Abstract
The development of pulmonary hypertension (PH) involves the uncontrolled proliferation of pulmonary smooth muscle cells via increased growth factor receptor signaling. However, the role of epidermal growth factor receptor (EGFR) signaling is controversial, as humans with advanced PH exhibit no changes in EGFR protein levels and purpose of the present study was to determine whether there are post-translational mechanisms that enhance EGFR signaling in PH.
The EGFR inhibitor, gefinitib, significantly attenuated EGFR signaling and prevented the development of PH in monocrotaline (MCT)-exposed rats, confirming the contribution of EGFR activation in MCT induced PH. There was an early MCT-mediated increase in hydrogen peroxide, which correlated with the binding of the active metabolite of MCT, monocrotaline pyrrole, to catalase Cys377, disrupting its multimeric structure. This early oxidative stress was responsible for the oxidation of EGFR and the formation of sodium dodecyl sulfate (SDS) stable EGFR dimers through dityrosine cross-linking. These cross-linked dimers exhibited increased EGFR autophosphorylation and signaling. The activation of EGFR signaling did not correlate with pp60src dependent Y845 phosphorylation or EGFR ligand expression. Importantly, the analysis of patients with advanced PH revealed the same enhancement of EGFR autophosphorylation and covalent dimer formation in pulmonary arteries, while total EGFR protein levels were unchanged. As in the MCT exposed rat model, the activation of EGFR in human samples was independent of pp60src phosphorylation site and ligand expression. This study provides a novel molecular mechanism of oxidative stress stimulated covalent EGFR dimerization via tyrosine dimerization that contributes into development of PH.
Keywords: EGFR, Pulmonary hypertension, Oxidative stress, Proliferation, Catalase
1. Introduction
Pulmonary hypertension (PH) is characterized by progressive endothelial dysfunction and uncontrolled vascular intimal and smooth muscle cell proliferation. These events ultimately induce right ventricle dysfunction and death. Recent studies confirm the involvement of oxidative stress in the development of PH [1–3]. Under pathological conditions the excessive formation of reactive oxygen species (ROS) occurs through a number of pathways including increased activity of ROS producing enzymes, such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [4] and xanthine oxidase [5], enzymatic dysfunctions, such as endothelial nitric oxide synthase (eNOS) uncoupling [6], dysfunction of the mitochondrial respiratory chain [7], or reductions in anti-oxidant scavenging via superoxide dismutase [8], catalase [9], glutathione reductase [10], thioredoxin system [11] and other enzymes maintaining cellular redox homeostasis. The primary ROS involved in cell signaling are superoxide and H2O2. Superoxide is charged and highly reactive, which significantly limits its diffusion. Thus, it is considered to be an intracellular messenger. The effective way to control the levels of cellular superoxide is to convert it into H2O2 through a dismutation reaction via superoxide dismutases. H2O2 is less reactive and is capable of diffusing through cellular membranes, thus it can induce redox changes distant from its generation and can exert both intracellular and extracellular signaling events [12–14]. Uncontrolled H2O2 accumulation has been shown to be associated with various cardiovascular diseases. The primary targets for H2O2 are protein cysteine and tyrosine residues, which, upon oxidation, form disulfide or dityrosine covalent bonds respectively [13]. Cysteine modifications are usually reversible, however, tyrosine modifications are irreversible, and, thus, permanently change the activity of the modified protein. The activity of a number of cellular proteins, including metabolic enzymes, ion channels, antioxidant enzymes, stress mediated protein kinases, growth factors, and transcriptional factors can be modified by H2O2 [14,15]. In vascular cells, H2O2-mediated protein oxidation has been shown to contribute both to vascular constriction and vascular remodeling [16–18].
Epidermal growth factor receptor (EGFR) is a member of the ERBB receptor tyrosine kinase superfamily [19]. EGFR controls a wide variety of biological functions including cell proliferation. Activation of EGFR occurs through direct binding of ligands to the receptor’s extracellular domain, which leads to dimerization of two receptor subunits and subsequent autophosphorylation of key tyrosine residues within the C-terminal region. The resulting phosphotyrosine residues act as docking sites for the proteins involved in regulating downstream cellular signaling cascades [19]. EGFR was found to be involved in pulmonary vasoconstriction in chronic hypoxia-induced PH [20], while EGFR antagonists have been shown to be an effective tool to stop the proliferation of vascular cells in cell culture and various preclinical animal models of PH [21–23]. However, the absence of significant changes in EGFR expression in lungs from human patients with idiopathic PH has led to the conclusion that EGFR does not represent a promising target for the treatment of PH [21], although the posttranslational regulation of EGFR activity and its possible role in the development of PH have not been investigated. In this study we elucidate a new mechanism by which H2O2 activates EGFR signaling by formation of dityrosine crosslinks that does not result in changes in the expression level of the receptor. Further, we show that the major H2O2 scavenger, catalase, is itself subject to post-translational inhibition. Together our data represent a new mechanism by which EGFR signaling contributes to the development of PH.
2. Experimental procedures
2.1. Animals
A total of 32 male Sprague Dawley rats (SD; 220–270 g) were used in this study (n=8 per group). Animals were housed at Georgia Regents University animal care facility for at least 1 week before being used in the experiments. Animals were kept in a 12-h light/dark cycle at an ambient temperature of 22 °C and received standard rodent food and water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Georgia Regents University.
2.2. Rat model of pulmonary hypertension
We utilized a rat monocrotaline model of PH in which the disease develops after a single administration of monocrotaline (60 mg/kg). Before beginning the study, rats were randomly allocated to one of four experimental groups: (1) Control group: SD rats receiving only the vehicle for MCT; (2) Acute study group: rats examined 3 days after MCT injection; (3) Chronic study group: rats examined 14 days after MCT injection; (4) Gefitinib group: rats subjected to a single injection of MCT and daily treatment by gefitinib (30 mg/kg by oral gavages) and examined 14 days after MCT injection and (5) Fully developed PH study group: rats examined 28 days after MCT injection.
2.3. Acute measurement of hemodynamic parameters
On Day 3, Day 14, and Day 28 of study, subsets of animals were anesthetized (Inactin, 100 mg/kg i.p.) and instrumented for measurement of right ventricle (RV) and systemic blood pressure. Briefly, a PE-240 polyethylene tube was inserted into the trachea to facilitate breathing. A customized pressure transducer catheter (SPR-513, Millar Instruments, Houston, TX), connected to a Millar Transducer Control Unit TC-510 and PL3504 PowerLab 4/35 data acquisition system (ADInstruments, Inc., Colorado Springs, CO) was inserted into the right ventricle via the right jugular vein and right atrium. For systemic blood pressure measurement, the right carotid artery was cannulated with a fluid-filled PE-50 polyethylene catheter connected to a blood pressure transducer and, through a bridge amplifier, to the PowerLab system. A 30 min stabilization period was permitted before a 30 min recording of the right ventricular pressure was conducted. At the end of pressure recording, the trachea catheter was connected to a Harvard Rodent Ventilator (Model 683; Harvard Apparatus, South Natick, MA), the thorax was opened and the lungs were flashed with saline (0.9% sodium chloride) via catheter inserted into the right atrium to remove the blood from pulmonary vessels. Animals were euthanized by an anesthetic overdose, heart and lungs were dissected and weighed. The right ventricle free wall (RV) was separated from the left ventricle and septum (LV+S) to determine the wet weights and the RV to LV+S weight ratio (RV/LV+S). One lung was immersed in 10% buffered formalin for at least 72 h before being embedded, while the other was stored on −80 °C and used for biochemical studies.
2.4. Human specimens
We selected five bilateral lung explants from human patients who underwent lung transplantation because of Eisenmenger’s syndrome (“associated pulmonary arterial hypertension”, NYHA IV). All lung specimens showed prominent plexiform vasculopathy (age at transplantation: 36.5 ± 11.04 years; female:male ratio – 4:1). All the specimens were inflated with formalin via the main bronchi and were formalin-fixed overnight before being extensively sampled and paraffin-embedded (FFPE). Subsequently, they were histologically evaluated, graded according to the Heath-Edwards classification (all grade 5), and correlated with clinical data to confirm the (histopathologic) diagnosis. The FFPE samples were retrieved from the archives of the Institute of Pathology of Hannover Medical School and were handled anonymously, following the requirements of the local ethics committee [24].
2.5. Confocal immunofluorescence microscopy and matrix RNA (mRNA) expression analysis of pulmonary tissue from human specimens
Tissue paraffin sections (5 µm) were mounted on slides and placed in a 55 °C oven for 10 min, deparaffinized in xylene (3 ×, 5 min), hydrated using an alcohol series—100%, 95%, 70% alcohol (each 3 × , 5 min) and rinsed in water. The sections were processed for antigen retrieval by boiling the slides in 10 mM Citrate Buffer (pH 6.0). The slides were cooled at room temperature for 20 min, washed in PBS and blocked in 10% normal serum overnight at 4 °C. Immunofluorescence was performed on serial sections from each group using rabbit anti-EFGR, anti-pY845EGFR and anti-pY1068EGFR antibodies (Cell Signaling) and mouse anti-EGF (R&D Systems). The sections were incubated with primary antibodies for 1 h at room temperature. Subsequently, sections were stained with Alexa Fluor® 546 goat anti-rabbit and anti-mouse secondary antibodies (Molecular Probes, Inc.). Sections were washed several times in PBS, mounted and cover slipped in anti-fading aqueous mounting medium. The fluorescent-stained sections were analyzed using the appropriate excitation and emission wavelengths by performing confocal microscopy using a computer-based DeltaVision imaging system (Applied Precision Inc.). Software Soft-Worx 3.6.2 was used to trace the pulmonary artery walls, to calculate the mean fluorescent signal per area and to normalize the fluorescent signals against the background. From each slide 20 pulmonary arteries were imaged and quantified to assess the final mean fluorescent signal for each sample.
Bilateral lung explants from human patients who underwent lung transplantation because of Eisenmenger’s syndrome were used. Tissue paraffin sections were deparaffinized, incubated with primary antibody, stained with Alexa Fluor® 546 secondary antibodies, and analyzed using a DeltaVision imaging system. Laser-assisted microdissection, RNA extraction and expression analysis were performed as previously described [25].
2.6. Synthesis of monocrotaline pyrrole
Monocrotaline (Sigma) 20 mg was dissolved in chloroform (5 ml) and was added at room temperature to o-chloranil (25 mg) in chloroform (CHCl3, 5 ml). After 2 min of incubation the mixture was shaken with cold LiBH4 2% solution in 70% KOH. Immediately filtered and dried with Na2SO4 (anhydrous), then separated from precipitate and CHCl3 was evaporated. Dried crystals were re-crystallized in acetonitrile.
2.7. Cell monoculture and co-culture
Primary cultures of ovine fetal PAEC and ovine fetal pulmonary artery smooth muscle cells (PASMC) were isolated and identified, as previously described [26,27]. Cells between passages 5 and 12 were maintained in DMEM supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT), antibiotics and antimycotics (MediaTech, Herndon, VA) at 37 °C with 5% CO2-95% air. For cell co-cultures, our previously described protocol was utilized [28]. Briefly, six-well Transwell plates (Corning Life Sciences, Pittston, PA) containing inserts with permeable polycarbonate membrane inserts (pore size 0.4 µM) were used. Transwell plates separate the cell monolayers by ~1 mm while allowing intercellular signaling via a microporous membrane. One day before the experiments the Transwell inserts were activated by incubation in cell culture media at 37 °C overnight according to manufacturer’s protocol. PAEC were seeded into the pre-activated inserts, allowed to attach overnight, and treated with MCTP (200 µM). After 30 min of MCTP treatment the media was removed from both compartments, PAEC were washed with PBS and transferred into the dishes containing PASMC to start the co-culture. In additional experimental groups either polyethylene glycol (PEG)-Catalse (200 U/ml) (Sigma), or AG1478 (100 nM) (Cayman Chemical) were added into the PASMC media 30 min before co-culture. 48 hours after co-culture PASMC were collected by trypsinization, washed with PBS and stored at −80 °C for Western blot analysis.
