Supplemental digital content is available in the text.
Key Words: 12-Month survival rate, ADXS11-001, Cervical cancer, Cisplatin, Immunotherapy
Abstract
Objectives
A global unmet medical need exists for effective treatments for persistent, recurrent, or metastatic cervical cancer, as patients have a short life expectancy. Recently, immunotherapies have shown promising survival benefits for patients with advanced forms of cancer. Axalimogene filolisbac (ADXS11-001), a Listeria monocytogenes immunotherapy with a broad effect on the immune system, is under investigation for treatment of human papillomavirus–associated cancers including cervical cancer.
Methods
This phase 2 study evaluated the safety and efficacy of ADXS11-001, administered with or without cisplatin, in patients with recurrent/refractory cervical cancer following prior chemotherapy and/or radiotherapy. A total of 109 patients were treated, and 69 were evaluable for tumor response at equal to or more than 3 months postbaseline.
Results
Median overall survival (OS) was comparable between treatment groups (ADXS11-001: 8.28 months; 95% confidence interval [CI], 5.85–10.5 months; ADXS11-001 + cisplatin: 8.78 months; 95% CI, 7.4–13.3 months). The 12- and 18-month milestone OS rates were 30.9% versus 38.9%, and 23.6% versus 25.9% for each group, respectively (34.9% and 24.8% combined). Median progression-free survival (6.10 vs 6.08 months) and the overall response rate (17.1% vs 14.7%) were similar for both groups. ADXS11-001 was generally well tolerated; adverse events were predominantly mild to moderate in severity and not related to treatment. More adverse events were reported in the combination group (429 vs 275).
Conclusions
These promising safety and efficacy results, including the encouraging 12-month 34.9% combined OS rate, warrant further investigation of ADXS11-001 for treatment of recurrent/refractory cervical cancer.
Cervical cancer is the fourth most common cancer and cause of cancer deaths in women worldwide.1 Most cases are diagnosed in less-developed countries, at an advanced stage, because of lack of effective screening programs. In India, cervical cancer ranks as the second most common cancer among women, with approximately 122,844 annual diagnoses and 67,477 reported deaths.2 Cervical cancer is attributable to infection with high-risk human papillomavirus (HPV)3; HPV types 16 (HPV-16) and HPV-18 account for more than 70% of invasive cervical cancer cases.4,5
Advanced cervical cancer has a poor prognosis, with median survival of 4 to 7 months in previously treated patients.6–9 Doublet chemotherapy (with/without bevacizumab) is currently recommended by the National Comprehensive Cancer Network as standard of care for patients with recurrent disease not eligible for surgery or radiation.10 In a pivotal phase 3 study, addition of bevacizumab to chemotherapy in patients with persistent, recurrent, or metastatic cervical cancer (PRmCC) was associated with a 3.7-month improvement in median overall survival (OS).6 On the basis of these results, bevacizumab received US Food and Drug Administration approval in 2014 for the treatment of PRmCC in combination with chemotherapy.
Axalimogene filolisbac (ADXS11-001) is a live, irreversibly attenuated Listeria monocytogenes (Lm)–listeriolysin O (LLO) immunotherapy bioengineered to secrete an antigen-adjuvant fusion protein consisting of a truncated, nonhemolytic fragment of LLO fused to human HPV-16 E7 (tLLO-HPV-16 E7). Upon administration, ADXS11-001 is phagocytized by antigen-presenting cells. The tLLO-HPV-16 E7 fusion protein, along with other secreted Lm proteins, activates the major histocompatibility complex class I pathway, whereas nonsecreted ADXS11-001 proteins activate the major histocompatibility complex class II pathway. In addition, ADXS11-001 has been shown to alter the tumor microenvironment, facilitate T-cell infiltration, and reduce immune suppression mediated by regulatory T cells and myeloid-derived suppressor cells.11–13
To better assess the effects of immunotherapies on targeted cancers, clinical end points such as OS and overall response rate (ORR) must be reconsidered. Survival curves in randomized immunotherapy studies can show a “plateauing effect” that may prolong the time needed to achieve an OS benefit. Therefore, intermediate clinical end points (ICEs), which have shown to strongly correlate with OS,14 need to be evaluated in immunotherapy clinical studies.
