Abstract
Talimogene laherparepvec is a genetically modified herpes simplex virus type 1–based oncolytic immunotherapy for the local treatment of unresectable subcutaneous and nodal tumors in patients with melanoma recurrent after initial surgery. We report on two patients with melanoma who, after progression on numerous systemic therapies, derived clinical benefit from talimogene laherparepvec in an expanded-access protocol (ClinicalTrials.gov, NCT02147951). Intralesional talimogene laherparepvec (day 1, ≤4 ml 106 PFU/ml; after 3 weeks, ≤4 ml 108 PFU/ml every 2 weeks) was administered until complete response, no injectable tumors, progressive disease, or intolerance occurred. Patient 1 was 71 years old, had stage IIIB disease, and had previously received granulocyte–macrophage colony-stimulating factor, vemurafenib, metformin, ipilimumab, dabrafenib, trametinib, and pembrolizumab. Patient 2 was 45 years old, had stage IIIC disease, and had previously received nivolumab/ipilimumab combination therapy. There were marked reductions in the number and size of melanoma lesions during treatment with talimogene laherparepvec. Both patients experienced mild-to-moderate nausea and vomiting, which were managed using ondansetron, metoclopramide, and pantoprazole. Both patients completed treatment with talimogene laherparepvec in the expanded-access protocol on 24 November 2015, but received talimogene laherparepvec in clinical practice. Patient 1 continues to receive therapy (>60 weeks); patient 2 experienced a complete response at 23 weeks. Immunohistochemistry of a biopsied dermal metastasis from patient 1 showed a marked infiltration of CD4+ and CD8+ T cells after 1 year of treatment. Talimogene laherparepvec was active in patients with advanced melanoma with disease progression following multiple previous systemic therapies; no new safety signals were identified.
Keywords: cutaneous malignant melanoma, immunotherapy, oncolytic viruses, talimogene laherparepvec
Introduction
Several newly approved treatments have shown improved outcomes in advanced melanoma. These therapies include small-molecule agents (e.g. dabrafenib 1, vemurafenib 2, trametinib 1,3, and cobimetinib 4), immunotherapies (e.g. ipilimumab 5, pembrolizumab 6, and nivolumab 7), and the oncolytic immunotherapy talimogene laherparepvec 8.
Talimogene laherparepvec is a genetically modified herpes simplex virus type 1 designed to selectively replicate in and lyse tumor cells, causing the release of tumor-derived antigens, promoting a regional and systemic antitumor response 9. In the phase 3 OPTiM study, intralesional talimogene laherparepvec treatment resulted in a significant improvement in the durable response rate (DRR; the primary endpoint) versus subcutaneous granulocyte–macrophage colony-stimulating factor (GM-CSF; 16 vs. 2%; P<0.0001) 8. DRR was higher among patients who received talimogene laherparepvec as first-line therapy (23.9%; n=138) than those who received it as second-line or later therapy (9.6%; n=157) 8. Talimogene laherparepvec is approved in the USA for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in melanoma recurrent after initial surgery; talimogene laherparepvec has not been shown to improve overall survival or have an effect on visceral metastases 9. In addition, in January 2016, the European Medicines Agency approved talimogene laherparepvec for the treatment of unresectable melanoma that is regionally or distantly metastatic (stage IIIB/IIIC/IVM1a) with no bone, brain, lung, or other visceral disease 10.
Given the range of therapies available for advanced melanoma, patients may receive multiple lines of therapy and have varying responses to each. Notably, evidence suggests that outcomes with some agents may differ on the basis of previous treatment exposure 11. In the OPTiM study, 47% had not received previous systemic therapy and, because patients were enrolled between May 2009 and July 2011, the extent of previous treatment with immunotherapy or targeted agents for those who had was likely to be limited. Consequently, it has been unclear whether, or how, previous therapy might influence response to talimogene laherparepvec. We present two patients with melanoma who had received multiple therapies before receiving talimogene laherparepvec in an expanded-access protocol (ClinicalTrials.gov, NCT02147951). Results from the full analysis of the extended-access protocol have been published 12.
