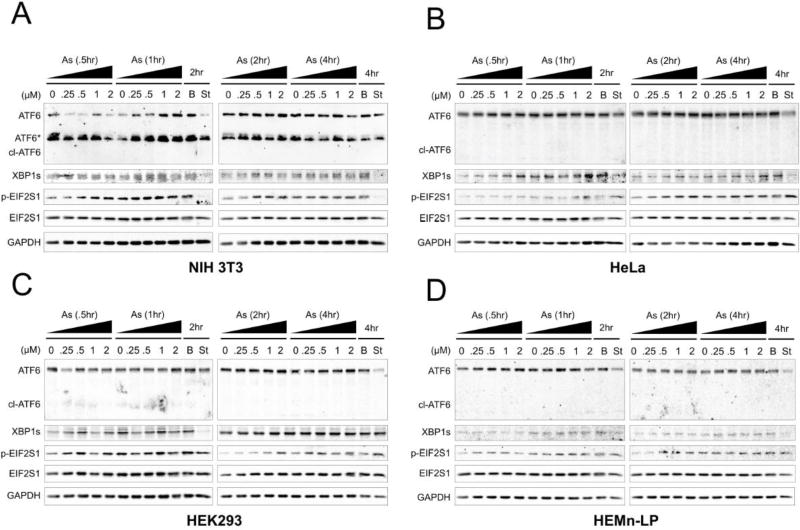
Since sodium arsenite has been shown to cause ER stress in certain cell types (Hou et al., 2013; Chen et al., 2015), we next analyzed the three major ER stress response pathways (ATF6, IRE1, and PERK) to determine if sodium arsenite blockage of autophagy was associated with increased ER stress. We treated NIH 3T3, HeLa, HEK 293, and HEMn-LP cells with sodium arsenite (0, 0.25, 0.5, 1, or 2 µM) for 0.5, 1, 2 or 4 h, and measured ATF6 cleavage, an indicator of activation of the ATF6 arm, XBP1s levels, an indicator of activation of the IRE1 arm, and phosphorylation of EIF2S1/eIF2α, an indicator of activation of the PERK arm, by immunoblot analysis (Figure 4). ATF6 cleavage was not observed in any of the arsenite treated cell lines (Figure 4A–D). Expression levels were very low for XBP1s in all four of the cell lines tested; however, arsenite did cause a slight concentration-dependent increase in XBP1s levels at 1 and 2 h in 3T3 cells (Figure 4A), 0.5, 1, 2, and 4 h in HeLa cells (Figure 4B), and 2 h in 293 cells (Figure 4C). HEMn-LP cells showed no significant changes in XBP1s levels at any time or concentration of sodium arsenite (Figure 4D). Phosphorylation of EIF2S1 also occurred in a concentration-dependent manner at 0.5, 1, and 2 h in NIH 3T3, HeLa, and HEMn-LP cells, and 0.5 and 2 h in 293 cells (Figure 4A–D). Thus, while sodium arsenite does not induce ROS, it does cause a slight increase in ER stress at early time points, typically returning to basal levels by 4 h.
Figure 4. Low-level sodium arsenite causes transient ER stress.
(A–D) Immunoblot analysis of changes in cleaved ATF6, XBP1s, phosphorylated EIF2S1 (p-EIF2S1), and total EIF2S1 protein levels following treatment with the indicated time points and concentrations of sodium arsenite in NIH 3T3 (A), HeLa (B), HEK 293 (C), or HEMn-LP (D) cells. Starvation (HBSS), or 0.1 µM BAF for 2 or 4 h were used as controls. GAPDH was used as an internal loading control.