2.8. Cell proliferation
The 24-well plates with the same Transwell permeable polycarbonate membrane inserts were activated as described above. PAEC (60,000 cells per insert) were seeded into each pre-activated insert and allowed to attach overnight. PASMC (10,000 cells per well) were seeded into a separate 24-well plate and allowed to grow for 48 h. 4 hours before co-culture, the media in PASMC was changed into a low serum media (0.2% FBS). PAEC were treated by MCTP as described above. Thirty minutes after treatment the MCTP was washed out with PBS, and the inserts with PAEC were added to the dishes with PASMC to start the co-cultures. Additional wells with non-co-cultured PASMC were used to analyze cell numbers at the moment when the co-cultures were started (T=0 h). PASMC proliferation was measured by utilizing according to manufacturer’s protocol. Briefly, at 0, 24 and 48 h after co-culture the inserts with PAEC were removed, the media was changed, and the fresh mixture of PMS and MTS solution (ratio 1:20) was added to the media (1:5 vol/vol) and incubated at 37 °C for 90 min. The optical density (OD) was subsequently analyzed at 520 nm. In additional experiments, a known cell number of PASMC were seeded onto 24-well plates and allowed to attach for 4 h. The cell proliferation for each well was then measured using the 3-(4,5-dimethylthiazol-2-yl) - 2,5-diphenyltetrazolium bromide (MTT) assay. After 24 h 10 µL of the MTT reagent was added. Cells were incubated for 2 h, then 100 µL of detergent reagent was added and the cells incubated for a further 2 h in the dark. The absorbance at 570 nm was then determined using a spectrophotometer. Different cell numbers were seeded on the plates to plot a calibration curve and used to transform OD values into viable cell number.
2.9. Detection of H2O2 in cell culture media and smooth muscle cells
A modified 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) oxidation method was used to detect H2O2 levels [28]. Briefly, PASMC were co-cultured with untreated PAEC, or PAEC treated by MCTP as outlined above. On day 0,1 and 2 of co-culture the media was collected and incubated with 25 µM H2DCFDA (Calbiochem) for 30 min in the dark with or without 100 U/ml catalase (Sigma, St. Louis, MO). Similarly, H2O2 levels were analyzed in PASMC by adding 25 µM H2DCFDA directly to the PASMC on day 0,1 and 2 of co-culture in the presence or absence of 100 U/ ml catalase. Purified catalase was used to confirm that the oxidation of H2DCFDA was H2O2 dependent. Samples were measured with an excitation at 485 nm and an emission at 530 nm in Fluoroskan Ascent FL (Thermo Electron, Waltham, MA). The catalase inhibitable H2DCFDA oxidation was obtained by subtracting the fluorescent intensity measured in the presence and absence of catalase.
2.10. Expression and purification of recombinant human catalase
For catalase purification, 50 ml of terrific broth was premixed with kanamycin (100 mg/ml) and chlorophenicol (50 mg/ml), and inoculated with E. coli BL21 cells transformed with the pET 28b plasmid containing a complete human catalase cDNA sequence [29]. Bacteria were grown overnight at 37 °C (260 rpm) then used to inoculate 2.8 L Fernabach flasks (6 × 1.5 L) containing terrific broth (52 g/L) as the culture medium and supplemented with kanamycin (100 mg/ml) and riboflavin (15 mg). Flasks were placed on an orbital shaker and were allowed to grow at 37 °C (200 rpm). The OD600 was checked periodically during the growth period until it reached 0.8–1.0 (4–5 h) then adenosine-5′-triphosphate (ATP, 200 µM final concentration) and isopropyl-beta-d-thiogalactopyranoside, dioxane free (IPTG, 1 mM final concentration, to induce the T7 promoter) was added and the cells incubated for 18–20 h at 25 °C (200 rpm). Bacteria were then harvested by centrifugation using a FiberLite F6 6 × 1000 rotor at 4 °C (3500 rpm/ 2700 g) for 20 min. The pellet was immediately transferred into lysis buffer (40 mM Tris-HCl, 5% glycerol, 1 mg/ml lysozyme) and a protease inhibitor cocktail for use with histidine-tagged proteins (Sigma), ribonuclease A from bovine pancreas (Sigma), and deoxyribonuclease I from bovine pancreas (106 units, Sigma) was added. The pellet was gently rocked for 30 min at 4 °C, sonicated on ice, then subjected to ultracentrifugation at 4 °C (60,000 rpm/ 37,1000g) for 1 hour and 45 min. The supernatant was loaded onto a Hisprep FF 16/10 column (charged with 0.1 M NiSO4) using binding buffer (40 mM Tris-HCl, 100 mM NaCl, 5% glycerol, 30 mM imidazole) at 0.1 ml/min flow. The column was washed with washing buffer (40 mM Tris-HCl, 300 mM NaCl, 5% glycerol, 30 mM imadizole) using a flow rate of 1.5 ml/min, and a base line was obtained resulting in the washing out of non-histidine-tagged proteins. Elution of histidine-tagged protein was accomplished using elution buffer (40 mM Tris-HCl, 300 mM NaCl, 5% glycerol, 400 mM imidazole) at 1.0 ml/min flow. Collected fractions were loaded for size-exclusion gel filtration on a HiLoad 26/60 Superdex 200 prep grade column using catalase gel filtration buffer (60 mM Tris-HCl, 100 mM NaCl, 5% glycerol) at 0.2 ml/min flow. Fractions were collected in 5 ml amounts for analysis by Coomassie blue staining and Western blot. Desalting was then performed for fractions containing catalase using a HiPrep 26/10 desalting column and catalase gel filtration buffer at flow rate of 0.5 ml/min. All purification steps were performed at 4 °C, and purified protein was stored at −80 °C. Protein homogeneity was confirmed using Coomassie blue staining and Western blot with anti-catalase antibody (Research Diagnostics Inc.) with dilution 1:1000. Final protein concentration was then measured in each fraction.
2.11. Determination of catalase activity in vitro and in vivo
In gel catalase activity was visualized by the inhibition of exogenous horseradish peroxidase/H2O2-mediated diaminobenzidine (DAB) oxidation due to H2O2 consumption by catalase and appeared as a colorless band against a dark background. Native gel contained catalase samples was soaked with DAB (0.7 mg/ml) and HRP (1 µg/ml) in PBS for 1 h then washed twice with deionized water and developed by applying H2O2 solution (3 mM). Catalase activity in PASMC was measured as previously described [30,31]. Briefly, this method is based on the rate of H2O2 degradation. Purified catalase (5 µg), cell lysate (20 µl) or tissue homogenate (20 µl) were added to 1 ml of 15 mM H2O2 in 50 mM phosphate buffer (pH 7.0). Decomposition of the H2O2 was recorded by the decrease in absorbance at 240 nm over a 60 s period. The signal was normalized to the sample protein concentration and catalase activity was expressed as a rate of H2O2 degradation per mg protein.
2.12. Gel filtration chromatography
To examine the oligomeric composition of the catalase treated with monocrotaline pyrrole we utilized analytical gel filtration. One hundred microliters of each sample, at a concentration 1 mg/ ml, were injected into a Tosoh TSKgel G3000SWxl gel filtration column. Using a flow rate of 0.5 ml/min, monomer, dimer, trimer and tetramer fractions were eluted in 100 mM phosphate buffer (pH=7.0) using a high performance liquid chromatography (HPLC) system (GE) and analyzed by measuring using the absorption at 260 nm.
2.13. MALDI-TOF-TOF mass spectrometry (MS)
Peptide calibration standards and matrix α-Cyano-4-hydro-xycinnamic acid (CHCA) were obtained from Applied Biosystems (Carlsbad, CA). All spectra were taken on an ABSciex 5800 MALDI-TOF-TOF mass spectrometer in positive reflector mode (10 kV) with a matrix of CHCA. At least 1000 laser shots were averaged to obtain each spectrum. Masses were calibrated to known peptide standards. Five microliter aliquots of the catalase (band from catalase immunoprecipitation above 60 kDa) tryptic digest (18 h incubation with 10 ng MS grade trypsin at 28 °C in 25 mM ammonium bicarbonate) were cleaned on a C18 ZipTip (Millipore, Bedford, MA) as per manufacturer’s instructions. Bound peptides were desalted with two 5-µl washes of 0.1% TFA and then eluted with 2.5 µl of aqueous, acidic acetonitrile (75% CH3CN, 0.1% TFA). The eluant was mixed with 2.5 µl of freshly prepared CHCA stock solution (20 mg/ml CHCA in aqueous acetonitrile as above), and 1.5-µl portions of this mixture were spotted onto a MALDI sample plate for air drying. 1.5 µl of crude peptides were additionally mixed with 1.5 µl of CHCA and spotted. MS and MS/MS spectra (2077.101m/z corresponded to LGPNYLHIPVNC377PYR sequence from catalase with monocrotaline adduct at C377) were analyzed in Protein Pilot 3.0, Mascot Distiller, and PEAKS software packages. For identification of EGFR dityrosine site, EGFR was over-expressed in human embryonic kidney (HEK) 293 cells then exposed to H2O2 (10 µM) and CuCl2(300 µM) for 30 min to induce dityrosine formation. EGFR from the cell lysates was immunoprecipitated using an antibody specific to EGFR then subjected to SDS-PAGE electrophoresis. Bands corresponding to the EGFR dimer and monomer were excised from Coomassie stained gels and subjected to in-gel trypsin digestion as described for catalase above. Peptides were further deglycosylated using in solution deglycosylation kit Glycoprofile II (Sigma).
2.14. Detection of dityrosine crosslinks by HPLC
Dityrosine formation in EGFR IP samples was monitored by fluorescence measurements at 300 nm excitation and 410 nm emission. Dityrosine produces a fluorescent spectrum that is different to other amino acids in proteins, calibration was done using Cu2+/H2O2 oxidized l-tyrosine. Detection of dityrosine was performed by HPLC reverse phase chromatography of tryptic digest samples of EGRF dimer or monomer. The HPLC AKTA purifier System (GE) was equipped with a fluorescence detector FL2020plus (Jasco), column Kromasil (100-5C18) with a guard column and UV–vis detector AKTA UV-900. The mobile phase consisted of 12% Acetonitrile in water and was eluted at 0.2 mL/ min. Eluent was monitored both UV at 280 nm and fluororescence at 410 nm emission with 300 nm excitation for measuring dityrosine fluorescence. Peptides were diluted in 100 µl of mobile phase and 30 µl were injected into HPLC.
2.15. Computational modeling
Analysis of the previously available crystal structure of catalase (PBD ID 1QQW) using YASARA software package[32] identified cysteine C377 as being located at the dimeric interface. Homology modeling of EFGR and PDGFR receptors extracellular domains were performed on YASARA Structure module with automated homology modeling script. The extracellular sequences of human EGFR or PDGFR without signaling peptide were obtained from Uniprot database (uniprot.org).
