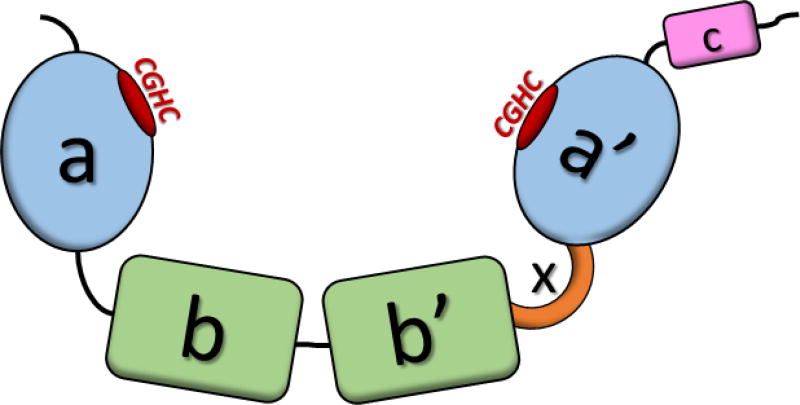Abstract
The mechanisms underlying the hypercoagulability of cancer are complex and include the upregulation coagulation factors or procoagulant proteins, shedding of microparticles, and direct activation of vascular cells. Protein disulfide isomerase (PDI) is a thiol isomerase secreted from activated platelets and endothelial cells and plays a critical role in both platelet aggregation and fibrin generation. A number of potential intravascular targets of PDI have been identified including cell surface receptors (e.g. β-integrins and glycoprotein Ib), receptor ligands (e.g. fibrinogen and von Willebrand factor), serine proteases (e.g. cathepsin G and kallekrein-14), and coagulation factors (e.g. factor XI and factor V). Recent clinical studies demonstrated that a small molecule inhibitor of PDI, isoquercetin, decreases platelet-dependent thrombin generation and PDI activity in plasma following oral administration. This review explores the mechanistic overlap between the molecular drivers of cancer associated thrombosis and the potential roles PDI plays in mediating thrombosis. These molecular insights provide rationale for clinical trials targeting PDI to prevent thrombosis in cancer patients.
Introduction
Cancer is associated with an increase in both venous and arterial thrombosis (1–4). Proposed mechanisms underlying the hypercoagulability of cancer include modulation of coagulation factor activity, increased adhesion of platelets, and elaboration of prothrombotic proteins or microparticles. Protein disulfide isomerase (PDI) is a thiol isomerase secreted by platelets and endothelial cells and plays a critical role in thrombus formation in vivo (5–7). A number of extracellular substrates of PDI have been identified (8–16). The mechanisms underlying the regulatory role of PDI in thrombus formation appear to intersect with the dysregulated thrombotic pathways of the malignant state. Clinical studies are underway to determine whether targeting extracellular PDI will prevent thrombosis in advanced cancer populations.
PDI and thrombus formation
PDI is the most critical, and most extensively studied, thiol isomerase (17–26). PDI is the head of the PDI superfamily, which currently includes 21 different thiol isomerases, all of which contain at least one thioredoxin-like domain (17–19, 25, 26). PDI is a 57 kD protein, structured in an a-b-b’-x-a’-c confirmation, displayed in Figure 1. PDI contains two active sites in the a and a’ domains, and two protein-substrate binding sites in the b and b’ domains (19, 26). The x linker region serves allows flexibility of the a’ domain, motion which is critical for catalysis (27, 28). Both active site domains consist of the classical Cys-X-X-Cys motif, and the intervening sequences vary between the family members (17, 18, 20). In PDI, both active sites have a Cys-Gly-His-Cys motif, which is common among several thiol isomerases expressed in humans (17).
Figure 1. Schematic Representation of Protein Disulfide Isomerase.
The a-b-b’-x-a’-c structure of PDI, with the CGHC active sites displayed in red.
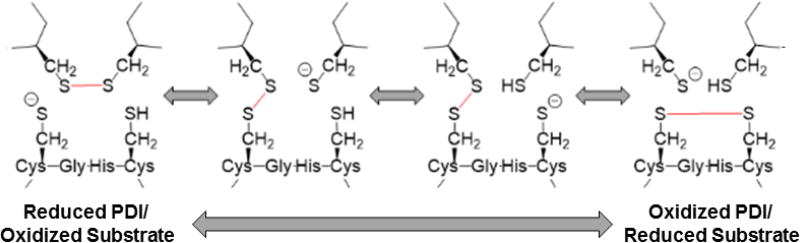
PDI acts as a necessary protein folding catalyst, with classic chaperone activity toward nascent peptides (20, 21, 29). The removal of PDI at the transcriptional or translational levels is lethal because of its importance in protein folding in the endoplasmic reticulum (ER) (21). While PDI is crucial for protein folding, it also is capable of carrying out multiple enzymatic functions. PDI can also serve as a thiol oxidoreductase and isomerase, and is capable of further post-translational cysteine modifications such as S-nitrosylation or S-glutathionylation (20, 22, 29–31). For oxidoreductase activity, the CXXC motif will cycle between a reduced state containing two free thiols (-SH), and an oxidized state in which both cysteines are linked via a disulfide bond (S-S) as shown in Figure 2. During reduction, the N-terminal cysteine (N-Cys) acts as the nucleophile to begin reducing a disulfide bond on another protein (22, 23). This is possible because the local pKa of the active site causes the N-Cys to be a stable thiolate anion (S-), instead of a true free thiol (-SH). The disulfide-linked PDI-substrate complex shuffles its electrons among the four cysteine residues, until the C-terminal free thiol of PDI (C-Cys) forms a disulfide bond with its active site partner. This results in an effective disulfide bond transfer between substrate and PDI. The oxidation reaction takes place similarly but in reverse, effectively transferring a disulfide bond from PDI to a substrate protein which requires oxidation (22, 23, 29).