Methods
Clinical
Patients received intralesional talimogene laherparepvec dosing [≤4 ml 106 plaque-forming units (PFU)/ml on day 1, then after 3 weeks, ≤4 ml 108 PFU/ml once every 2 weeks]. Treatment was continued until complete response, no injectable tumors remained, progressive disease, or intolerance. Photography (patient 1), microcaliper measurements (patients 1 and 2), and full-body computed tomography (CT) imaging (patients 1 and 2) were performed to assess clinical response every 3 months.
Immunohistochemistry
Immunohistochemistry (IHC) was performed using a Bond-III automated staining platform (Leica Biosystems, Buffalo Grove, Illinois, USA). Epitope retrieval was performed using an EDTA-based epitope retrieval solution (pH 9.0; 20 min), followed by incubation with H2O2 (5 min) and then the primary antibody (15 min) at room temperature. Detection was performed using the Bond Polymer Refine Detection kit (cat. DS9800), which required incubation with 3,3′-diaminobenzidine (10 min) and counterstaining with hematoxylin (10 min). Antibodies (Cell Marque Corporation, Rocklin, California, USA) used included mouse anti-HMB45 antibody (clone HMB-45, 282M-96; Cell Marque Corporation), mouse anti-CD4 antibody (4B12, PA0427; Cell Marque Corporation), and mouse anti-CD8 (4B11, PA0183; Cell Marque Corporation). The presence of melanoma cells and the phenotypic response to these cells were evaluated by an experienced pathologist (M.F.).
Case presentations
Patient 1
Patient 1 was a 71-year-old white man with stage IIIC melanoma with in-transit metastases who started talimogene laherparepvec treatment on 20 May 2015. His medical history included hypertension, chronic obstructive pulmonary disease, kidney cancer, benign prostate hypertrophy, pain, hypothyroidism, elevated cholesterol, nausea, coronary artery disease, gastroesophageal reflux disease, depression, myocardial infarction, itching, and dry skin.
The patient initially developed a 1.1 mm deep melanoma of the right calf in April 2009. He underwent wide local excision, and sentinel lymph node biopsy, which was negative for melanoma on 29 June 2009. A biopsy of a suspicious nodule on the right upper anterior tibia in October 2009 identified in-transit metastases of melanoma (stage IIIC). Fifteen biopsies of additional in-transit metastases were positive for melanoma between October 2009 and March 2013 (right anterior tibia, n=11; mid inner thigh and calf, n=2; knee, n=1; shin, n=1); all were resected. Additional biopsies from the left thigh, lower back, and right lateral bicep were negative for melanoma.
Beginning September 2011, the patient received subcutaneous recombinant human GM-CSF as adjuvant therapy, completing treatment in April 2012. After a fine-needle aspirate biopsy in 2012, he was found to have BRAF V600E mutant lesions; subsequently, vemurafenib and metformin on a phase 1/2 clinical trial were administered from June 2012 to October 2012, but were stopped because of toxicity. Ipilimumab 3 mg/kg was administered from December 2013 to March 2014.
After identification of recurrent disease, the patient initiated twice-daily dabrafenib 500 mg and trametinib 2 mg treatment in May 2014; treatment ended in June 2014 owing to toxicity. In November 2014, he received eight cycles of intravenous pembrolizumab 2 mg/kg once every 3 weeks; treatment was stopped in April 2015 because of progressive disease in the right groin identified by CT imaging.