Sodium arsenite blocks autophagy without causing significant cell death
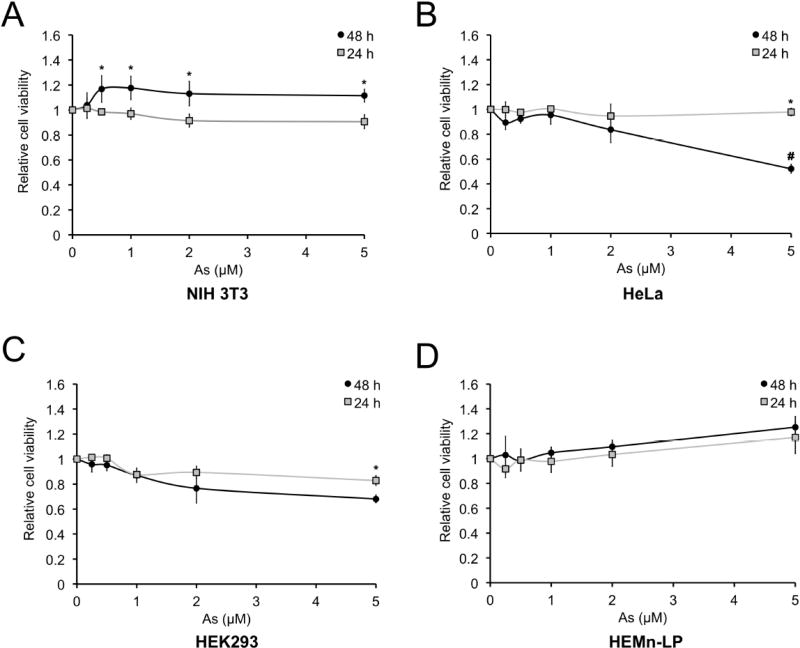
Since higher (micromolar) concentrations of sodium arsenite cause toxicity (Styblo et al., 2000), we tested the effects of low-level sodium arsenite on cell viability. We treated NIH 3T3, HeLa, HEK 293, and HEMn-LP cells with sodium arsenite (0, 0.25, 0.5, 1, 2, or 5 µM) for 24 and 48 h, and determined cell viability using an MTT assay (Figure 5). Minimal toxicity was observed across the different cell lines for 0.25–2 µM sodium arsenite. In NIH 3T3 and HEMn-LP cells, sodium arsenite slightly induced proliferation (Fig. 5A and D), consistent with previous reports (Chen et al., 2001; Graham-Evans et al., 2003). Only 5 µM sodium arsenite, a concentration 2.5–20X higher than our effective concentrations, caused ~40% cell death in HeLa cells and ~20% cell death in HEK 293 cells at 48 h (Figure 5B and C), indicating that acute, low-level exposure to sodium arsenite alters proteostasis without affecting ROS production or cell viability.
Figure 5. Low-level sodium arsenite does not cause cell death.
(A–D) MTT cell viability assay of NIH 3T3 (A), HeLa (B), HEK 293 (C), or HEMn-LP (D) cells treated with the indicated concentrations of sodium arsenite for 24 or 48 h. Data = mean ± SEM, n = 4. *p<0.05 compared to 24 h untreated control; #p<0.05 compared to 5 µM 24 h treatment. Two-way ANOVA.
Discussion
Due to the increased correlation between chronic arsenic exposure and the incidence of a number of diseases, it is important to determine the intracellular targets affected by arsenic at lower, more relevant concentrations. Furthermore, defining the pathways affected by arsenic during both the onset/early stages, as well as at the later stages of disease progression, will be integral in developing treatments to prevent or mitigate arsenic-linked disease progression. As mentioned above, arsenite and its metabolites have been shown to have a number of deleterious effects depending on the concentration, time of exposure, or tissue/cell type tested. Two major stresses that are initiated by both acute and chronic arsenic exposure are increased oxidative damage and proteotoxic stress. However, many of the studies demonstrating increased oxidative damage or enhanced proteotoxicity utilize concentrations of NaAsO2 or As2O3 in the >5 µM micromolar range (375–750 ppb As(III)). As such, determining the acute cellular response to low-level arsenite will greatly enhance our understanding of the early stages of disease progression.
Here, we show that low concentrations of arsenite do not cause oxidative stress at acute time points in NIH 3T3, HeLa, HEK 293, and HEMn-LP primary melanocytes, and that no significant ROS production was observed at 24, 48, or 72 h in low-level arsenite treated NIH 3T3 cells (Figure 1). However, low-level arsenite does inhibit autophagy (Figure 2 and 3), and initiates a transient ER stress response up to 4 h of exposure (Figure 4), without causing toxicity up to 48 h post-exposure (Figure 5). These results indicate that lower concentrations of arsenite at early time points do not increase oxidative stress, and instead can significantly alter proteostasis through autophagy inhibition and rapid, but manageable, initiation of ER stress response pathways.