2.16. Western blot analysis
Whole lung tissues were pulverized with mortar and pestle under liquid nitrogen and homogenized in lysis buffer (1 M TRIS pH 7.4, 0.5% Triton X-100, 20% glycerol, 200 mM Na3VO4, 1 M NaF, 100 mM PMSF, plus a protease inhibitor cocktail (Sigma) and phosphatase inhibitor cocktail (Sigma)), centrifuged at 10,000 rpm for 10 min at 4 °C and stored at −80 °C until further use. Samples (100 µg of total protein) were incubated with 5 × sample buffer at 100 °C for 5 min and separated on 4–20% denaturating polyacrylamide gels (Mini-PROTEAN® TGX™, BioRad) using TRIS/Glycine running buffer with 10% SDS on 200 V for 30 min. For western blot analysis of EGFR the samples were heated with sample buffer at 75 °C to prevent receptor conglomeration. For seminative gel electrophoresis to measure catalase tetramer/mononer ratio the samples were mixed with ice cold PBS and ice cold 5 × sample buffer, incubated on ice for 10 min, centrifuged and loaded into 4–20% denaturating polyacrylamide gels (Mini-PROTEAN® TGX™, BioRad). The samples were separated on ice using icecold TRIS/Glycine running buffer with 0.1% SDS on 50 V for 3 h. EGF levels were measured using the Novex® 10–20% Tricine Gels (Invitrogen) to resolve low molecular weight proteins and peptides using Tricine running buffer. All gels were then electrophoretically transferred to PVDF membranes. The membranes were blocked with 1% bovine serum albumin (BSA) in Tris-buffered saline (TBS) containing 0.1% Tween 20 for 1 h and incubated overnight at 4 °C with an appropriate dilution of primary antibody. The antibodies used were: anti-pY1068EGFR, anti-pY845EGFR, anti-EGFR (Cell Signaling), anti-EGF (R&D Systems) and anti-amphiregulin (Santa Cruz Biotechnology) to study the levels of EGFR activation, anti-Ras (Cell Signaling), and anti-GRB2 (Abnova) to evaluate EGFR signaling, anti-pp60src, anti-pY416pp60src and anti-pY845EGFR (Cell Signaling) to measure the contribution of pp60src-mediated pathways, anti-caldesmon antibodies (Sigma) to estimate the levels of pulmonary SMC proliferation; and monoclonal anti-PCNA antibody (Cayman chemical company) to evaluate overall cellular proliferation of the lung. After washing with TBS containing 0.1% Tween 20 for three times, membranes were incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (1:5000) for 1 h at room temperature. Membranes were washed three times with TBS containing 0.1% Tween 20 and protein bands were visualized using chemiluminescent procedures and the SuperSignal West Femto Maximum Sensitivity Substrate (Pierce). The band intensities were recorded using a Kodak ImageStation and analyzed using AlphaView software or Image J. Blots were re-probed with β-actin to normalize for protein loading. Some membranes were stripped and re-probed for more than one protein (pY845EGFR/total EGFR, pY1068EGFR/ PCNA), therefore they share the same β-actin.
2.17. Immunoprecipitation
Tissue lysates (500 µg) were incubated with 5 µl anti-EGFR (Cell Signaling) at 4 °C overnight. Then protein G PLUS/Protein A Agarose beads (Calbiochem) was added and incubated at 4 °C for a further 2 h. Immunoprecipitates were pelleted and washed 4 times with lysis buffer. Pellets were suspended in 2 × sample buffer, boiled for 5 min at 75 °C and subjected to immunobloting using an antibody specific for GRB2 (1:1000, Abnova). Blots were re-probed with Ras antibodies (1:1000, Cell Signaling), and again - with EGFR antibodies to normalize for immunoprecipitation efficiency.
2.18. Direct and indirect enzyme-linked immunosorbent assay (ELISA) to measure the levels of EGFR ligands
To measure EGF protein levels in lung tissue homogenates by indirect ELISA, the previously published protocol [33] was slightly modified. Briefly, lung tissues kept on −80°C were homogenized and centrifuged as described for Western blot analysis. The samples were diluted with PBS to a final protein concentration of 10 µg per 200 µl. Samples and standards were added to the Reacti-Bind™ White Opaque 96-well plate (TermoScientific) and incubated for 2 h on ice. After incubation the samples were removed and the well surface was blocked by cold 5% BSA solution for 1 hour. The primary anti-rat EGF specific antibodies (ProSci Inc.) were added (1.5 µg/ml in 100 µl of 5% BSA) and incubated for 1 hour. The wells were rinsed twice with cold PBS containing 0.1% Tween20 and incubated with secondary anti-rabbit horseradish peroxidase (HRP)-conjugated antibody for another 45 min. The HRP signal was developed using SuperSignal West Pico Luminol/ Enhancer Solution (TermoFisher) and chemiluminescence was read using a Synergy™ HT Multi-Mode Microplate Reader (BioTek Instruments). To analyze the levels of tumor growth factor α (TGFα) the rat TGFα ELISA kit (Biotang Inc.) was used. The assay procedure was performed according to manufacturer’s protocol. Briefly, 10 µl of blood plasma (obtained by blood collection in heparinized tubes, centrifugation at 2000 rpm for 10 min, quick frozen in liquid nitrogen and stored at −80 °C) diluted in 40 µl of sample solution or standards were added into the provided 96-well plate, sealed and incubated at 37 °C for 30 min. After incubation the wells were washed four times with washing buffer, and 50 µl of HRP-conjugate reagent was added into each well. The wells were sealed, incubated at 37 °C for 30 min and washed four times with washing buffer. After the last wash 50 µl of chromogen substrate A and chromogen substrate B were added into each well and incubated at 37 °C for 30 min. The reaction was stopped by adding 50 µl of stop solution. The optical density was read at 450 nM using the Synergy™ HT Multi-Mode Microplate Reader (BioTek Instruments).
2.19. Measurement of H2O2 in vivo
The Amplex Red Reagent (Molecular Probes) was used to detect H2O2 levels in rat peripheral lung tissues. Briefly, 10 mg of rat peripheral lung tissue was incubated at room temperature for 30 min in a master mix solution containing Amplex Red Reagent, horseradish peroxidase, and a buffer solution, according to the manufacturer’s protocol. The supernatant was then collected by brief centrifugation, added to a black 96-well plate, and fluorescence was read spectrophotometrically at excitation/emission 530/ 590 nm. The concentration of H2O2 was determined through extrapolation from a standard curve. The fluorescent signal was normalized to the total protein concentration and reported as nanomols/min/microgram protein.
2.20. Statistical analysis
Statistical calculations were performed using the GraphPad Prism V. 4.01 software. The means ± SE was calculated for all samples, and significance was determined by either the unpaired or paired t-test or analysis of variance (ANOVA). For ANOVA, Newman-Keuls post hoc testing was also utilized. A value of P < 0.05 was considered significant.
3. Results
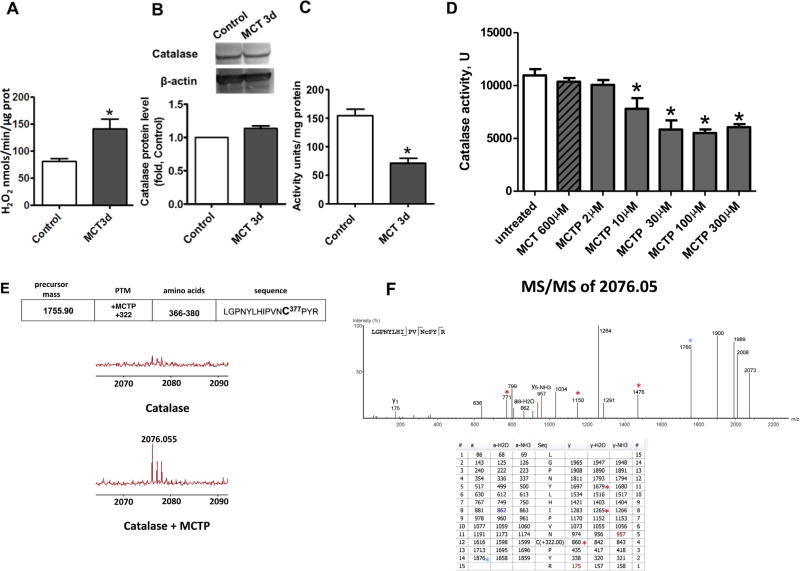
Although increases in oxidative stress have been identified in the lungs of patients with advanced PH [1], we hypothesized that uncontrolled accumulation of ROS could start much earlier and actually contribute to the development of PH. Thus, we initially determined the level of H2O2 in lung tissue 3 days after MCT injection in rats. We found a significant increase in H2O2 (Fig. 1A) that was not associated with alterations in catalase protein (Fig. 1B), but correlated with a decrease in catalase activity (Fig. 1C). It has been established that the active MCT metabolite is monocrotaline pyrrole (MCTP) [34]. MCTP is a functional cross-linking agent capable of binding to cysteine residues, resulting in protein inhibition [35]. To determine if the decrease in catalase activity induced by MCT exposure was dependent on MCTP–catalase interactions, we exposed purified human catalase to increasing concentrations of MCTP. MCTP concentrations as low as 10 µM induced catalase inhibition, while even 600 µM of MCT did not alter catalase activity (Fig. 1D). To determine which cysteine residue is sensitive to cross-linking with MCTP, we performed mass spectrometry (MS) analysis of recombinant catalase in the presence or absence of MCTP. MS/MS revealed a single modified cysteine residue located at position 377 (C377) (Fig. 1E and F). The same cysteine residue was found to be modified in catalase in endothelial cells treated with MCTP (not shown).
Fig. 1.
Monocrotaline pyrrole inhibits catalase activity through adduct formation at cysteine 377. The level of H2O2 produced in snap frozen peripheral lung tissue is increased 3 days after MCT injection (A). Despite no changes in catalase protein as determined by Western blot analysis and normalized to β-actin (B), catalase activity is significantly attenuated in MCT-treated rats. MCTP, but not MCT, attenuates recombinant human catalase (D) and this corresponds to the addition of the pyrole adduct to cysteine 377 located in the peptide sequence 366-LGPNYLHIPVNC*PYR-380 as determined by MS (E). MS/MS spectra (F, upper panel) were analyzed with PEAKS 5.2 software and the resulting ion table is shown (F, lower panel). Due to instability of MCTP modification, the fragmentation was calculated manually. Colored numbers represent predicted y,b ions in MS/MS with MCTP attached to cysteine 377 and colored *-symbols indicate predicted collision products with fragmented MCTP modification. Results are expressed as mean±SEM; n=3–8. *P<0.05 vs. control or untreated group.