At the time of enrollment in the talimogene laherparepvec expanded-access protocol, after progressing on anti-CTLA-4 therapy, BRAF/MEK inhibitors, and anti-PD-1 therapy, the patient had an Eastern Cooperative Oncology Group performance status of 1. He had four large clusters of dermal melanoma metastases on the right lower extremity, from groin to thigh. The clusters measured 105×21 mm (groin), 65×40 mm (medial thigh), 41×52 mm (lower thigh), and 65×52 mm (posterior thigh). CT imaging of the chest, abdomen, and pelvis (29 April 2015) identified an enlarging inguinal nodule (10 mm) and a right upper inguinal node (7 mm) adjacent to the femoral artery. The patient consented to receive talimogene laherparepvec on 5 May 2015. On 20 May 2015, he received the first of 14 treatments with intralesional talimogene laherparepvec (initial dose, 106 PFU/ml; subsequent doses, 108 PFU/ml). The second dose was administered 20 days after the first, and subsequent doses were administered every 14 days. The drug was not administered during two cycles. Talimogene laherparepvec treatment on the expanded-access protocol was completed on 24 November 2015, following approval by the US Food and Drug Administration. He then started talimogene laherparepvec in the clinical practice setting.
Photographs of lesions were taken at baseline (21 April 2015; Fig. 1a and c) and after talimogene laherparepvec treatment for 1 year (17 April 2016; Fig. 1b and d). Following intralesional administration, there was a significant reduction in lesion size on the right lower extremity, consistent with a partial response by Response Evaluation Criteria in Solid Tumors version 1.1, that has continued through submission of this manuscript. Three-month full-body CT imaging was performed, and no visceral metastases have developed to date during talimogene laherparepvec treatment (most recent imaging occurred on 5 May 2016).
Fig. 1.
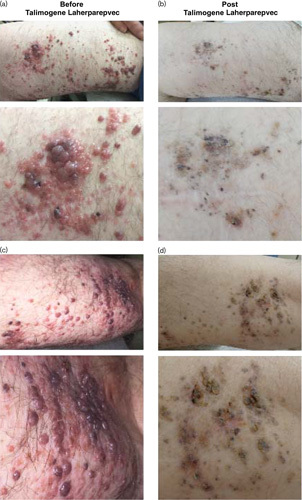
Regression of multiple in-transit melanoma metastases in patient 1 who had previously received BRAF/MEK inhibitors and immune checkpoint inhibitors. Photographs of lesions on the right lower extremity of patient 1 at baseline (a, c) and after intralesional administration of talimogene laherparepvec for 1 year (b, d).
Adverse events (AEs) that occurred during talimogene laherparepvec treatment included vomiting, fever, weakness, and a fractured femur. Concomitant therapies included betamethasone and clotrimazole for itching, and prochlorperazine and ondansetron for nausea. Other therapies administered during the study were hydrocodone, levothyroxine, atorvastatin, lisinopril, hydrocodone/acetaminophen, pantoprazole, metoclopramide, furosemide, macrogol 3350, bupropion, tiotropium, tamsulosin, cholecalciferol, carvedilol, aspirin, and fluticasone/salmeterol.
To better understand why the patient responded to talimogene laherparepvec treatment after progressing with treatment from most other available FDA-approved agents, including immune checkpoint inhibitors and BRAF/MEK inhibitors, we analyzed a biopsy taken for diagnostic reasons for the presence of CD4+ and CD8+ T cells after 1 year of therapy. We found that the dermal metastasis only consisted of small clusters of melanoma cells, and there was extensive peripheral infiltration of CD4+ and CD8+ T cells (Fig. 2). Higher magnification showed that certain clusters of melanoma cells were surrounded by both CD4+ and CD8+ T cells (Fig. 2).
Fig. 2.
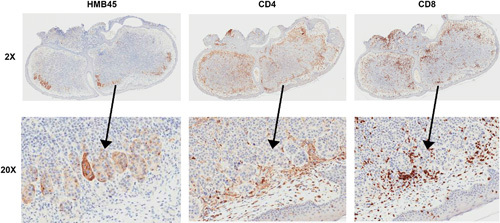
CD4+ and CD8+ T cell infiltration of in-transit melanoma metastasis after 1 year of intralesional administration of talimogene laherparepvec. A solitary in-transit melanoma metastasis was biopsied and analyzed by IHC for the presence of HMB45+ melanoma cells, CD4+ T cells, and CD8+ T cells. Infiltration of CD4+ T cells and CD8+ T cells was noted in the peripheral aspects of the mass, which was largely devoid of melanoma cells determined by HMB45 staining.