Figure 2. Reduction/Oxidation Mechanism of Protein Disulfide Isomerase.
Shown is a reaction scheme diagramming the PDI active site transitioning between a reduced (left) and oxidized (right) state performing disulfide bond reduction (left to right) or oxidation (right to left) on a protein substrate. PDI active site (lower) and protein substrate (upper) are shown. Modified from (8)
The activities of PDI are known to extend beyond the ER as PDI is required for thrombus formation in vivo. Following a laser-induced vascular injury in an mouse model of thrombosis, PDI will accumulate rapidly at the site of injury in a growing thrombus (7). The enzymatic activity of PDI, and not just its localization, is essential, because blocking PDI activity with inhibitory antibody (RL90) or small molecule inhibitors like bacitracin, diminishes platelet accumulation and fibrin formation (7, 15, 32). PDI is released from platelets, leukocytes, and endothelial cells upon activation and is capable of “binding-back” to the extracellular membrane through β3 integrin complexes such as αIIbβ3 on platelets and αVβ3 on endothelial cells (14). On the endothelium, PDI also associates with the β1 integrin (33). While the exact mechanism of PDI action is still controversial, multiple studies have shown interaction between a myriad of proteins known to be important for blood cell activation and thrombosis (8, 10, 11, 34, 35). Several other thiol isomerase family members such as ERp5, ERp57, and ERp72 have also been shown to affect thrombus formation when inhibited (5, 6)
To identify proteins substrates of PDI or other thiol isomerases, a technique called kinetic substrate trapping has proven useful (8, 11, 24, 25). The majority of these studies utilized PDI variants which lack the second active site cysteine (CGHA) which forms stable covalent disulfide-linked intermediates between PDI and substrate proteins to enable isolation and identification via mass spectroscopy. One limitation of this approach is the requirement for substrates to undergo PDI-mediated disulfide reduction and not oxidation or rearrangement. Our group recently developed PDI variants capable of performing kinetic substrate trapping in both the oxidation and reduction directions (8). These PDI variants with amino acid substitutions in the intervening sequences between the cysteines, alter the pKa and spacial geometry of the active site to slow catalysis enough to trap the disulfide-linked intermediate complexes. This study identified several proteins as PDI substrates, from multiple classes of proteins involved with thrombosis, including coagulation factors, extracellular scaffolds, activating proteases, and cell surface receptors.
The binding substrates of extracellular PDI and how PDI regulates thrombus formation are not clearly defined. PDI binds to cell surface receptors such β-integrins, annexin V, and GP1β (8, 35) as well as cell surface receptor protein ligands such as fibrinogen, fibrin, and collagen VI (8, 36, 37). Previous studies identified von Willebrand’s factor and thrombospondin-1 as substrates for thiol isomerases (10, 12, 38). Cell surface receptors can be activated via binding, but also through cleavage by proteases; kallekrein-14 and cathepsin G are PDI substrates and are known to cleave and activate members of the PAR receptor family (8, 39, 40). PDI can modulate phosphatidylserine (PS) exposure (16, 41) and may regulate coagulation through disulfide regulation of tissue factor, activation of factor XI, maturation of platelet factor V, and attenuating factor XIII cross-linking activity toward fibrin (8, 9, 13, 42, 43). More globally, PDI is believed to sense and regulate the redox state of the extracellular membrane, by interacting with small molecule effectors like glutathione and nitric oxide (NO), and modulating the redox state of other extracellular thiol isomerases (5, 6, 44). Recently identified was glutaredoxin-1, a regulator of NO synthetase, and also the other thiol isomerases thioredoxin and ERp57 (8, 45).
Hypercoagulability of cancer and potential interface with PDI
Certain coagulation factors and other procoagulant proteins are upregulated in expression and secretion in cancer. For example, many cancers are capable of constitutively expressing tissue factor (TF) at an elevated level (46, 47). Tissue factor initiates the extrinsic pathway of blood coagulation forming a complex with activated factor VII (VIIa), which then activates factor X. Tumor cells not only express TF on their cell surface but also generate circulating microparticles which express TF (47, 48). These tissue factor-bearing microparticles are associated with a heightened risk of venous thrombeombolism (49, 50). The regulation of tissue factor activation is complex and incompletely understood (42, 51, 41, 52). The reduction of an allosteric disulfide bond converting “cryptic” TF to the active form may serve as a key regulatory step (51). This disulfide bond reduction may be mediated by PDI, which is also overexpressed and secreted by cancer cells (34, 44), but this hypothesis has not been proven and remains controversial (51, 53, 54). PDI can alter TF activity indirectly through PS exposure (16, 41, 55) or through the enzyme heparanase which increases the procoagulant activity of tissue factor by preventing the binding of tissue factor pathway inhibitor (TFPI) (52, 56). Heparanase requires disulfide bond oxidation for activation and was identified as a potential substrate for extracellular PDI (8, 57). Taken together, elevated levels of tissue factor and PDI in cancer may lead to hypercoagulability of cancer through increased TF activity and down-regulation of TFPI through heparanase activity.