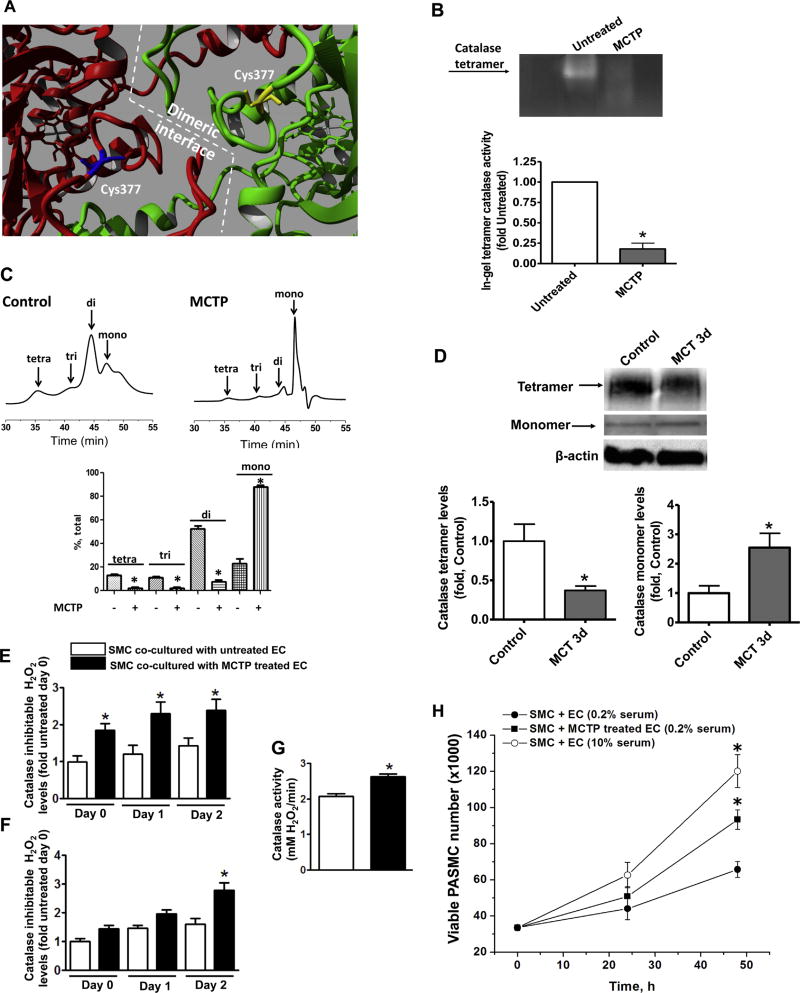
Computational modeling of the catalase tetrameric structure suggested that C377 lies at the dimeric catalase interface (Fig. 2A). Catalase is most active as a tetramer [36], suggesting that cross-linking with MCTP could attenuate catalase tetramer formation and reduce activity of the enzyme. Using an in gel catalase activity assay we found that MCTP treatment induces a complete disruption of the catalytically active catalase tetramer (Fig. 2B). Further, gel filtration analysis confirmed that untreated purified catalase has tetra-, tri- and dimeric forms, while exposure to MCTP results in a predominantly monomeric catalase (Fig. 2C). Finally, we found that in the lungs of rats exposed to MCT for 3d, there is a significant decrease in the active tetrameric form of catalase and a corresponding increase in the level of catalase monomers (Fig. 2D). Together these data suggest that MCT cross-linking to C377 disrupts the catalase tetrameric structure and inhibits its activity.
Fig. 2.
The monocrotaline pyrrole adduct disrupts the oligomeric structure of catalase. Analysis of the crystal structure of catalase (PBD ID 1QQW) identified C377 as being located at the dimeric interface (A). Semi-native zymography (in gel activity) demonstrates that the activity of recombinant catalase tetramer is significantly attenuated by MCTP (30 µM, 30 min) (B). Analytical gel filtration confirms the disruption of the catalase multimeric structure by MCTP (C). Semi-native gel electrophoresis of peripheral lung tissues of MCT-treated rats and the following western blot analysis identified a significant reduction in the tetrameric form of catalase and accumulation of catalase monomer (D). Pretreatment of PAEC with MCTP (200 µM, 30 min), followed by complete removal of MCTP, causes a significant increase in H2O2 in the media within 2 h and this persists for at least 48 h (E). H2O2 levels increase in the PASMC co-cultured with PAEC pretreated with MCTP after 48 h (F) despite a significant increase in catalase activity (G). The increase in H2O2 in the PASMC correlates with an increase in proliferation (MTS proliferation assay) (H). Results are expressed as mean±SEM; N=3–4. *P<0.05 vs. control or untreated group.
To elucidate the contribution of MCTP mediated H2O2 accumulation to pulmonary artery smooth muscle cells (PASMC) proliferation, pulmonary artery endothelial cells (PAEC) were pre-treated with MCTP and then co-cultured with PASMC. Our data indicate that H2O2 levels are significantly increased in the media of the co-cultures within a few hours, and this increase is maintained over the next 2 days (Fig. 2E). Interestingly, the intracellular levels of H2O2 in PASMC co-cultured with MCTP pre-treated PAEC, increased only after 2 days of co-culture (Fig. 2F). This delayed increase of H2O2 in PASMC was probably due to an elevation in catalase activity in the PASMC (Fig. 2G), which may occur in response to oxidative stress. The proliferation rate of PASMC co-cultured with MCTP treated PAEC was significantly higher compared to PASMC co-cultured with untreated PAEC (Fig. 2H), and similar to PASMC co-cultured in serum media containing 10% FBS.
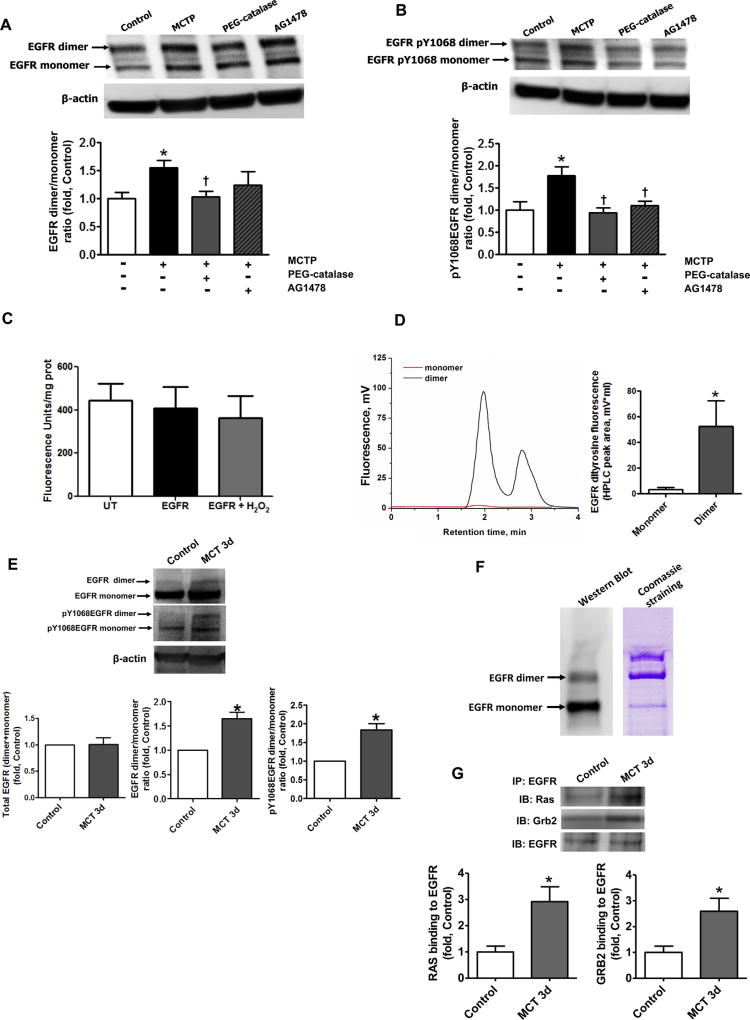
It has previously been reported that in the presence of oxidants, EGFR can undergo a tyrosine–tyrosine covalent dimerization [37]. Using Western blot analysis, we found that co-culture of PASMC with PAEC pre-treated with MCTP induced the formation of an SDS stable high molecular weight form of EGFR in PASMC (Fig. 3A), confirmed as EGFR by MS (data not shown). The increase in the high molecular weight SDS stable form of EGFR was attenuated by pre-treatment with PEG-catalase (Fig. 3A), but not by EGFR kinase inhibitor, AG1478 (Fig. 3A). The high molecular weight SDS stable form of EGFR was found to be highly autophosphorylated (Fig. 3B) and this autophosphorylation was attenuated in the presence of both PEG-catalase and AG1478 (Fig. 3B). As the cells were cultured in a low (0.2% FBS) serum media, the increased level of EGFR auto-phosphorylation suggests growth factor independent EGFR activation.
Fig. 3.
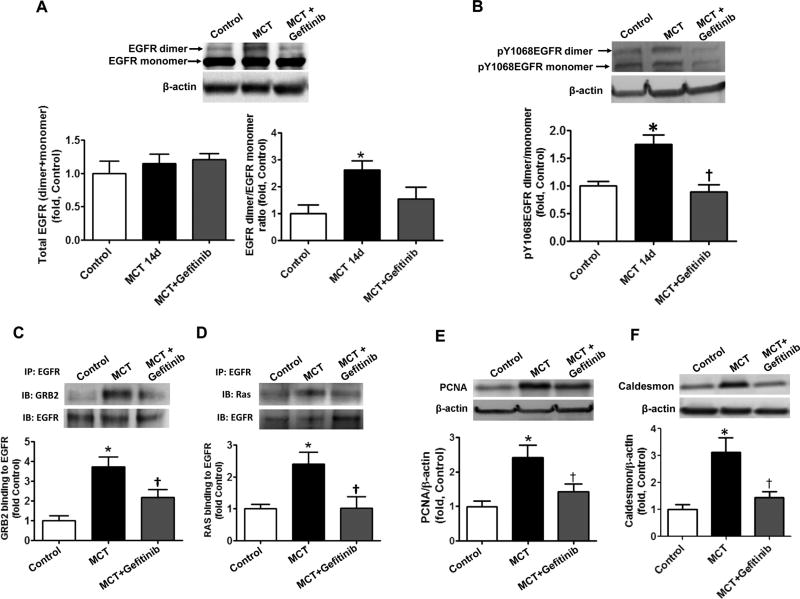
Covalent dimerization of EGFR increases EGFR signaling in vitro and in vivo. The levels of EGFR dimer and monomer in PASMC co-cultured with MCTP pretreated PAEC were obtained by performing Western blot analysis of PASMC lysates. The presence of SDS-resistant an EGFR dimer was significantly increased in PASMC co-cultured with PAEC pretreated with MCTP and this was attenuated by pretreatment with PEG-catalase (250 U/ml), but not by the EGFR inhibitor AG1478 (100 nM, A). The ratio of auto-phosphorylated EGFR dimer/auto-phosphorylated EGFR monomer was also significantly increased in PASMC co-cultured with PAEC pretreated with MCTP and attenuated by PEG-catalase or the EGFR kinase inhibitor, AG1478 (B). HEK cells over-expressing EGFR were treated with CuCl2/H2O2 (10 µM/300 µM) to induce oxidative stress. Whole cell lysates were then examined for specific dityrosine fluorescence. Global tyrosine oxidation was unchanged (C) but HPLC analysis of the peptide fragments from dimer and monomer bands of immunoprecipitated and digested EGFR revealed the presence of dityrosine crosslinks only in the EGFR dimer (D). Western blot analysis revealed no change in total EGFR protein levels in peripheral lung tissue lysates prepared from control and MCT-treated rats at 3 days (E, left panel). Loading was normalized to β-actin. However, the EGFR dimer/monomer ratio (E, middle panel) and the levels of auto-phosphorylation, assessed by measuring pY1068EGFR (E, right panel), were both significantly in MCT treated rats. The levels of EGFR dimer assessed by Western blot analysis of EGFR immunoprecipitation (F, left panel) appear to be much less when compared to the band present with Coomassie staining (F, right panel). The paradox may be due to reduced ability of antibodies to recognize the covalently cross-linked EGFR protein. Thus, the amount of EGFR dimer and its contribution into uncontrolled cell growth may be underestimated based on the data obtained by Western blot. The level of EGFR signaling was evaluated by immunopreciptating EGFR and probing for Grb2 and Ras. EGFR/Grb2 and EGFR/Ras interactions were significantly increased in MCT-treated rats (G). Results are expressed as mean±SEM; N=3–7. *P<0.05 vs. control group; †P<0.05 vs. PASMC co-cultured with MCTP treated PAEC.