Patient 2
Patient 2 was a 45-year-old white man with stage IIIC melanoma with in-transit dermal metastases who started talimogene laherparepvec on 26 August 2015. His medical history included seizures, adrenal insufficiency, hearing loss, swollen right ankle and leg, dizziness, anxiety, and attention-deficit disorder with hyperactivity. He initially developed melanoma of the right foot in June 2010, detected by excisional biopsy. This was followed by sentinel lymph node dissection in July 2010 that tested negative for melanoma. The patient underwent a tumor dissection of the right inguinal lymph node in August 2010 that tested negative for melanoma. In May 2014, he had an excisional biopsy of the right leg and thigh that tested positive for melanoma. In November 2014, combination ipilimumab and nivolumab therapy was started; the patient completed treatment in January 2015, but experienced autoimmune hepatitis and discontinued maintenance nivolumab. He suffered from progression of dermal metastases, proven by biopsy in June 2015.
The patient consented to receive talimogene laherparepvec on 18 August 2015. At enrollment, he had an Eastern Cooperative Oncology Group performance status of 0. Beginning 28 August 2015, he received intralesional talimogene laherparepvec on seven occasions (initial dose, 106 PFU/ml; subsequent doses, 108 PFU/ml). The first dose was administered 21 days before the second, and subsequent doses were administered every 14 days. CT imaging showed no baseline visceral metastases.
The patient completed study treatment on 24 November 2015, when talimogene laherparepvec was approved. He subsequently began talimogene laherparepvec treatment outside the study and completed 10 weeks of treatment until he experienced a complete response, as determined by biopsy and full-body imaging in February 2016.
AEs occurring during talimogene laherparepvec treatment included nausea, vomiting, rash, and itching. Concomitant therapies included ondansetron, promethazine, metoclopramide, pantoprazole, and famotidine for nausea/vomiting; vancomycin for rash; triamcinolone for rash/itching; and hydrocortisone for nausea, vomiting, and adrenal insufficiency. Other therapies administered during the study were epinephrine, methylsulfonylmethane, lorazepam, herbal preparations, supplements, and curcuma longa rhizome.
Discussion
This report presents two cases of melanoma from an expanded-access protocol and highlights the clinical benefit derived from talimogene laherparepvec treatment after progression on multiple previous therapies. Both patients had received standard previous systemic therapies, including GM-CSF, checkpoint inhibitors (pembrolizumab, ipilimumab), BRAF inhibitors (dabrafenib, vemurafenib), and MEK inhibitors (tramentinib; patient 1), and the most potent immune checkpoint inhibitor combination (nivolumab/ipilimumab; patient 2). Notwithstanding this extensive previous therapy, both had significant reductions in the number and size of melanoma lesions during talimogene laherparepvec treatment. Given that it is an oncolytic immunotherapy designed to selectively replicate and lyse tumor cells, it is not surprising that previous exposure to systemic therapies did not result in resistance to this oncolytic immunotherapy. Both patients continued to receive talimogene laherparepvec after the completion of the expanded-access protocol. AEs occurring during talimogene laherparepvec treatment (including vomiting and nausea) were consistent with those previously reported in talimogene laherparepvec clinical studies 8,13,14.
The patients described here presented significant clinical challenges, and the outcomes indicate the feasibility and efficacy of talimogene laherparepvec, even after extensive previous systemic therapy. This is an area of significant unmet need: patients who have progressed on current therapies often have limited remaining treatment options and may have a performance status that renders them ineligible for standardized clinical trials. Our results show that clinical benefit can be achieved in such patients with talimogene laherparepvec. Although outcomes in these heavily pretreated patients are encouraging, it is important to note that in the phase 3 OPTiM study, improvements in DRR and overall survival were most pronounced in patients with stage IIIB/IIIC/IVM1a disease and who had not previously received systemic therapy for melanoma 8.