Tumor cells also exert their procoagulant state by interacting with vascular cells such as endothelial cells and platelets through direct activation (46, 58–60). These platelet-tumor cell interactions are mediated through multiple receptors, most notably the P-selectin receptor and β3 integrin (3, 58, 60, 61). Interaction with either of these receptors leads to platelet activation which can enhance the metastatic potential of the tumor through shielding from an anti-tumor immune response (59, 60, 62). Several cell surface receptors, receptor agonist proteases, and large scaffolding proteins which bind to these receptors across multiple cells and strengthen the thrombus have all been identified as PDI substrates (9, 12, 14, 33, 35, 38, 43).
Cancer cells elaborate growth factors and cytokines to promote tumor growth and aggressiveness (46, 60, 63). These growth factors will activate other cells to begin to remodel the extracellular matrix. In cancer, this increase in metalloproteinases and circulating growth factors leads to extracellular remodeling but also to vascular permeability and metastasis (64). One of the more important metalloproteinases is ADAM17, which is responsible for the cleavage/shedding of multiple EGFR ligands and TNF-α, has been shown to be regulated by extracellular PDI (65). Following disulfide isomerization catalyzed by PDI, ADAM17 is able to adopt an active conformation facilitating tumor growth and metastasis thus potentially contributing to the hypercoagulable state caused by interaction of tumors and platelets.
Targeting PDI with quercetin flavonoids
In light of the central role PDI plays in thrombosis, our group and others have investigated targeting PDI as an antithrombotic therapeutic (32, 64, 66–68). In a screen of compounds for PDI inhibitors, the bioactive small molecule quercetin-3-rutinoside (rutin) was identified (32). Rutin is a member of the quercetin family of compounds, which are naturally occurring in many fruits and vegetables. Epidemiologic studies have shown that populations with an increased amount of quercetin-rich foods in their diets show a decreased prevalence of thrombosis-related death, such as stroke (69). Rutin, and other similarly structured quercetin family members with a glycosyl moiety at the 3-position, inhibit PDI with a half maximal inhibitory concentration (IC50) in the micromolar range (32). Rutin binds to PDI in the x-linker region between the b’ and a’ domains, preventing any flexibility between the two domains (27, 28). This “locking” of PDI inhibits catalysis, and the binding to the x-linker region gives these quercetins increased specificity to PDI over the other thiol isomerase family members. In a laser-injury model of mouse thrombosis, rutin administered either intravenously or orally decreased thrombus formation in a dose-dependent manner (32). Notably, in a tail bleeding model, rutin did not affect the bleeding time indicating that PDI may be required for thrombosis but not hemostasis (6, 32, 70).
Quercetin-3-glucoside (isoquercetin) has better bioavailability than rutin when administered orally (71) and inhibits PDI in vitro with the same low micromolar IC50 affinity as rutin (32). To evaluate whether isoquercetin could be a viable antithrombotic in humans, we conducted a pharmacokinetic-pharmacodynamic study in healthy volunteers. The results are summarized in Table 1. Quercetin aglycone (500 mg), which lacks the necessary glycosyl group did not inhibit PDI. Notably, significant PDI inhibition was only observed in the volunteers which received 1000 mg of isoquercetin two hours after ingestion, whereas the quercetin aglycone or isoquercetin at 500 mg did not inhibit PDI relative to the pre-ingestion sample.
Table 1.
Pharmacokinetics and PDI inhibition following ingestion of quercetin flavonoids (9)
| Quercetin Flavonoid | Median Peak Conc. (µM) | AUC (µM hr/l) | PDI Inhibition (%) | Thrombin Gen. (%) |
|---|---|---|---|---|
| Quercetin Aglycone (500 mg) | 0.8 | 3.8 | N.S. | N.D. |
| Isoquercetin (500 mg) | 4.22 | 18.3 | N.S. | N.S. |
| Isoquercetin (1000 mg) | 9.2 | 39.4 | 38% (p = 0.03) | 51% (p = 0.0004) |
N.S. = Not Significant (P>0.05)
N.D. = Not Determined
Platelet-dependent thrombin generation, where thrombin generation is propagated through the activation of platelets, is increased in hypercoagulable states (72, 73). Treatment with isoquercetin (1000 mg) decreased the platelet-dependent thrombin generation after isoquercetin ingestion by 51% compared to the pre-ingestion time zero sample. The observed effect on platelet-dependent thrombin generation was reversed with the addition of exogenous PDI, indicating that the effect of isoquercetin is a PDI-dependent process. Isoquercetin also did not affect coagulation times, thrombin activity, prothrombin cleavage, or Xa activity using isoquercetin-spiked plasma at the peak concentration observed in the volunteer samples.