To study whether SDS stable EGFR dimer is formed through dityrosine crosslinking, we developed an HPLC based assay. To stimulate oxidation of EGFR, HEK 293 cells over-expressing EGFR were exposed to CuCl2/H2O2. This condition is known to stimulate tyrosine oxidation and dityrosine crosslink formation. First, we measured total dityrosine fluorescence in HEK cells lysates to measure total tyrosine oxidation. Interestingly, we found no increase in dityrosine formation (Fig. 3C) suggesting that CuCl2/H2O2 exposure does not simply induce the global non-specific oxidation of cellular proteins. Next, EGFR was immunoprecipitated from cell lysates and the monomer and SDS resistant dimer of EGFR were separated by SDS-PAGE. Bands corresponding to EGFR monomer and dimer were then digested with trypsin and the resulting peptides were analyzed by HPLC. The fluorescence of the eluted peptides was then monitored to detect a dityrosine signal. No dityrosine fluorescence was observed in the EGFR monomer band in the cells treated with CuCl2/H2O2 (Fig. 3D). In contrast, the SDS-resistant EGFR dimer showed a significant accumulation of peptides specific to dityrosine fluorescence (Fig. 3D). From these data we conclude that the SDS-resistant EGFR dimer represents a covalently modified EGFR formed by dityrosine cross-links between two EGFR monomers.
To determine if the increase in covalent EGFR dimerization takes place in vivo, we measured the levels of EGFR dimer in the lungs of rats treated with MCT, either acutely (3 days) or chronically (14 days) (Fig. 4). In accordance with previously published data [21] we did not find any changes in total EGFR levels at either 3- (Fig. 3E, left panel) or 14-days (Fig. 5A, left panel). However, the levels of the EGFR dimer (Fig. 3E, middle panel and Fig. 5A, right panel) were significantly increased. It is important to mention that the antibodies used to analyze the level of EGFR bind less well to the EGFR dimer as compared to the EGFR monomer (Fig. 3F) suggesting that the levels of EGFR dimerization may be significantly underestimated when analyzed by Western blotting. The same results were obtained with other EGFR antibodies from different sources (data not shown). As in the cell culture studies, the EGFR dimer was highly auto-phosphorylated (Fig. 3E right panel and Fig. 5B), although there was no evidence of increased auto-phosphorylation of the EGFR monomer (a subset of which represents the non-covalent EGFR dimer, Fig. 3E right panel and Fig. 5B). To determine if auto-phosphorylated EGFR dimer is capable of inducing downstream signaling, we examined the ability of EGFR to form a complex with two of its important signaling intermediates, Ras and Grb2. Our immunoprecipitation results indicate that there are enhanced interactions between EGFR/Ras and EGFR/Grb2 after both 3- (Fig. 3G) and 14-days (Fig. 5C and D). Moreover, we observed a significantly increased proliferation in the lungs 14 days after MCT treatment, as estimated by measuring the levels of PCNA (Fig. 5E) and elevated amounts of the smooth muscle cells marker, caldesmon (Fig. 5F), indicating the activation of a mitogenic pathway in pulmonary SMC.
Fig. 4.
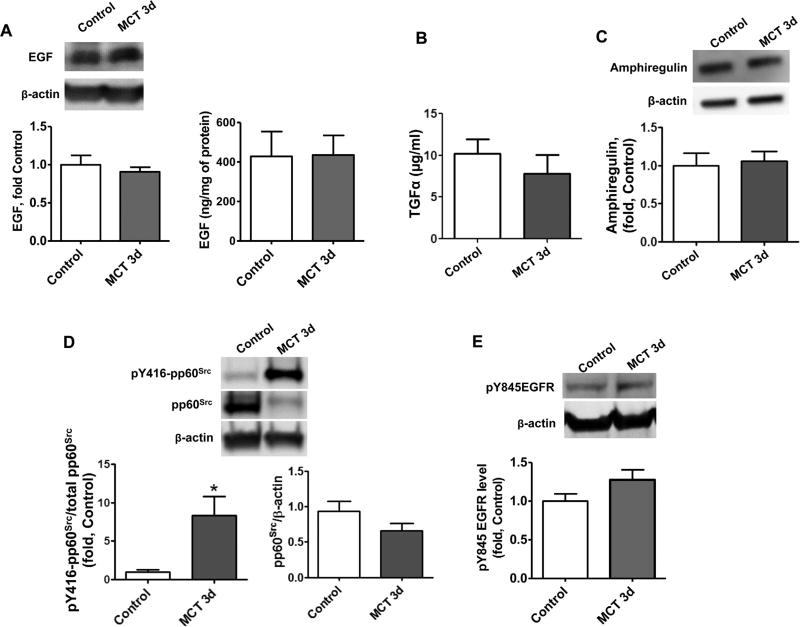
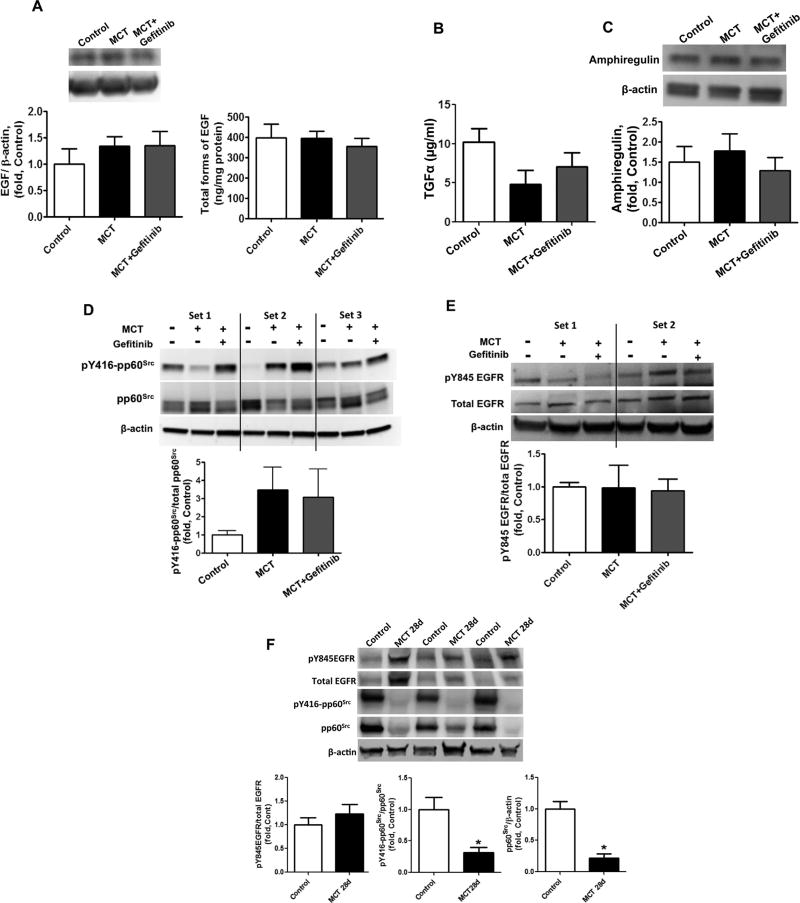
Acute MCT exposure stimulates EGF, TGFα, Amphiregulin- and pp60Src-independent activation of EGFR signaling. The levels of EGF in peripheral lung tissue lysates were measured by Western blot analysis (A, left panel) and ELISA (A, right panel) and no significant changes were observed between control rats and MCT-treated rats at 3d. No changes were observed in the alternate EGFR receptor ligands TGFα (B) or amphiregulin (C). Although pp60Src activity was significantly increased in MCT-treated rats as estimated by Western blot analysis of pY416-pp60Src (D) no significant increase was observed in pp60Src dependent activation of EGFR as estimated by measuring the phosphorylation of Y845EGFR, a pp60Src dependent site of EGFR, by Western blot analysis and normalization to the total level of EGFR in peripheral lung tissue (E). Results are expressed as mean±SEM; N¼6–8. *Po0.05 vs. control group.
Fig. 5.
Stimulation of EGFR signaling is attenuated by selective EGFR kinase inhibition. Total EGFR protein levels were measured by Western blot analysis and normalized with β-actin in peripheral lung lysates prepared from control rats and rats treated for 14 days with MCT in the presence or absence of the EGFR kinase inhibitor, gefitinib (30 mg/kg by oral gavages, daily). No differences in EGFR protein levels were observed between groups (A, left panel). The levels of SDS-resistant EGFR dimer were also evaluated by performing Western blot analysis. The level of SDS-resistant EGFR dimer was significantly increased in lung tissues of MCT treated rats and this increase was not significantly attenuated by gefitinib (A, right panel). However, the increased ratio of autophosphorylated EGFR dimer/autophosphorylated EGFR monomer in lung tissues of MCT treated rats was significantly attenuated by treatment with gefitinib (B). The level of EGFR signaling was evaluated by immunopreciptating EGFR and probing for Grb2 and Ras. EGFR/Grb2 (C) and EGFR/Ras (D) interactions were significantly increased in MCT-treated rats and this was attenuated by gefitinib. The total level of proliferation in peripheral lung tissue was evaluated by measuring PCNA protein levels by Western blot analysis. The levels of PCNA were normalized to β-actin. The levels of PCNA are significantly increased in MCT-treated rats and this is attenuated by gefitinib (E). The proliferation of pulmonary SMC was also evaluated by measuring the levels of the SMC marker, caldesmon by Western blot analysis. The levels of caldesmon were normalized to β-actin. The levels of caldesmon are significantly increased in MCT-treated rats and this is attenuated by gefitinib (F). Results are expressed as mean±SEM; N=3–7. *P<0.05 vs. control group. †P<0.05 vs. MCT alone.
Although MCT treatment increased EGFR signaling, the levels of the primary EGFR ligands epidermal growth factor (EGF), TGFα and amphiregulin—were found to be unchanged, compared to healthy control animals, suggesting the ligand-independent activation of EGFR in our models (Fig. 4A – C and Fig. 6A – C). Oxidative stress is known to activate pp60src-mediated signaling and the formation of a complex between pp60src and EGFR can induce phosphorylation of EGFR at Y845 and increase EGFR signaling [38,39]. Therefore, it was important to investigate, whether EGFR signaling up-regulation occurs through pp60src mediated pathways. As we expected, the severe oxidative stress induced a significant increase in pp60src activation in our acute MCT model, as estimated by increases in the Y416 pp60src phosphorylation (Fig. 4D). However, by day 14 of the study the activity of pp60src was not significantly different from control levels (Fig. 6D). Further analyses demonstrated that 28 days after MCT injection, pp60src protein and activity were significantly reduced (Fig. 6F). Importantly, the phosphorylation of EGFR at Y845 was similar to control values at 3-days (Fig. 4E), 14 days (Fig. 6E) and 28 days (Fig. 6F) after exposure to MCT, suggesting that EGFR phosphorylation at Y845 does not correlate with pp60src activity.
Fig. 6.
EGF, TGFα, Amphiregulin- and pp60Src -independent activation of EGFR signaling after chronic exposure to MCT. The levels of EGF in peripheral lung tissue lysates were measured by Western blot analysis (A, left panel) and ELISA (A, right panel) and no significant changes were observed between control rats or MCT-treated rats in the presence or absence of gefitinib. No changes were observed in the alternate EGFR receptor ligands TGFα (B) or amphiregulin (C). The activity of pp60Src was also analyzed by Western blot analysis. Shown are the Western blot data from three different sets of rats. The levels of p-Y416pp60Src were normalized to the level of pp60Src. The activity of pp60Src was not significantly different between groups (D). No significant differences were observed between groups in pp60Src dependent activation of EGFR, as estimated by measuring the phosphorylation of Y845EGFR, a pp60Src dependent site of EGFR, by Western blot analysis and normalization to the total level of EGFR in peripheral lung tissue (E). Shown are the Western blot data from two different sets of rats (E). No significant differences were observed in phosphorylation of Y845EGFR between control and the 28 days MCT group by Western blot analysis normalized to the total level of EGFR in peripheral lung tissue (F). The levels of p-Y416pp60Src and total pp60Src were significantly reduced between control and the 28 MCT group (F). Results are expressed as mean±SEM; N=4–7. P<0.05 vs. control group. †P<0.05 vs. MCT alone.