The objectives of this phase 2 study were to evaluate the safety and efficacy of ADXS11-001, alone or in combination with cisplatin, for the treatment of patients with recurrent/refractory cervical cancer (RRCC) following primary treatment with chemotherapy, radiotherapy, or chemoradiotherapy. In addition, the use of an ICE (12-month milestone survival) was also evaluated as a clinically meaningful efficacy end point in this immunotherapy study.
MATERIALS AND METHODS
The reported phase 2 study enrolled patients (November 2010–July 2013) across 25 centers in India. All investigators were licensed medical practitioners (Supplemental Digital Content Table 1, http://links.lww.com/IGC/A674). The study was registered at Clinical Trials Registry–India (CTRI/2010/091/001232). The original protocol and its amendment, as well as patient information sheets (including informed consent forms), were reviewed and/or approved by competent authorities and independent ethics committees/institutional review boards according to local regulations, prior to study initiation. All patients provided written informed consent before study enrollment.
Patients
Patients (18–60 years old) had squamous cell RRCC confirmed by histology and computed tomography (CT)/radiologic scan, following prior treatment with chemotherapy, radiotherapy, or chemoradiotherapy, and measurable disease with at least 1 “target lesion” (by Response Evaluation Criteria In Solid Tumors [RECIST] v1.0). In addition, patients had Eastern Cooperative Oncology Group (ECOG) performance status (PS) 2 or less, and adequate immunologic, renal, hepatic, and neurologic functions.
Study Design
The intent of this proof-of-concept study was to investigate the safety, tolerability, and effectiveness of ADXS11-001 administered as a monotherapy and in combination with cisplatin in patients with RRCC. Cisplatin was selected for combination therapy as it is a standard treatment for RRCC. The ADXS11-001 dose used in this study was selected on the basis of results from a previous phase 1 safety study in patients with late-stage RRCC, where doses of 1 × 109, 3.3 × 109, and 1 × 1010 colony-forming units (CFUs) were safely administered and well tolerated.15 The dose of 1 × 109 CFUs was the lowest dose not associated with any dose-limiting toxicity. Under the 1 × 109-CFU dose regimen, ADXS11-001 would be administered at a rate that delivered fewer organisms per minute as well as fewer organisms per milliliter. Administering the infusion at the rate of 5.3 mL/min (80 mL/15 min) would allow ample time to dilute the infusion within the circulation and not administer a bolus of microbes. In the cisplatin-containing arm, the time period between the first ADXS11-001 dose (day 1) and the first cisplatin dose (day 29) was chosen based on the following rationale: (i) to allow ADXS11-001 to fully exert its immunologic priming effect, (ii) to allow cytotoxic chemotherapy to release tumor antigens and enhance the activity of ADXS11-001, and (iii) to avoid potential inhibition of T-cell proliferation by cisplatin.
The power for the study was set at 50% to detect a 3-month difference in OS between the 2 treatment groups. The objective was to expose the minimum number of patients necessary to detect a clinically meaningful difference in efficacy (eg, 3 months) as this was the initial study conducted to evaluate ADXS11-001 alone or in combination. Patients were randomized 1:1 to ADXS11-001 monotherapy or ADXS11-001 + cisplatin. Patients randomized to ADXS11-001 monotherapy received 1 cycle (3 intravenous infusions) of ADXS11-001 (1 × 109 CFUs as an 80-mL intravenous infusion over 15 minutes) on days 1, 29, and 57. Patients randomized to ADXS11-001 + cisplatin received a single intravenous infusion of ADXS11-001 on day 1, followed 4 weeks later by 5 weekly cisplatin doses (40 mg/m2 intravenous infusion over 2 hours), followed by 1 cycle of ADXS11-001 (Supplemental Digital Content Figure 1, http://links.lww.com/IGC/A669). Each patient received oral ampicillin (500 mg 4 times a day for 7 days) or trimethoprim/sulfamethoxazole in case of penicillin allergy, beginning 72 hours after each dose of ADXS11-001, to ensure clearance of Lm. Before each dose of ADXS11-001, patients received up to 1 L of normal saline for hydration and before and after administration of oral naproxen and promethazine to help decrease the frequency and severity of adverse events (AEs) related to cytokine release. Patients with disease progression after initiating study treatment did not receive further treatments. Patients were followed at 3, 6, 9, 12, and 18 months to assess safety and efficacy (tumor response assessed through CT scans; survival status) (Supplemental Digital Content Table 2, http://links.lww.com/IGC/A675). Patients who consented to long-term-survival follow-up (a separate written informed consent was requested during the 18-month visit and obtained for patients who were followed beyond the 18-month time point) were followed by telephone or face-to-face contact by site personnel until study closure.