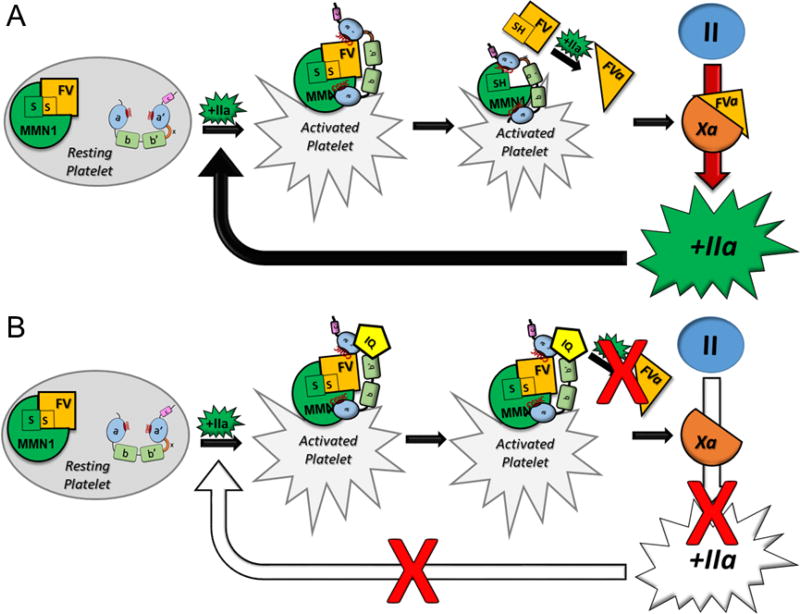
Platelet factor V was identified as a potential substrate of PDI, and factor V is a necessary cofactor in the prothrombinase complex, where factors Xa and Va convert prothrombin to thrombin (55, 74). Platelet factor V is associated with multimerin-1 in the platelet alpha-granule, where they are linked through disulfide interactions (75). This interaction is thought to inhibit platelet factor V from being activated by thrombin, and the complex must be dissolved before full factor V activation is possible (76). Multimerin-1 was also identified as a PDI substrate using the same kinetic-substrate trapping technique, and like PDI, multimerin-1 also binds back to β3 integrins on the cell surface (8, 9, 14, 77). We hypothesize that PDI and the multimerin-factor V complex are released during platelet activation and bind back on the cell surface via interaction with β3 integrins thus permitting PDI to release the disulfide linkage between multimerin-1 and factor V to provide additional factor V for thrombin generation (Figure 3).
Figure 3. Inhibition of PDI with isoquercetin decreases thrombin-induced thrombin generation through FVa.
Diagram of the proposed role of PDI in the activation of platelet factor V and the downstream generation of thrombin in the absence (Panel A) and presence (Panel B) of isoquercetin (9) MMN1: Multimerin-1; FV: Platelet Factor V; IIa: Thrombin; FVa: Factor Va; Xa: Factor Xa; II: Prothrombin; IQ: Isoquercetin; a-b-b’-a’: PDI.
To test this hypothesis, factor Va levels were measured from washed platelets activated in the presence of increasing concentrations of isoquercetin (9). As isoquercetin concentration increased, factor Va levels decreased, correlating with levels of PDI inhibition and platelet-dependent thrombin generation. Factor Va levels were also measured from platelets in samples before and after oral administration of isoquercetin (1000 mg). Plasma samples from both time points were obtained and immunodepleted of plasma factor V. Platelet rich plasma was then reconstituted with healthy donor platelets, using both the FV-depleted and FV-replete plasma samples, and FVa levels were measured after activation. While isoquercetin inhibited factor Va generation in the FV-replete samples (26% reduction), the decrease in levels was enhanced in the FV-depleted samples (55% reduction), indicating a greater effect of isoquercetin on factor Va generation from platelets. The addition of factor Va was able to fully restore thrombin generation after isoquercetin treatment. In summary, these data indicate that the inhibition of PDI with isoquercetin decreases platelet-dependent thrombin generation by decreasing the amount of available factor Va generated from platelets. Platelet factor V accounts for approximately 20% of the total factor V in circulation, but the absence of plasma factor V does not significantly reduce endogenous thrombin potential (78, 79). In contrast, patients which lack platelet factor V have a higher risk of bleeding, suggesting platelet factor V plays a more critical role in supporting hemostasis (80).
Clinical trial of isoquercetin to prevent venous thromboembolism in cancer
Due to the fortuitous discovery that the small molecule flavonoid, isoquercetin, inhibited PDI activity and thrombus formation in animal models, we are able to transition to later stage clinical trials in humans. In light of the potential intersection between PDI and cancer associated thrombosis, we initiated a multi-center clinical trial (CATIQ) to evaluate the potential efficacy of isoquercetin to prevent thrombosis in advanced cancer patients (NCT02195232). Eligible patients are required to have locally advanced or metastatic adenocarcinoma of the pancreas, metastatic colorectal or unresectable or metastatic non-small lung cancer. As the greatest risk for venous thromboembolism is the within the first few months following the initiation of chemotherapy (1), we limited enrollment only to those patients newly initiated first or second line chemotherapy. All patients are monitored for the development of venous thromboembolism that includes a bilaterally lower extremity duplex ultrasound at two months. These inclusion and exclusion criteria are similar to our prior phase II thromboprophylaxis study where the observed rate of venous thromboembolism in the non-anticoagulated arm exceeded 25% at two months (49). We anticipate completion of the phase II study in 2018.