To confirm the role of EGFR signaling in the development of PH an additional group of animals was treated with a selective EGFR kinase inhibitor, gefitinib for 14 days, by daily oral dosing starting at day 0. EGFR inhibitor ablated EGFR auto-phosphorylation (Fig. 5B) and almost completely abolished activation of EGFR signaling (Fig. 5C and D) and accumulation of proliferative markers (Fig. 5E and F). In addition, although there was no significant right ventricle hypertrophy at this early stage of PH (data not shown), gefitinib treatment attenuated the MCT induced increase in right ventricle peak systolic pressure (RVPSP) (Table 1). Together with previously published data, which underlines an ability of EGFR kinase inhibitors to markedly attenuate PH at advanced stages of MCT induced PH [21], our results indicate that EGFR signaling is involved in the development of PH.
Table 1.
Right ventricle and systemic hemodynamic parameters. RVPSP=right ventricle peak systolic pressure, RVPDP=right ventricle peak diastolic pressure, +dP/dt=maximum dP/dt, −dP/dt=minimum dP/dt, HR=heart rate, mean BP=mean blood pressure. Results are expressed as mean ± SEM: N=8 in each group.
| Treatment | RVPSP (mmHg) | RVPDP (mmHg) | +dP/dt (mmHg/s) | −dP/dt (mmHg/s) | HR (B/s) | Mean-BP (mmHg) |
|---|---|---|---|---|---|---|
| Control | 28.12±2.12 | −3.71 ± 0.64 | 2604±192 | −2604 ± 856 | 365±24 | 104±8 |
| MCT | 44.73±7.49a | −1.91 ± 1.35a | 3741±1461a | −3384 ± 946 | 365±12 | 100 ±13 |
| MCT+Gefitinib | 30.42±4.45b | −4.11 ± 1.67b | 2557±384 | −2623 ± 288 | 354±28 | 97±10 |
| a1F-ANOVA, P< | 0.00006 | 0.0049 | 0.047 | 0.118 | 0.97 | 0.42 |
| b1F-ANOVA, P< | 0.0013 | 0.019 | 0.06 | 0.064 | 0.38 | 0.69 |
P<0.05 vs. Control group.
P<0.05 vs. MCT group.
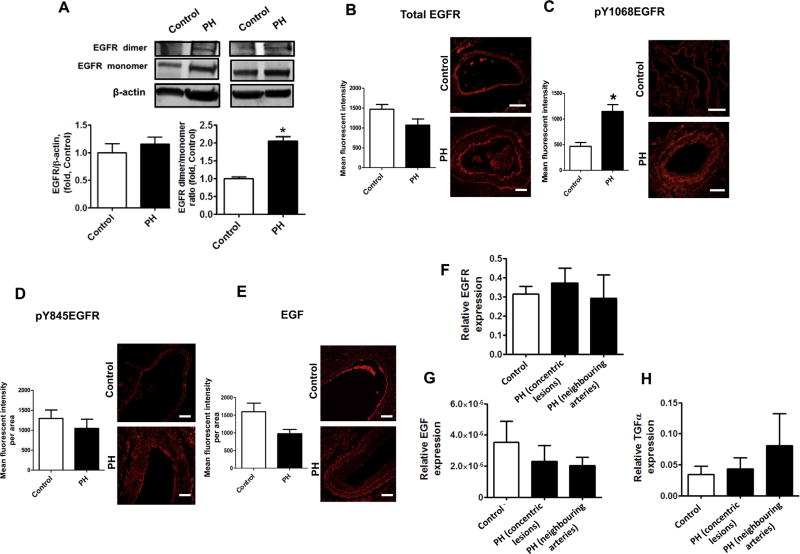
To study whether the activation of EGFR signaling occurs in human PH, lung samples obtained from patients with advanced PH and control subjects were used to measure the levels of EGFR dimer and its autophosphorylation. Although total EGFR protein levels in human lung tissue from PH patients were not increased (Fig.7A, left panel), the covalently modified EGFR dimer was increased (Fig. 7A, right panel). Further immunohistochemical studies confirmed the significant increase in EGFR auto phosphorylation in pulmonary hypertensive humans and provided evidence that the activated EGFR signaling originates in the pulmonary arteries (Fig. 7B and C). This increase in EGFR activation was also pp60src and EGF independent (Fig. 7D and E). Possible ligand dependent EGFR activation was further evaluated by measuring the expression of EGFR and its primary ligands inside concentric lesions and neighboring pulmonary arteries using laser-assisted microdissection followed by quantitative reverse transcription polymerase chain reaction (qRT-PCR). We observed no increases in mRNAs for EGFR (Fig. 7F), EGF (Fig. 7G) or TGFα (Fig. 7H) in either concentric lesions, or pulmonary arteries of PH subjects.
Fig. 7.
Ligand independent EGFR activation in pulmonary arteries of patients with advanced pulmonary hypertension. The levels of total EGFR in human peripheral pulmonary tissue were measured by Western blot analysis and normalized on the level of β-actin. There was no significant difference between total EGFR levels in controls and patients with advanced PH (A, left panel). Two representative sets are shown The levels of SDS-resistant EGFR dimer were also evaluated by performing Western blot analysis. Due to differences in expression levels, two different exposure times were used for each blot to measure EGFR monomers and EGFR dimers. Thus, each box represents the same gel but different exposure times. The levels of SDS-resistant EGFR dimer (A, right panel) was significantly increased in lung tissues patients with advanced PH. Immunofluorescence was performed on paraffin tissue sections using antibodies against EGFR (B), pY1068EGFR (C), pY845EGFR (D) and EGF (E) followed by an Alexa Fluor® 546 secondary antibody. Pulmonary artery walls of controls and patients with PH were traced to obtain mean fluorescent signal per area (n=60 pulmonary arteries). Representative images are shown. The fluorescent signal for EGFR (B), pY845EGFR (D) and EGF (E) were not different between controls and patients with advanced PH. However, EGFR auto-phosphorylation (pY1068EGFR) was significantly increased in the pulmonary artery wall of patients with advanced PH (C). Messenger RNA levels were also measured using laser-assisted microdissection followed by qRT-PCR in pulmonary arteries of control and concentric lesions and neighboring arteries in patients with advanced PH. EGFR (F) and EGF (G) and TGFα (H) mRNA levels were not different between controls and patients with advanced PH. Results are expressed as mean±SEM; N=3–17. *P<0.05 vs. controls. Scale bars are equal to 200 µm.
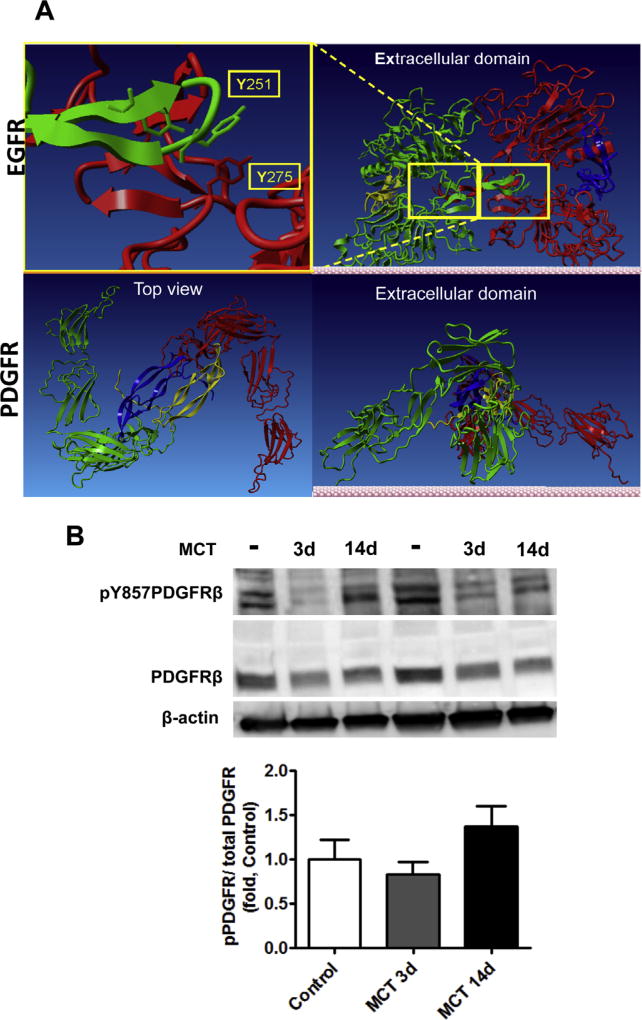
Finally, to evaluate whether the covalent dimerization found for the EGFR is a common mechanism of oxidative stress induced growth receptor activation, we evaluated the level of platelet-derived growth factor receptor (PDGFR) activation and dimerization in rat pulmonary tissue 3 and 14 days after MCT treatment (Fig. 8A). PDGF signaling has been previously implicated into PH induced pulmonary vascular remodeling [40,41]. However, we found that at early stages of PH (3 and 14 days after MCT injection), the phosphorylation level of PDGFRβ was not significantly different comparing to controls. Nevertheless, this does not contradict to the previously published data [40], as the activation of PDGFR in MCT model was evaluated only on the developed stages of PH. Besides, the level of PDGFR phosphorylation was normalized on a loading control, not on the total PDGFR level, although the expression of PDGFR was significantly increased. Interestingly, we did not find any evidence of PDGFRβ dimerization neither 3, nor 14 days after MCT injection (Fig. 8A), suggesting different mechanisms of EGFR and PDGFR activation. Indeed, our molecular analysis of extracellular and intracellular domains of EGFR have revealed the existence of at least two tyrosine residues (one in each domain) that could be involved in dityrosine bond formation upon their activation (Y251–Y275) in extracellular domain (Fig. 8B). This assumption is based on the computational models of the EGFR dimer, which showed a distance between these tyrosine residues is sufficient to form dityrosine bond upon oxidation. In contrast to EGFR, the homology model of PDGFR indicates no dimeric interface formed between dimerized PDGFR subunits due to separation by ligands (Fig. 8B).
Fig. 8.
PDGF receptor activation. Structural comparison of EGFR and PDGFR dimerization (A). Homology modeling of both EGFR and PDGFR revealed different dimerization characteristics of these receptors. Two EGFR subunits (red and green, upper insert) forms dimeric interface between subunits with stabilization by EGF ligands (yellow and blue). Upper insert indicates two tyrosine residues (Y251 and Y275) from adjacent subunits on the dimeric interface that could involve in dityrosine crosslink. In contrast PDGFR forms a dimer (red and green, lower insert) without direct contact between subunits. Top view (lower insert) indicates that adjacent monomeric subunits of PDGFR (green and red) are separated by PDGF ligands (blue and yellow) and are not capable to form the covalent crosslinks. The activity of PDGFR in pulmonary tissue was analyzed by the level of PDGFR phosphorylation p-Y857 PDGFRβ by Western blot analysis and normalized to the levels of total PDGFRβ. The activity of PDGFRβ was not significantly different between Control and MCT treated animals (B). Results are expressed as mean±SEM; N=6. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
It has been previously suggested that EGFR does not represent a promising target for the treatment of PH, based on the fact that the EGFR protein levels in the lungs of patients with idiopathic PH do not increase [21]. However, here for the first time, we provide evidence of increased EGFR signaling via formation of covalent crosslinks, in both an animal model of PH and in patients with advanced PH. We show that in PH, an activation of EGFR occurs via H2O2-mediated oxidation and the formation of a covalently modified dimeric EGFR. This oxidation event, just like ligand binding, is able to stimulate the intrinsic intracellular protein-tyrosine kinase activity of EGFR with subsequent activation of signal transduction cascades, leading to DNA synthesis and cell proliferation. EGFR dimerization plays an important role in stimulating SMC proliferation. Therefore, the formation of this covalently modified and, thus, constitutively active EGFR dimer may be important in the uncontrolled SMC proliferation observed in PH. In contrast, the level of EGFR expression in PAEC is relatively low. The survival signaling in EC is more dependent from VEGF signaling, rather than from EGFR. However, although this possibility was not explored in this study, the upregulation of EGFR may also contribute to EC growth.