Efficacy Assessments
The primary end point was OS. Secondary efficacy end points were tumor response (defined per RECIST v1.0), reported as complete response (CR), partial response (PR), progressive disease (PD), and stable disease (SD) at 3 months or longer; investigator's assessment of best overall response (OR); ORR (CR + PR) and disease control rate (DCR; CR + PR + SD) at 3 months or longer; duration of OR and duration of SD; progression-free survival (PFS); and milestone OS at 3, 6, 9, 12, and 18 months.
The efficacy survival follow-up population was defined as all randomized patients who received at least 1 dose of ADXS11-001 (n = 109). One patient from the randomized population (n = 110) was not included because of voluntary withdrawal on day 1 before ADXS11-001 dosing. Overall survival, assessed in the efficacy survival follow-up population, was calculated from randomization date to date of death. Overall survival was further explored by time to disease recurrence after last administration of primary therapy (≤2 or >2 years), prior therapy (chemotherapy and/or radiation therapy), HPV genotypes (where available), and baseline ECOG PS.
Secondary analyses (ORR, DCR, PFS, and milestone OS rate) were conducted on the randomized (n = 110) population and on the subgroup of patients from the efficacy-survival follow-up population who received at least 1 dose of ADXS11-001 and at least 1 postbaseline tumor evaluation scan at 3 months or more postbaseline (n = 69). Tumor burden was considered as the sum of the largest linear diameters of measurable index lesions in accordance with RECIST v1.0. Overall response rate and DCR were based on best measured tumor response from treatment initiation until disease progression/recurrence, utilizing RECIST v1.0. Duration of OR was measured from the date of CR/PR to date of first progression. Duration of SD was measured from treatment initiation until the criteria for progression were met from the smallest tumor burden measurement recorded. Progression-free survival was defined as length of time after randomization until first tumor burden measurement meeting the RECIST v1.0 definition of PD or death. Milestone OS was measured at 3, 6, 9, 12, and 18 months after first treatment, and survival status was dichotomized as either alive or dead at a time point.
Safety Assessments
Changes in laboratory parameters (hematologic and serum chemistry), vital signs, and physical examination were reported from baseline. Injection site reactions (swelling, irritation, immune reactions, or other abnormalities) were recorded at each visit. Adverse events were reported, and toxicities were graded per National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.
Exploratory Cytokine and Chemokine Assessments
Serum cytokine and chemokine samples were collected predose and at 2 and 4 hours postdose of ADXS11-001. Samples from a subset of 18 patients who experienced an AE possibly related to ADXS11-001 administration and a control group without symptoms were planned for analysis. However, analysis was not performed because the collected samples were not viable upon receipt by the central clinical laboratory.
Statistical Analyses
Sample size calculation was based on the following assumptions: a 2-sided significance level of 0.05, duration of recruitment between 12 and 24 months, duration of follow-up of 18 months, and median OS for ADXS11-001 monotherapy of 6 months. If median OS was 6 months in the ADXS11-001 group and 9 months in the ADXS11-001 + cisplatin group, then a theoretical sample size of 110 patients (55/group) would yield 50% power to detect a difference in survival at a 2-sided significance level of 0.05. The power was set to 50%, which is the minimal value necessary to yield a resultant P = 0.05, provided the data showed the hypothesized result. Considering that median OS would exceed the hypothesized duration in the ADXS11-001 monotherapy group, the power would increase from 50% to 70% in the case that median OS was 11 months or from 50% to 80% in the case that median OS was 12 months.