Summary
The mechanisms contributing to the hypercoagulability of cancer are multi-faceted. PDI has been shown to play a key role in thrombosis and recent advances in our understanding of the extracellular substrates of PDI suggests considerable mechanistic overlap with the molecular drivers of cancer associated thrombosis. Clinical trials with anti-PDI small molecules are currently underway which may lead to novel therapeutics for the prevention and treatment of thrombosis in cancer patients.
Acknowledgments
Funding: NHLBI U54 HL112302 (JIZ); NHLBI T32 HL007917 (J.S.)
Conflicts: JIZ has received research support from Quercegen Pharma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648–655. doi: 10.1002/cncr.27772. [DOI] [PubMed] [Google Scholar]
- 2.Di Nisio M, Ferrante N, Feragalli B, De Tursi M, Iacobelli S, Cuccurullo F, Porreca E. Arterial thrombosis in ambulatory cancer patients treated with chemotherapy. Thromb. Res. 2011;127:382–383. doi: 10.1016/j.thromres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood. 2017;130:1499–1506. doi: 10.1182/blood-2017-03-743211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blom JW, Vanderschoot JPM, Oostindiër MJ, Osanto S, van der Meer FJM, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J. Thromb. Haemost. JTH. 2006;4:529–535. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 5.Flaumenhaft R, Furie B. Vascular thiol isomerases. Blood. 2016;128:893–901. doi: 10.1182/blood-2016-04-636456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulman S, Bendapudi P, Sharda A, Chen V, Bellido-Martin L, Jasuja R, Furie BC, Flaumenhaft R, Furie B. Extracellular Thiol Isomerases and Their Role in Thrombus Formation. Antioxid. Redox Signal. 2016;24:1–15. doi: 10.1089/ars.2015.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J. Clin. Invest. 2008;118:1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stopa JD, Baker KM, Grover SP, Flaumenhaft R, Furie B. Kinetic-based trapping by intervening sequence variants of the active sites of protein-disulfide isomerase identifies platelet protein substrates. J. Biol. Chem. 2017;292:9063–9074. doi: 10.1074/jbc.M116.771832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stopa JD, Neuberg D, Puligandla M, Furie B, Flaumenhaft R, Zwicker JI. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI Insight. 2017;2:e89373. doi: 10.1172/jci.insight.89373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essex DW, Miller A, Swiatkowska M, Feinman RD. Protein disulfide isomerase catalyzes the formation of disulfide-linked complexes of vitronectin with thrombin-antithrombin. Biochemistry (Mosc.) 1999;38:10398–10405. doi: 10.1021/bi990694s. [DOI] [PubMed] [Google Scholar]
- 11.Bowley SR, Fang C, Merrill-Skoloff G, Furie BC, Furie B. Protein disulfide isomerase secretion following vascular injury initiates a regulatory pathway for thrombus formation. Nat. Commun. 2017;8:14151. doi: 10.1038/ncomms14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippok S, Kolšek K, Löf A, Eggert D, Vanderlinden W, Müller JP, König G, Obser T, Röhrs K, Schneppenheim S, Budde U, Baldauf C, Aponte-Santamaría C, Gräter F, Schneppenheim R, Rädler JO, Brehm MA. von Willebrand factor is dimerized by protein disulfide isomerase. Blood. 2016;127:1183–1191. doi: 10.1182/blood-2015-04-641902. [DOI] [PubMed] [Google Scholar]
- 13.Zucker M, Seligsohn U, Yeheskel A, Mor-Cohen R. An allosteric disulfide bond is involved in enhanced activation of factor XI by protein disulfide isomerase. J. Thromb. Haemost. JTH. 2016;14:2202–2211. doi: 10.1111/jth.13488. [DOI] [PubMed] [Google Scholar]
- 14.Cho J, Kennedy DR, Lin L, Huang M, Merrill-Skoloff G, Furie BC, Furie B. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of β3 integrins. Blood. 2012;120:647–655. doi: 10.1182/blood-2011-08-372532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinhardt C, von Brühl M-L, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J. Clin. Invest. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurk K, Lahav J, VAN Aken H, Brodde MF, Nofer J-R, Kehrel BE. Extracellular protein disulfide isomerase regulates feedback activation of platelet thrombin generation via modulation of coagulation factor binding. J. Thromb. Haemost. JTH. 2011;9:2278–2290. doi: 10.1111/j.1538-7836.2011.04509.x. [DOI] [PubMed] [Google Scholar]