Ligand independent EGFR activation has been reported previously [42,43], although these were in vitro studies using a serum free cell culture model. In the present study we have obtained additional evidence that ligand independent EGFR activation can stimulate cell growth in cultured PASMC via the formation of covalent cross-link between EGFR monomers. Further, for the first time, our data demonstrate the presence of cross-linked EGFR in rats with MCT-induced PH and in humans with advanced PH. Previous studies have shown that increased levels of H2O2 correlate with EGFR activation [44,45] which could be dependent on pp60src-family kinases activation [46]. However, it appears that in PH, although pp60src activity is increased early in the disease process (3 days after MCT injection), the level of pY845 EGFR was not found to significantly increased either at the early-, mid-, or late-stages of the disease (3-, 14- and 28-days after MCT injection respectively). The differences pp60src-EGFR signaling is unclear. However, it is possible that in PH, the phosphorylation of EGFR at Y845 is also modulated by a yet unknown, increased tyrosine phosphatase. It should be noted that EGFR activation independent of pp60src in PH is supported by our data in humans with advanced PH. Further, our animal and human data in combination, suggest that the up-regulation of EGFR signaling may be a common mechanism associated with the development of PH. It should be noted that, although we found that several EGFR ligands were unchanged in rats with PH, EGFR is promiscuous. Thus, it is possible that, besides its activation by covalent cross-linking, it is possible that ligand dependent activation may also be involved in increasing EGFR signaling during PH. Further, there is also a possibility that activation of pp60src could contribute to EGFR activation during PH development and progression but outside of the time points we evaluated.
Importantly, in our studies we have described a rare mechanism whereby covalent modifications in proteins are occurring through the formation of a dityrosine bond. Tyrosyl radicals are rapidly formed by the peroxidase-mediated decomposition of H2O2 or via the Fenton reaction. The tyrosyl radical is highly unstable and can react rapidly with surrounding water molecules or other free radicals. However, when tyrosyl radicals are formed in close proximity, they can recombine much faster than reacting with water or other free radicals. In our study we confirmed that the SDS resistant EGFR dimer contained a dityrosine bond. Covalent dityrosine modifications have been previously observed in other proteins, such as manganese superoxide dismutase (MnSOD) [47] and alpha-synuclein [48]. However, they have been considered to be involved in the oxidative damage to the protein resulting in a loss of activity. Thus, we have, for the first time, been able to demonstrate a biological role for a dityrosine cross-linked protein, both an in vivo model of PH and in the human disease, resulting in an increase in activity. Moreover, these covalently cross-linked EGFR dimers become constitutively active and independent of ligand stimulation. Although further studies will be required to identify the sites of dityrosine formation, we speculate that covalent dimerization may inhibit EGFR internalization and its proteosomal degradation due to covalent nature of the bond between subunits. Therefore, this will increase protein lifespan, leading to prolonged growth signaling.
PDGFR signaling was also shown to be important in PH vascular remodeling; however, our computational homology modeling provides the evidence that EGFR and PDGFR utilize a significantly different molecular structure that would result in the different mechanisms of the receptor’s activation. Indeed, EGFR has a dimeric interface between subunits and its activation is normally initiated by mechanic interaction of one subunit tail with kinase domain of another. Our analysis predicts that the dimeric interface of EGFR has also at least one pair of tyrosine residues that come close enough to form the dityrosine bond upon EGFR oxidation. In contrast, PDGFR does not have any dimeric interface between subunits and even upon ligand binding the subunits of PDGFR have no contact between each other, which prevent PDGFR from covalent dimerization. Besides, while EGFR has at least 11 ligands currently identified to initiate EGFR activation [49], each isoform of PDGFR recognizes only a specific type of PDGF. A selective PDGFR activation is achieving due to similarity of PFGBR binding region to immunoglobulin-like domain [50]. This precisely regulated mechanism would prevent any environmental factors, like oxidative stress, to initiate the PDGFR signaling.
In contrast, the oxidative modification of EGFR may represent an adaptive response of the cell to a potentially damaging environment. This response could be triggered by any pathological event that induces uncontrolled ROS accumulation. In our model the oxidative stress was induced by MCTP mediated inhibition of catalase and, perhaps, other cysteine-sensitive antioxidant enzymes. Indeed, the non-selective nature of MCTP binding to protein cysteine residues allows us to expect that not only catalase, but other proteins that have cysteines important for enzymatic activity can be affected. For example, cysteine residues of anti-oxidant enzymes such as glutathione reductase, glutathione per-oxidase, thioredoxin, thioredoxin reductase, peroxiredoxins, as well as cysteines in albumin and gluthathione that are also contribute to the overall redox homeostasis, can be directly modified by MCTP. However, apart from these proteins, for which the importance of their cysteine residues for anti-oxidant activity is well established, the role of cysteines in assembling of the active tetrameric form of catalase has never been reported before.
Indeed, despite the growing body of research dedicated to the problem of oxidative stress in various pathological conditions, the mechanisms involved in regulation of catalase, one of the primary antioxidant enzymes, is poorly understood. To our knowledge, there is only one published link between catalase cysteine oxidation and catalase inactivation [51]. Although this study identified the same cysteine (C377) as in our study, the molecular mechanism involved in catalase inactivation has until now not been elucidated. In the present study we provide a clear evidence of the critical role of C377 in catalase oligomerization due to its position on the dimeric interface of catalase (Fig. 3). Since catalase monomers are not catalytically active, this shift inevitably leads to a decrease in catalase activity. Although urea [52], low or high pH [53,54] and high temperature have been shown to induce dissociation of tetrameric catalase into a monomeric form, for the first time our study provides evidence of catalase tetramer disruption due to a catalase post-translational modification in a biological system. Even though humans are not exposed to the alkaloid MCT or its active metabolite, MCTP, our results provide a novel and very important evidence of the involvement of C377 in assembly of catalase tetramer and, thus, preservation of enzyme activity. Indeed, this new mechanism of catalase inactivation adds another source for the increased H2O2 levels observed in cardiovascular and other diseases. For example, NO donors or free thiols may potentiate H2O2 formation due to catalase monomerization. Thus, we have previously shown that NO donors can attenuate catalase activity in cultured PAEC [55].
In addition, in cancer there is a need to understand the mechanisms that contribute to the resistance of some tumors to tyrosine kinase inhibitors (TKI) targeting EGFR signaling [56]. Interestingly, there is a clear association between smoking and responsiveness to EGFR TKI therapy, with non-smokers benefiting the most [56]. Smoking history is now considered the most relevant factor that determines the sensitivity to TKI, although the reasons for this association remain unclear. Based on our results one can speculate that the high levels of H2O2, contained in gas phase of cigarette smoke, would oxidize EGFR and produce a covalently modified, constantly active EGFR dimer that could be less sensitive to EGFR TKI therapy. In PH, the treatment with novel TKI, dasatinib, was found to be associated with development of precapillary PH [57]. The non-selective nature of dasatinib, which inhibits several kinases including Src family, may severely impair the adaptive response to constant oxidative/nitrative stress, known to occur in PH patients. Therefore, the selective therapy that will inhibit only hyper-activated signaling may be needed.
In conclusion, our data have elucidated new mechanisms involved in the development of PH involving catalase inhibition and oxidative EGFR modification. Our data not only deepen our understanding regarding the importance of redox regulated post-translational mechanisms in the uncontrolled proliferation of SMC in PH, but suggest ways in which current therapies could be adjusted in order to increase their effectiveness. With regard to PH, we suggest that our data provide a robust background for applying anti-EGFR therapy and, perhaps, a rationale for combination therapy that includes both EGFR antagonism and antioxidants.
The role of epidermal growth factor receptor (EGFR) signaling in PH is controversial. EGFR induces pulmonary vasoconstriction [20], and EGFR antagonism prevents progression of animal model of PH. However, humans with advanced PH exhibit no changes in EGFR protein levels. In the present study we elucidated for the first time post-translational redox modification of EGFR that enhance its signaling in PH. We found that covalent dimerization of tyrosines stabilizes dimeric EGFR and increases its level of phosphorylation. This reflects in enhanced downstream signaling. Thus, inhibition of EGFR signaling has clinical importance in treatment of PH patients.
Acknowledgments
The authors would like to thank Marius M. Hoeper (Department of Respiratory Medicine, Hannover Medical School) and Gregor Warnecke (Department of Thoracic Surgery, Hannover Medical School) for providing clinical data and surgical samples from human patients, as well as Lavinia Maegel and Jonhanna Rische (both Institute of Pathology, Hannover Medical School) for their excellent technical support. This research was supported in part by grants HL60190 (to S.M.B.), HL67841 (to S.M.B.), HL084739 (to S.M.B.), and P01HL0101902 (to S.M.B.) all from the National Institutes of Health, a Transatlantic Network Development Grant from the Fondation Leducq (to S.M.B.), by an Entelligence Actelion Young Investigator Award for Research Excellence in Pulmonary Hypertension (to O.R.) and by a Scientist Development Grant from the American Heart Association National Office (to S.S.). O.R. was supported in part by National Institutes of Health Training Grant 5T32-HL06699 and in part by F32HL103136. R.R. was supported by a Scientist Development Grant from the American Heart Association 14SDG20480354. D.J. and B.L. are supported by the “In-tegriertes Forschungs- und Behandlungszentrum Transplantation” (IFB-Tx, German Federal Ministry of Education, reference number: 01EO0802).
Abbreviations
- PH
pulmonary hypertension
- EGFR
epidermal growth factor receptor
- EGF
epidermal growth factor
- MCT
monocrotaline
- SDS
sodium dodecyl sulfate
- ROS
reactive oxygen species
- NADPH
nicotinamide adenine dinucleotide phosphate
- eNOS
endothelial nitric oxide synthase
- MCTP
monocrotaline pyrrole
- MS
mass spectrometry
- PASMC
pulmonary artery smooth muscle cells
- PAEC
pulmonary artery endothelial cells
- PEG
polyethylene glycol
- FBS
fetal bovine serum
- HEK
human embryonic kidney cells
- HPLC
high performance liquid chromatography
- PAGE
polyacrylamide gel electrophoresis
- TGFα
tumor growth factor α
- RVPSP
right ventricle peak systolic pressure
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- mRNA
matrix RNA
- MnSOD
manganese superoxide dismutase
- TKI
tyrosine kinase inhibitors
- FFPE
formalin fixed paraffin-embedded
- PBS
phosphate buffer saline
- MTT
3-(4,5-dimethylthiazol-2-yl)—2,5-diphenyltetrazolium bromide
- H2DCFDA
2′,7′-dichlorodihydrofluorescein diacetate
- ATP
adenosine-5′-triphosphate
- DAB
diaminobenzidine
- CHCA
α-Cyano-4-hydroxycinnamic acid
- ELISA
enzyme-linked immunosorbent assay
- BSA
bovine serum albumin
- CHCl3
chloroform
- ANOVA
analysis of variance
Footnotes
Disclosures
The authors have nothing to disclose.