Elucidating the mechanisms by which low concentrations of arsenic affect homeostasis at acute time points is of particular importance in designing therapeutics to treat arsenic-linked diseases. While increased oxidative damage is observed at higher concentrations or chronic treatments with arsenic, early time points are associated with ER stress and autophagy inhibition, without displaying any signs of increased ROS production. It will be interesting in future studies to determine how the balance between proteostasis and redox homeostasis is affected by repetitive exposure to environmentally relevant concentrations of arsenite. It is also important to note that autophagy and metabolism are intimately linked (Dodson et al., 2013), and that prolonged autophagic dysfunction could also contribute to changes in redox homeostasis and overall cellular metabolism during the progression of arsenic-linked pathologies. Therefore, therapeutic regimes designed to treat arsenic exposure in less to moderately affected areas, or early during exposure, should focus on restoring proper proteostasis, as opposed to preventing oxidative stress.
Highlights.
Acute, low-level arsenite does not induce oxidative stress
Low-level arsenite inhibits autophagy
Restoring autophagy may be a viable therapy to treat arsenic-linked disease
Acknowledgments
Funding:
This work was supported by the following grants from the National Institutes of Health: ES023758 (DDZ and EC), ES026845 (DDZ) and DK109555 (DDZ), and ES004940-28 (DDZ).
Abbreviations
- ATF6
activating transcription factor 6
- DCF
2',7'-dichlorofluorescein
- H2DCFDA
2',7'-dichlorodihydrofluorescein diacetate
- EIF2S1/eIF2α
eukaryotic initiation factor 2, subunit 1 alpha
- IRE-1
inositol-requiring enzyme 1
- LC3
microtubule-associated protein 1A/1B-light chain 3
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- PERK
eukaryotic translation initiation factor 2 alpha kinase 3
- ROS
reactive oxygen species
- SQSTM1/p62
sequestosome 1
- XBP1s
X-box-binding protein 1, spliced
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have no conflicts of interests to disclose
References
- Alarifi S, Ali D, Alkahtani S, Siddiqui MA, Ali BA. Arsenic trioxide-mediated oxidative stress and genotoxicity in human hepatocellular carcinoma cells. Onco Targets Ther. 2013;6:75–84. doi: 10.2147/OTT.S38227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew AS, Burgess JL, Meza MM, Demidenko E, Waugh MG, Hamilton JW, Karagas MR. Arsenic exposure is associated with decreased DNA repair in vitro and in individuals exposed to drinking water arsenic. Environ Health Perspect. 2006;114:1193–1198. doi: 10.1289/ehp.9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic Biol Med. 1999;27:1405–1412. doi: 10.1016/s0891-5849(99)00186-0. [DOI] [PubMed] [Google Scholar]
- Buschmann J, Berg M, Stengel C, Winkel L, Sampson ML, Trang PT, Viet PH. Contamination of drinking water resources in the Mekong delta floodplains: arsenic and other trace metals pose serious health risks to population. Environ Int. 2008;34:756–764. doi: 10.1016/j.envint.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Chang Q, Pan J, Wang X, Zhang Z, Chen F, Shi X. Reduced reactive oxygen species-generating capacity contributes to the enhanced cell growth of arsenic-transformed epithelial cells. Cancer Res. 2010;70:5127–5135. doi: 10.1158/0008-5472.CAN-10-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Gu S, Jiang X, Zhang Z. Arsenite-induced endoplasmic reticulum-dependent apoptosis through disturbance of calcium homeostasis in HBE cell line. Environ Toxicol. 2015 doi: 10.1002/tox.22226. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Zhao CQ, Diwan BA, Merrick BA, Waalkes MP. Association of c-myc overexpression and hyperproliferation with arsenite-induced malignant transformation. Toxicol Appl Pharmacol. 2001;175:260–268. doi: 10.1006/taap.2001.9253. [DOI] [PubMed] [Google Scholar]
- Cheng YY, Huang NC, Chang YT, Sung JM, Shen KH, Tsai CC, Guo HR. Associations between arsenic in drinking water and the progression of chronic kidney disease: A nationwide study in Taiwan. J Hazard Mater. 2017;321:432–439. doi: 10.1016/j.jhazmat.2016.09.032. [DOI] [PubMed] [Google Scholar]