- 17.Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appenzeller-Herzog C, Ellgaard L. The human PDI family: versatility packed into a single fold. Biochim. Biophys. Acta. 2008;1783:535–548. doi: 10.1016/j.bbamcr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Darby NJ, Kemmink J, Creighton TE. Identifying and characterizing a structural domain of protein disulfide isomerase. Biochemistry (Mosc.) 1996;35:10517–10528. doi: 10.1021/bi960763s. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim. Biophys. Acta. 2004;1699:35–44. doi: 10.1016/j.bbapap.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Laboissiere MC, Sturley SL, Raines RT. The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J. Biol. Chem. 1995;270:28006–28009. doi: 10.1074/jbc.270.47.28006. [DOI] [PubMed] [Google Scholar]
- 22.Darby NJ, Freedman RB, Creighton TE. Dissecting the mechanism of protein disulfide isomerase: catalysis of disulfide bond formation in a model peptide. Biochemistry (Mosc.) 1994;33:7937–7947. doi: 10.1021/bi00191a022. [DOI] [PubMed] [Google Scholar]
- 23.Walker KW, Lyles MM, Gilbert HF. Catalysis of oxidative protein folding by mutants of protein disulfide isomerase with a single active-site cysteine. Biochemistry (Mosc.) 1996;35:1972–1980. doi: 10.1021/bi952157n. [DOI] [PubMed] [Google Scholar]
- 24.Walker KW, Gilbert HF. Oxidation of kinetically trapped thiols by protein disulfide isomerase. Biochemistry (Mosc.) 1995;34:13642–13650. doi: 10.1021/bi00041a045. [DOI] [PubMed] [Google Scholar]
- 25.Darby NJ, Creighton TE. Characterization of the active site cysteine residues of the thioredoxin-like domains of protein disulfide isomerase. Biochemistry (Mosc.) 1995;34:16770–16780. doi: 10.1021/bi00051a027. [DOI] [PubMed] [Google Scholar]
- 26.Darby NJ, Penka E, Vincentelli R. The multi-domain structure of protein disulfide isomerase is essential for high catalytic efficiency. J. Mol. Biol. 1998;276:239–247. doi: 10.1006/jmbi.1997.1504. [DOI] [PubMed] [Google Scholar]
- 27.Bekendam RH, Bendapudi PK, Lin L, Nag PP, Pu J, Kennedy DR, Feldenzer A, Chiu J, Cook KM, Furie B, Huang M, Hogg PJ, Flaumenhaft R. A substrate-driven allosteric switch that enhances PDI catalytic activity. Nat. Commun. 2016;7:12579. doi: 10.1038/ncomms12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin L, Gopal S, Sharda A, Passam F, Bowley SR, Stopa J, Xue G, Yuan C, Furie BC, Flaumenhaft R, Huang M, Furie B. Quercetin-3-rutinoside Inhibits Protein Disulfide Isomerase by Binding to Its b’x Domain. J. Biol. Chem. 2015;290:23543–23552. doi: 10.1074/jbc.M115.666180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert HF. Protein disulfide isomerase. Methods Enzymol. 1998;290:26–50. doi: 10.1016/s0076-6879(98)90005-2. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Zhang X, Li C, Guan T, Shang H, Cui L, Li X-M, Kong J. S-nitrosylated protein disulfide isomerase contributes to mutant SOD1 aggregates in amyotrophic lateral sclerosis. J. Neurochem. 2013;124:45–58. doi: 10.1111/jnc.12046. [DOI] [PubMed] [Google Scholar]
- 31.Townsend DM, Manevich Y, He L, Xiong Y, Bowers RR, Hutchens S, Tew KD. Nitrosative stress-induced s-glutathionylation of protein disulfide isomerase leads to activation of the unfolded protein response. Cancer Res. 2009;69:7626–7634. doi: 10.1158/0008-5472.CAN-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jasuja R, Passam FH, Kennedy DR, Kim SH, van Hessem L, Lin L, Bowley SR, Joshi SS, Dilks JR, Furie B, Furie BC, Flaumenhaft R. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J. Clin. Invest. 2012;122:2104–2113. doi: 10.1172/JCI61228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgess JK, Hotchkiss KA, Suter C, Dudman NP, Szöllösi J, Chesterman CN, Chong BH, Hogg PJ. Physical proximity and functional association of glycoprotein 1balpha and protein-disulfide isomerase on the platelet plasma membrane. J. Biol. Chem. 2000;275:9758–9766. doi: 10.1074/jbc.275.13.9758. [DOI] [PubMed] [Google Scholar]
- 34.Täger M, Kröning H, Thiel U, Ansorge S. Membrane-bound proteindisulfide isomerase (PDI) is involved in regulation of surface expression of thiols and drug sensitivity of B-CLL cells. Exp. Hematol. 1997;25:601–607. [PubMed] [Google Scholar]
- 35.Chiu J, Passam F, Butera D, Hogg PJ. Protein Disulfide Isomerase in Thrombosis. Semin. Thromb. Hemost. 2015;41:765–773. doi: 10.1055/s-0035-1564047. [DOI] [PubMed] [Google Scholar]
- 36.Weisel JW, Litvinov RI. Fibrin Formation, Structure and Properties. Subcell. Biochem. 2017;82:405–456. doi: 10.1007/978-3-319-49674-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manon-Jensen T, Kjeld NG, Karsdal MA. Collagen-mediated hemostasis. J. Thromb. Haemost. JTH. 2016;14:438–448. doi: 10.1111/jth.13249. [DOI] [PubMed] [Google Scholar]
- 38.Milev Y, Essex DW. Protein disulfide isomerase catalyzes the formation of disulfide-linked complexes of thrombospondin-1 with thrombin-antithrombin III. Arch. Biochem. Biophys. 1999;361:120–126. doi: 10.1006/abbi.1998.0963. [DOI] [PubMed] [Google Scholar]