References
- 1.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 2.Tabima DM, Frizzell S, Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic. Biol. Med. 2012;52:1970–1986. doi: 10.1016/j.freeradbiomed.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Vizcaino F, Cogolludo A, Moreno L. Reactive oxygen species signaling in pulmonary vascular smooth muscle. Respir. Physiol. Neurobiol. 2010;174:212–220. doi: 10.1016/j.resp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol. Life Sci. 2012;69:2327–2343. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins P, Dawson J, Lees KR, McArthur K, Quinn TJ, Walters MR. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovasc. Ther. 2012;30:217–226. doi: 10.1111/j.1755-5922.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 6.d’Uscio LV. eNOS uncoupling in pulmonary hypertension. Cardiovasc. Res. 2011;92:359–360. doi: 10.1093/cvr/cvr270. [DOI] [PubMed] [Google Scholar]
- 7.Kang J, Pervaiz S. Mitochondria: redox metabolism and dysfunction. Biochem. Res. Int. 2012:896751. doi: 10.1155/2012/896751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walton PA, Pizzitelli M. Effects of peroxisomal catalase inhibition on mi-tochondrial function. Front. Physiol. 2012;3:108. doi: 10.3389/fphys.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 12.Schroder E, Eaton P. Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations. Curr. Opin. Pharmacol. 2008;8:153–159. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 14.Forman HJ. Use and abuse of exogenous H2O2 in studies of signal transduction. Free Radic. Biol. Med. 2007;42:926–932. doi: 10.1016/j.freeradbiomed.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nedeljkovic ZS, Gokce N, Loscalzo J. Mechanisms of oxidative stress and vascular dysfunction. Postgrad. Med. J. 2003;79:195–198. doi: 10.1136/pmj.79.930.195. (quiz pp. 198-200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao GN, Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ. Res. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- 17.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science (New York, NY) 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 18.Pourmahram GE, Snetkov VA, Shaifta Y, Drndarski S, Knock GA, Aaronson PI, Ward JP. Constriction of pulmonary artery by peroxide: role of Ca2+ release and PKC. Free Radic. Biol. Med. 2008;45:1468–1476. doi: 10.1016/j.freeradbiomed.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Herbst RS. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Norton CE, Broughton BR, Jernigan NL, Walker BR, Resta TC. Enhanced depolarization-induced pulmonary vasoconstriction following chronic hypoxia requires EGFR-dependent activation of NAD(P)H oxidase 2. Antioxid. Redox Signal. 2013;18:1777–1788. doi: 10.1089/ars.2012.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahal BK, Cornitescu T, Tretyn A, Pullamsetti SS, Kosanovic D, Dumitrascu R, Ghofrani HA, Weissmann N, Voswinckel R, Banat GA, Seeger W, Grimminger F, Schermuly RT. Role of epidermal growth factor inhibition in experimental pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2010;181:158–167. doi: 10.1164/rccm.200811-1682OC. [DOI] [PubMed] [Google Scholar]
- 22.Merklinger SL, Jones PL, Martinez EC, Rabinovitch M. Epidermal growth factor receptor blockade mediates smooth muscle cell apoptosis and improves survival in rats with pulmonary hypertension. Circulation. 2005;112:423–431. doi: 10.1161/CIRCULATIONAHA.105.540542. [DOI] [PubMed] [Google Scholar]
- 23.Toby IT, Chicoine LG, Cui H, Chen B, Nelin LD. Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am. J. Physiol. 2010;298:L600–L606. doi: 10.1152/ajplung.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonigk D, Golpon H, Bockmeyer CL, Maegel L, Hoeper MM, Gottlieb J, Nickel N, Hussein K, Maus U, Lehmann U, Janciauskiene S, Welte T, Haverich A, Rische J, Kreipe H, Laenger F. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am. J. Pathol. 2011;179:167–179. doi: 10.1016/j.ajpath.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonigk D, Golpon H, Bockmeyer CL, Maegel L, Hoeper MM, Gottlieb J, Nickel N, Hussein K, Maus U, Lehmann U, Janciauskiene S, Welte T, Haverich A, Rische J, Kreipe H, Laenger F. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am. J. Pathol. 2011;179:167–179. doi: 10.1016/j.ajpath.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wedgwood S, Black SM. Molecular mechanisms of nitric oxide-induced growth arrest and apoptosis in fetal pulmonary arterial smooth muscle cells. Nitric Oxide. 2003;9:201–210. doi: 10.1016/j.niox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Kelly LK, Wedgwood S, Steinhorn RH, Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am. J. Physiol. 2004;286:L984–L991. doi: 10.1152/ajplung.00224.2003. [DOI] [PubMed] [Google Scholar]
- 28.Wedgwood S, Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am. J. Physiol. 2005;288:L480–L487. doi: 10.1152/ajplung.00283.2004. [DOI] [PubMed] [Google Scholar]
- 29.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc. Natl. Acad. Sci. USA. 2004;101:2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Sud N, Fonseca FV, Hou Y, Black SM. Shear stress stimulates nitric oxide signaling in pulmonary arterial endothelial cells via a reduction in catalase activity: role of protein kinase C delta. Am. J. Physiol. 2010;298:L105–L116. doi: 10.1152/ajplung.00290.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catalase Aebi H. Methods Enzymatic Analysis. 1974;2:121–126. [Google Scholar]
- 32.Krieger E, Vriend G. New ways to boost molecular dynamics simulations. J. Comput. Chem. 2015;36:996–1007. doi: 10.1002/jcc.23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science (New York, NY) 1999;286:2507–2510. doi: 10.1126/science.286.5449.2507. [DOI] [PubMed] [Google Scholar]
- 34.Mattocks AR. Toxicity of pyrrolizidine alkaloids. Nature. 1968;217:723–728. doi: 10.1038/217723a0. [DOI] [PubMed] [Google Scholar]
- 35.Lame MW, Jones AD, Wilson DW, Segall HJ. Monocrotaline pyrrole targets proteins with and without cysteine residues in the cytosol and membranes of human pulmonary artery endothelial cells. Proteomics. 2005;5:4398–4413. doi: 10.1002/pmic.200402022. [DOI] [PubMed] [Google Scholar]
- 36.Ueda M, Kinoshita H, Maeda SI, Zou W, Tanaka A. Structure-function study of the amino-terminal stretch of the catalase subunit molecule in oligomerization, heme binding, and activity expression. Appl. Microbiol. Biotechnol. 2003;61:488–494. doi: 10.1007/s00253-003-1251-5. [DOI] [PubMed] [Google Scholar]
- 37.van der Vliet A, Hristova M, Cross CE, Eiserich JP, Goldkorn T. Peroxynitrite induces covalent dimerization of epidermal growth factor receptors in A431 epidermoid carcinoma cells. J. Biol. Chem. 1998;273:31860–31866. doi: 10.1074/jbc.273.48.31860. [DOI] [PubMed] [Google Scholar]
- 38.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leu TH, Maa MC. Functional implication of the interaction between EGF receptor and c-Src. Front. Biosci. 2003;8:s28–s38. doi: 10.2741/980. [DOI] [PubMed] [Google Scholar]
- 40.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J. Clin. Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barst RJ. PDGF signaling in pulmonary arterial hypertension. J. Clin. Invest. 2005;115:2691–2694. doi: 10.1172/JCI26593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997;16:7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai W, Morielli AD, Peralta EG. The m1 muscarinic acetylcholine receptor transactivates the EGF receptor to modulate ion channel activity. EMBO J. 1997;16:4597–4605. doi: 10.1093/emboj/16.15.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan EM, Lanir R, Danielson AR, Goldkorn T. Epidermal growth factor receptor exposed to cigarette smoke is aberrantly activated and undergoes perinuclear trafficking. FASEB J. 2008;22:910–917. doi: 10.1096/fj.06-7729com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao GN. Hydrogen peroxide induces complex formation of SHC-Grb2-SOS with receptor tyrosine kinase and activates Ras and extracellular signal-regulated protein kinases group of mitogen-activated protein kinases. Oncogene. 1996;13:713–719. [PubMed] [Google Scholar]
- 46.Filosto S, Khan EM, Tognon E, Becker C, Ashfaq M, Ravid T, Goldkorn T. EGF receptor exposed to oxidative stress acquires abnormal phosphorylation and aberrant activated conformation that impairs canonical dimerization. PLoS One. 2011;6:e23240. doi: 10.1371/journal.pone.0023240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch. Biochem. Biophys. 1999;366:82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 48.Lee EN, Lee SY, Lee D, Kim J, Paik SR. Lipid interaction of alpha-synuclein during the metal-catalyzed oxidation in the presence of Cu2+ and H2O2. J. Neurochem. 2003;84:1128–1142. doi: 10.1046/j.1471-4159.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 49.Bazley LA, Gullick WJ. The epidermal growth factor receptor family. Endocr. Relat. Cancer. 2005;12(Suppl. 1):S17–S27. doi: 10.1677/erc.1.01032. [DOI] [PubMed] [Google Scholar]
- 50.Heidaran MA, Pierce JH, Jensen RA, Matsui T, Aaronson SA. Chimeric alpha- and beta-platelet-derived growth factor (PDGF) receptors define three immunoglobulin-like domains of the alpha-PDGF receptor that determine PDGF-AA binding specificity. J. Biol. Chem. 1990;265:18741–18744. [PubMed] [Google Scholar]
- 51.Ghosh S, Janocha AJ, Aronica MA, Swaidani S, Comhair SA, Xu W, Zheng L, Kaveti S, Kinter M, Hazen SL, Erzurum SC. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J. Immunol. 2006;176:5587–5597. doi: 10.4049/jimmunol.176.9.5587. [DOI] [PubMed] [Google Scholar]
- 52.Takeda A, Hirano K, Shiroya Y, Samejima T. On the denaturation of porcine erythrocyte catalase with alkali, urea, and guanidine hydrochloride in relation to its subunit structure. J. Biochem. 1983;93:967–975. doi: 10.1093/oxfordjournals.jbchem.a134252. [DOI] [PubMed] [Google Scholar]
- 53.Prajapati S, Bhakuni V, Babu KR, Jain SK. Alkaline unfolding and salt-induced folding of bovine liver catalase at high pH. Eur. J. Biochem./FEBS. 1998;255:178–184. doi: 10.1046/j.1432-1327.1998.2550178.x. [DOI] [PubMed] [Google Scholar]
- 54.Samejima T, Miyahara T, Takeda A, Hachimori A, Hirano K. On the acid denaturation of porcine erythrocyte catalase in relation to its subunit structure. J. Biochem. 1981;89:1325–1332. [PubMed] [Google Scholar]
- 55.Brennan LA, Wedgwood S, Black SM. The overexpression catalase reduces NO-mediated inhibition of endothelial NO synthase. IUBMB life. 2002;54:261–265. doi: 10.1080/15216540215679. [DOI] [PubMed] [Google Scholar]
- 56.Hammerman PS, Janne PA, Johnson BE. Resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin. Cancer Res. 2009;15:7502–7509. doi: 10.1158/1078-0432.CCR-09-0189. [DOI] [PubMed] [Google Scholar]
- 57.Montani D, Bergot E, Gunther S, Savale L, Bergeron A, Bourdin A, Bouvaist H, Canuet M, Pison C, Macro M, Poubeau P, Girerd B, Natali D, Guignabert C, Perros F, O’Callaghan DS, Jais X, Tubert-Bitter P, Zalcman G, Sitbon O, Simonneau G, Humbert M. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125:2128–2137. doi: 10.1161/CIRCULATIONAHA.111.079921. [DOI] [PubMed] [Google Scholar]