In summary, talimogene laherparepvec showed efficacy in two patients with advanced melanoma who had disease progression following multiple previous systemic therapies and had a toxicity profile consistent with previously reported clinical trials. Phase 1/3 clinical trials of talimogene laherparepvec in combination with pembrolizumab for the treatment of melanoma and squamous cell carcinoma of the head and neck are ongoing 15,16. In addition, a phase 1 trial of intrahepatic injection of talimogene laherparepvec for the treatment of liver tumors is also currently evaluating the first patient cohort. The observation reported here that a talimogene laherparepvec–injected dermal metastasis had significant CD4+ and CD8+ T cell infiltration indicates that talimogene laherparepvec may induce tumor immunity. Talimogene laherparepvec, alone or in combination with immune checkpoint inhibitors, may have utility as treatment for multiple solid tumor types 15–19.
Acknowledgements
The authors thank Jennifer Venzie, PhD (Complete Healthcare Communications, LLC, West Chester, Pennsylvania, USA) for medical writing assistance in the preparation of this manuscript.
This research was supported by the James Graham Brown Foundation and Amgen Inc.
Conflicts of interest
J.C. has received consulting fees/honoraria and travel/accommodation expenses from Amgen Inc. and has been a scientific advisory board member and speakers bureau member for Amgen Inc. S.R. has received consulting fees/honoraria and travel/accommodation expenses from Amgen Inc. N.B. was an employee of Amgen Inc. at the time this study was carried out. Y.I-F., S.T., M.B., and M.F. have no conflicts of interest.
References
- 1.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380:358–365. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015; 386:444–451. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371:1867–1876. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320–330. [DOI] [PubMed] [Google Scholar]
- 8.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015; 33:2780–2788. [DOI] [PubMed] [Google Scholar]
- 9.IMLYGIC® (Talimogene Laherparepvec). Full prescribing information. Thousand Oaks, CA: Amgen Inc.; 2015. [Google Scholar]
- 10.IMLYGIC® (Talimogene Laherparepvec). Summary of product characteristics. Breda, The Netherlands: Amgen Europe B.V.; 2016. [Google Scholar]
- 11.Ackerman A, Klein O, McDermott DF, Wang W, Ibrahim N, Lawrence DP, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer 2014; 120:1695–1701. [DOI] [PubMed] [Google Scholar]
- 12.Chesney J, Awasthi S, Curti B, Hutchins L, Linette G, Triozzi P, et al. Phase IIIb safety results from an expanded-access protocol of talimogene laherparepvec for patients with unresected, stage IIIB-IVM1c melanoma. Melanoma Res 2018; 28:44–51. [DOI] [PubMed] [Google Scholar]
- 13.Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 2009; 27:5763–5771. [DOI] [PubMed] [Google Scholar]
- 14.Hu JCC, Coffin RS, Davis CJ. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 2006; 12:6737–6747. [DOI] [PubMed] [Google Scholar]
- 15.Harrington KJ, Kong A, Mach N, Rordorf T, Corral J, Esperli V, et al. Early safety from phase 1b/3, multicenter, open-label, randomized trial of talimogene laherparepvec (T-VEC)+pembrolizumab (pembro) for recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): MASTERKEY-232. Ann Oncol 2017; 28:v428–v448. [Google Scholar]
- 16.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 2017; 170:e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res 2010; 16:4005–4015. [DOI] [PubMed] [Google Scholar]
- 19.Chang KJ, Senzer NN, Binmoeller K, Goldsweig H, Coffin R. Phase I dose-escalation study of talimogene laherparepvec (T-VEC) for advanced pancreatic cancer (ca). J Clin Oncol 2012; 30:e14546. [Google Scholar]