- 39.Oikonomopoulou K, Hansen KK, Saifeddine M, Tea I, Blaber M, Blaber SI, Scarisbrick I, Andrade-Gordon P, Cottrell GS, Bunnett NW, Diamandis EP, Hollenberg MD. Proteinase-activated receptors, targets for kallikrein signaling. J. Biol. Chem. 2006;281:32095–32112. doi: 10.1074/jbc.M513138200. [DOI] [PubMed] [Google Scholar]
- 40.Sambrano GR, Huang W, Faruqi T, Mahrus S, Craik C, Coughlin SR. Cathepsin G activates protease-activated receptor-4 in human platelets. J. Biol. Chem. 2000;275:6819–6823. doi: 10.1074/jbc.275.10.6819. [DOI] [PubMed] [Google Scholar]
- 41.Langer F, Ruf W. Synergies of phosphatidylserine and protein disulfide isomerase in tissue factor activation. Thromb. Haemost. 2014;111:590–597. doi: 10.1160/TH13-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Versteeg HH, Ruf W. Thiol pathways in the regulation of tissue factor prothrombotic activity. Curr. Opin. Hematol. 2011;18:343–348. doi: 10.1097/MOH.0b013e32834981de. [DOI] [PubMed] [Google Scholar]
- 43.Lahav J, Tvito A, Bagoly Z, Dardik R, Inbal A. Factor XIII improves platelet adhesion to fibrinogen by protein disulfide isomerase-mediated activity. Thromb. Res. 2013;131:338–341. doi: 10.1016/j.thromres.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Ceccarelli J, Delfino L, Zappia E, Castellani P, Borghi M, Ferrini S, Tosetti F, Rubartelli A. The redox state of the lung cancer microenvironment depends on the levels of thioredoxin expressed by tumor cells and affects tumor progression and response to prooxidants. Int. J. Cancer. 2008;123:1770–1778. doi: 10.1002/ijc.23709. [DOI] [PubMed] [Google Scholar]
- 45.Chen C-A, De Pascali F, Basye A, Hemann C, Zweier JL. Redox Modulation of eNOS by Glutaredoxin-1 through Reversible Oxidative Post-translational Modification. Biochemistry (Mosc.) 2013 doi: 10.1021/bi400404s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falanga A, Russo L, Milesi V, Vignoli A. Mechanisms and risk factors of thrombosis in cancer. Crit. Rev. Oncol. Hematol. 2017;118:79–83. doi: 10.1016/j.critrevonc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit. Rev. Oncol. Hematol. 2007;62:126–136. doi: 10.1016/j.critrevonc.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Zwicker JI. Tissue factor-bearing microparticles and cancer. Semin. Thromb. Hemost. 2008;34:195–198. doi: 10.1055/s-2008-1079260. [DOI] [PubMed] [Google Scholar]
- 49.Zwicker JI, Liebman HA, Bauer KA, Caughey T, Campigotto F, Rosovsky R, Mantha S, Kessler CM, Eneman J, Raghavan V, Lenz H-J, Bullock A, Buchbinder E, Neuberg D, Furie B. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: a randomized-controlled phase II trial (the Microtec study) Br. J. Haematol. 2013;160:530–537. doi: 10.1111/bjh.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:6830–6840. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry (Mosc.) 2006;45:12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 52.Nadir Y. Heparanase and coagulation-new insights. Rambam Maimonides Med. J. 2014;5:e0031. doi: 10.5041/RMMJ.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kothari H, Nayak RC, Rao LVM, Pendurthi UR. Cystine 186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption. Blood. 2010;115:4273–4283. doi: 10.1182/blood-2009-09-241356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J. Biol. Chem. 2007;282:25416–25424. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 55.Mann KG, Nesheim ME, Hibbard LS, Tracy PB. The role of factor V in the assembly of the prothrombinase complex. Ann. N. Y. Acad. Sci. 1981;370:378–388. doi: 10.1111/j.1749-6632.1981.tb29750.x. [DOI] [PubMed] [Google Scholar]
- 56.Nadir Y, Brenner B. Heparanase procoagulant activity. Thromb. Res. 2012;129(Suppl 1):S76–79. doi: 10.1016/S0049-3848(12)70021-X. [DOI] [PubMed] [Google Scholar]
- 57.Simizu S, Suzuki T, Muroi M, Lai NS, Takagi S, Dohmae N, Osada H. Involvement of disulfide bond formation in the activation of heparanase. Cancer Res. 2007;67:7841–7849. doi: 10.1158/0008-5472.CAN-07-1053. [DOI] [PubMed] [Google Scholar]
- 58.Stegner D, Dütting S, Nieswandt B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb. Res. 2014;133(Suppl 2):S149–157. doi: 10.1016/S0049-3848(14)50025-4. [DOI] [PubMed] [Google Scholar]
- 59.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 60.Meikle CKS, Kelly CA, Garg P, Wuescher LM, Ali RA, Worth RG. Cancer and Thrombosis: The Platelet Perspective. Front. Cell Dev. Biol. 2016;4:147. doi: 10.3389/fcell.2016.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitagawa H, Yamamoto N, Yamamoto K, Tanoue K, Kosaki G, Yamazaki H. Involvement of platelet membrane glycoprotein Ib and glycoprotein IIb/IIIa complex in thrombin-dependent and -independent platelet aggregations induced by tumor cells. Cancer Res. 1989;49:537–541. [PubMed] [Google Scholar]