- Chowdhury UK, Biswas BK, Chowdhury TR, Samanta G, Mandal BK, Basu GC, Chanda CR, Lodh D, Saha KC, Mukherjee SK, Roy S, Kabir S, Quamruzzaman Q, Chakraborti D. Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environ Health Perspect. 2000;108:393–397. doi: 10.1289/ehp.00108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M, Darley-Usmar V, Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic Biol Med. 2013;63:207–221. doi: 10.1016/j.freeradbiomed.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eblin KE, Bredfeldt TG, Buffington S, Gandolfi AJ. Mitogenic signal transduction caused by monomethylarsonous acid in human bladder cells: role in arsenic-induced carcinogenesis. Toxicol Sci. 2007;95:321–330. doi: 10.1093/toxsci/kfl160. [DOI] [PubMed] [Google Scholar]
- Graham-Evans B, Tchounwou PB, Cohly HH. Cytotoxicity and proliferation studies with arsenic in established human cell lines: keratinocytes, melanocytes, dendritic cells, dermal fibroblasts, microvascular endothelial cells, monocytes and T-cells. International Journal of Molecular Sciences. 2003;4:13–21. [Google Scholar]
- Harvey CF, Swartz CH, Badruzzaman AB, Keon-Blute N, Yu W, Ali MA, Jay J, Beckie R, Niedan V, Brabander D, Oates PM, Ashfaque KN, Islam S, Hemond HF, Ahmed MF. Arsenic mobility and groundwater extraction in Bangladesh. Science. 2002;298:1602–1606. doi: 10.1126/science.1076978. [DOI] [PubMed] [Google Scholar]
- Hou Y, Xue P, Woods CG, Wang X, Fu J, Yarborough K, Qu W, Zhang Q, Andersen ME, Pi J. Association between arsenic suppression of adipogenesis and induction of CHOP10 via the endoplasmic reticulum stress response. Environ Health Perspect. 2013;121:237–243. doi: 10.1289/ehp.1205731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011;123:305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Chen C, Zhao W, Zhang Z. Sodium arsenite and arsenic trioxide differently affect the oxidative stress, genotoxicity and apoptosis in A549 cells: an implication for the paradoxical mechanism. Environ Toxicol Pharmacol. 2013;36:891–902. doi: 10.1016/j.etap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Gossai A, Pierce B, Ahsan H. Drinking Water Arsenic Contamination, Skin Lesions, and Malignancies: A Systematic Review of the Global Evidence. Curr Environ Health Rep. 2015;2:52–68. doi: 10.1007/s40572-014-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- Kumar S, Yedjou CG, Tchounwou PB. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J Exp Clin Cancer Res. 2014;33:42. doi: 10.1186/1756-9966-33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A, Zheng Y, Tao S, Wang H, Whitman SA, White E, Zhang DD. Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62- dependent manner. Mol Cell Biol. 2013;33:2436–2446. doi: 10.1128/MCB.01748-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen F. Oxidative stress, epigenetics, and cancer stem cells in arsenic carcinogenesis and prevention. Curr Pharmacol Rep. 2016;2:57–63. doi: 10.1007/s40495-016-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SX, Davidson MM, Tang X, Walker WF, Athar M, Ivanov V, Hei TK. Mitochondrial damage mediates genotoxicity of arsenic in mammalian cells. Cancer Res. 2005;65:3236–3242. doi: 10.1158/0008-5472.CAN-05-0424. [DOI] [PubMed] [Google Scholar]
- Lu TH, Tseng TJ, Su CC, Tang FC, Yen CC, Liu YY, Yang CY, Wu CC, Chen KL, Hung DZ, Chen YW. Arsenic induces reactive oxygen species-caused neuronal cell apoptosis through JNK/ERK-mediated mitochondria-dependent and GRP 78/CHOP-regulated pathways. Toxicol Lett. 2014;224:130–140. doi: 10.1016/j.toxlet.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL. Arsenic exposure and the induction of human cancers. J Toxicol. 2011;2011:431287. doi: 10.1155/2011/431287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D. Evaluation of the Association between Arsenic and Diabetes: A National Toxicology Program Workshop Review. Environ Health Perspect. 