- 62.Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–1300. [PubMed] [Google Scholar]
- 63.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan MMG, Simizu S, Kawatani M, Osada H. The potential of protein disulfide isomerase as a therapeutic drug target. Oncol. Res. 2011;19:445–453. doi: 10.3727/096504011x13123323849717. [DOI] [PubMed] [Google Scholar]
- 65.Willems SH, Tape CJ, Stanley PL, Taylor NA, Mills IG, Neal DE, McCafferty J, Murphy G. Thiol isomerases negatively regulate the cellular shedding activity of ADAM17. Biochem. J. 2010;428:439–450. doi: 10.1042/BJ20100179. [DOI] [PubMed] [Google Scholar]
- 66.Flaumenhaft R, Furie B, Zwicker JI. Therapeutic implications of protein disulfide isomerase inhibition in thrombotic disease. Arterioscler. Thromb. Vasc. Biol. 2015;35:16–23. doi: 10.1161/ATVBAHA.114.303410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flaumenhaft R. Protein disulfide isomerase as an antithrombotic target. Trends Cardiovasc. Med. 2013;23:264–268. doi: 10.1016/j.tcm.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zwicker JI. Unconventional approaches to the prevention of cancer associated thrombosis. Thromb. Res. 2014;133(Suppl 2):S44–48. doi: 10.1016/S0049-3848(14)50008-4. [DOI] [PubMed] [Google Scholar]
- 69.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet Lond. Engl. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 70.Kim K, Hahm E, Li J, Holbrook L-M, Sasikumar P, Stanley RG, Ushio-Fukai M, Gibbins JM, Cho J. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood. 2013;122:1052–1061. doi: 10.1182/blood-2013-03-492504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJ, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995;62:1276–1282. doi: 10.1093/ajcn/62.6.1276. [DOI] [PubMed] [Google Scholar]
- 72.Panova-Noeva M, Marchetti M, Spronk HM, Russo L, Diani E, Finazzi G, Finazzi G, Salmoiraghi S, Rambaldi A, Rambaldi A, Barbui T, Barbui T, Ten Cate H, Ten Cate H, Falanga A. Platelet-induced thrombin generation by the calibrated automated thrombogram assay is increased in patients with essential thrombocythemia and polycythemia vera. Am. J. Hematol. 2011;86:337–342. doi: 10.1002/ajh.21974. [DOI] [PubMed] [Google Scholar]
- 73.Tournier A, Wahl D, Chaouat A, Max J-P, Regnault V, Lecompte T, Chabot F. Calibrated automated thrombography demonstrates hypercoagulability in patients with idiopathic pulmonary arterial hypertension. Thromb. Res. 2010;126:e418–422. doi: 10.1016/j.thromres.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 74.Tracy PB. Regulation of thrombin generation at cell surfaces. Semin. Thromb. Hemost. 1988;14:227–233. doi: 10.1055/s-2007-1002782. [DOI] [PubMed] [Google Scholar]
- 75.Hayward CPM, Fuller N, Zheng S, Adam F, Jeimy SB, Horsewood I, Quinn-Allen MA, Kane WH. Human platelets contain forms of factor V in disulfide-linkage with multimerin. Thromb. Haemost. 2004;92:1349–1357. doi: 10.1160/TH03-02-0123. [DOI] [PubMed] [Google Scholar]
- 76.Jeimy SB, Fuller N, Tasneem S, Segers K, Stafford AR, Weitz JI, Camire RM, Nicolaes GAF, Hayward CPM. Multimerin 1 binds factor V and activated factor V with high affinity and inhibits thrombin generation. Thromb. Haemost. 2008;100:1058–1067. [PubMed] [Google Scholar]
- 77.Adam F, Zheng S, Joshi N, Kelton DS, Sandhu A, Suehiro Y, Jeimy SB, Santos AV, Massé J-M, Kelton JG, Cramer EM, Hayward CPM. Analyses of cellular multimerin 1 receptors: in vitro evidence of binding mediated by alphaIIbbeta3 and alphavbeta3. Thromb. Haemost. 2005;94:1004–1011. doi: 10.1160/TH05-02-0140. [DOI] [PubMed] [Google Scholar]
- 78.Gould WR, Simioni P, Silveira JR, Tormene D, Kalafatis M, Tracy PB. Megakaryocytes endocytose and subsequently modify human factor V in vivo to form the entire pool of a unique platelet-derived cofactor. J. Thromb. Haemost. JTH. 2005;3:450–456. doi: 10.1111/j.1538-7836.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 79.Duckers C, Simioni P, Spiezia L, Radu C, Dabrilli P, Gavasso S, Rosing J, Castoldi E. Residual platelet factor V ensures thrombin generation in patients with severe congenital factor V deficiency and mild bleeding symptoms. Blood. 2010;115:879–886. doi: 10.1182/blood-2009-08-237719. [DOI] [PubMed] [Google Scholar]
- 80.Bouchard BA, Chapin J, Brummel-Ziedins KE, Durda P, Key NS, Tracy PB. Platelets and platelet-derived factor Va confer hemostatic competence in complete factor V deficiency. Blood. 2015;125:3647–3650. doi: 10.1182/blood-2014-07-589580. [DOI] [PMC free article] [PubMed] [Google Scholar]