2012 doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MA, Gonzalez-Horta C, Sanchez-Ramirez B, Ballinas-Casarrubias L, Ceron RH, Morales DV, Terrazas FA, Ishida MC, Gutierrez-Torres DS, Saunders RJ, Drobna Z, Fry RC, Buse JB, Loomis D, Garcia-Vargas GG, Del Razo LM, Styblo M. Chronic Exposure to Arsenic and Markers of Cardiometabolic Risk: A Cross-Sectional Study in Chihuahua, Mexico. Environ Health Perspect. 2016;124:104–111. doi: 10.1289/ehp.1408742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales KH, Ryan L, Kuo TL, Wu MM, Chen CJ. Risk of internal cancers from arsenic in drinking water. Environ Health Perspect. 2000;108:655–661. doi: 10.1289/ehp.00108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300:814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- Padmaja Divya S, Pratheeshkumar P, Son YO, Vinod Roy R, Andrew Hitron J, Kim D, Dai J, Wang L, Asha P, Huang B, Xu M, Luo J, Zhang Z. Arsenic Induces Insulin Resistance in Mouse Adipocytes and Myotubes Via Oxidative Stress-Regulated Mitochondrial Sirt3-FOXO3a Signaling Pathway. Toxicol Sci. 2015;146:290–300. doi: 10.1093/toxsci/kfv089. [DOI] [PubMed] [Google Scholar]
- Parvez F, Chen Y, Brandt-Rauf PW, Bernard A, Dumont X, Slavkovich V, Argos M, D'Armiento J, Foronjy R, Hasan MR, Eunus HE, Graziano JH, Ahsan H. Nonmalignant respiratory effects of chronic arsenic exposure from drinking water among never-smokers in Bangladesh. Environ Health Perspect. 2008;116:190–195. doi: 10.1289/ehp.9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash C, Soni M, Kumar V. Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: A review. J Appl Toxicol. 2016;36:179–188. doi: 10.1002/jat.3256. [DOI] [PubMed] [Google Scholar]
- Shen S, Li XF, Cullen WR, Weinfeld M, Le XC. Arsenic binding to proteins. Chem Rev. 2013;113:7769–7792. doi: 10.1021/cr300015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg TJ, Chen AS, Wang L. Arsenic species in drinking water wells in the USA with high arsenic concentrations. Water Res. 2014;48:156–169. doi: 10.1016/j.watres.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Ferreccio C, Yuan Y, Acevedo J, Gonzalez F, Perez L, Cortes S, Balmes JR, Liaw J, Smith AH. Elevated lung cancer in younger adults and low concentrations of arsenic in water. Am J Epidemiol. 2014;180:1082–1087. doi: 10.1093/aje/kwu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus CM, Ferreccio C, Romo JA, Yuan Y, Cortes S, Marshall G, Moore LE, Balmes JR, Liaw J, Golden T, Smith AH. Drinking water arsenic in northern chile: high cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomarkers Prev. 2013;22:623–630. doi: 10.1158/1055-9965.EPI-12-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Sun Y, Pi J, Wang X, Tokar EJ, Liu J, Waalkes MP. Aberrant cytokeratin expression during arsenic-induced acquired malignant phenotype in human HaCaT keratinocytes consistent with epidermal carcinogenesis. Toxicology. 2009;262:162–170. doi: 10.1016/j.tox.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol. 2001;176:127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- Tseng CH, Chong CK, Tseng CP, Hsueh YM, Chiou HY, Tseng CC, Chen CJ. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicol Lett. 2003;137:15–21. doi: 10.1016/s0378-4274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- Wang S, Mulligan CN. Occurrence of arsenic contamination in Canada: sources, behavior and distribution. Sci Total Environ. 2006;366:701–721. doi: 10.1016/j.scitotenv.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Yen YP, Tsai KS, Chen YW, Huang CF, Yang RS, Liu SH. Arsenic induces apoptosis in myoblasts through a reactive oxygen species-induced endoplasmic reticulum stress and mitochondrial dysfunction pathway. Arch Toxicol. 2012;86:923–933. doi: 10.1007/s00204-012-0864-9. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yamauchi H, Fan Sun G. Chronic health effects in people exposed to arsenic via the drinking water: dose-response relationships in review. Toxicol Appl Pharmacol. 2004;198:243–252. doi: 10.1016/j.taap.2003.10.022. [DOI] [PubMed] [Google Scholar]