Statistical analyses were performed using Statistical Analysis System (SAS) software version 9.1.3 (SAS Institute, Cary, NC). Results were summarized descriptively. Adverse events were coded using Medical Dictionary for Regulatory Activities v16.1.
RESULTS
Patient Characteristics
A total of 110 patients were randomized 1:1 to ADXS11-001 monotherapy and ADXS11-001 + cisplatin. Both groups presented comparable demographics and baseline characteristics (Table 1). Thirty-nine patients (35.5%) received radiotherapy, 10 (9.1%) received chemotherapy, and 61 (55.4%) received chemoradiotherapy as their primary treatment. Of the patients who received chemotherapy, alone or in combination with radiotherapy (n = 71), 11.3% (8/71) received 2 or more prior chemotherapy regimens. Ninety patients in the randomized population (90/110 [81%]) presented with disease recurrence within 2 years following their last dose of primary treatment.
TABLE 1.
Summary of patient demographics
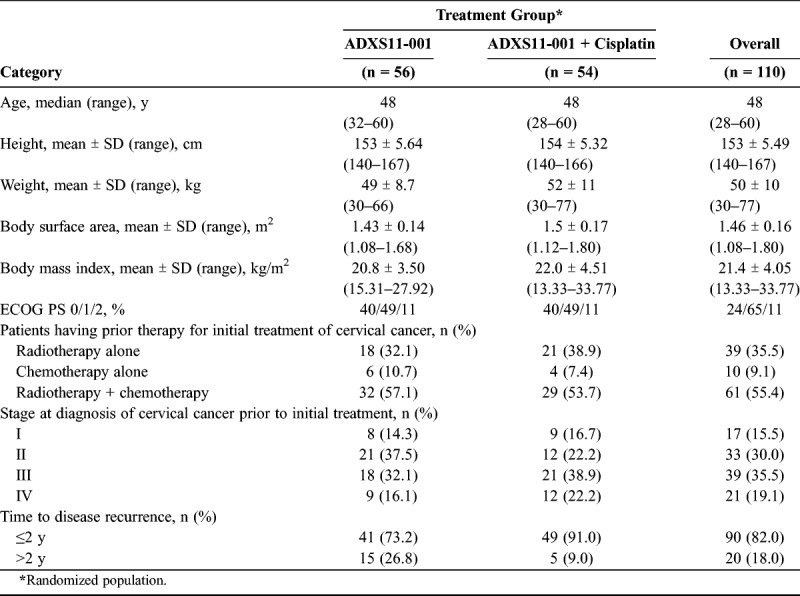
Scrapings from the cervix or from the vaginal vault for patients after hysterectomy were obtained from 99 (90%) of 110 patients for HPV testing; 97 patients were tested using type-specific primers by polymerase chain reaction. Human papillomavirus was detected in 64 of 97 samples, with HPV-16 (73.4%) and HPV-18 (15.6%) the predominant genotypes.
Overall, 69 of 110 randomized patients composed a subgroup who had at least 1 postbaseline imaging scan. The remaining 41 patients were not evaluated at the 3-month visit for the following reasons: 5 patients were lost to follow-up, 4 patients were discontinued from the study treatment, 15 patients died, 16 patients withdrew their consent, and 1 patient underwent CT instead of the magnetic resonance imaging scan.
One hundred five patients (105/110 [95.5%]) were discontinued before completing all scheduled tumor evaluation/assessment. Reasons for premature discontinuation are listed in Figure 1. Five (4.5%) of 110 patients completed their 18-month tumor evaluation/assessment visit (day 545). Thirty-nine patients in the monotherapy group (39/55 [70.9%]) received all doses of ADXS11-001, and 18 patients in the combination group (18/54 [33%]) received all doses of ADXS11-001 and cisplatin. Patients who withdrew consent or were lost to follow-up were censored at last date of known contact. Fifteen patients consented to long-term survival follow-up until study closure.
FIGURE 1.
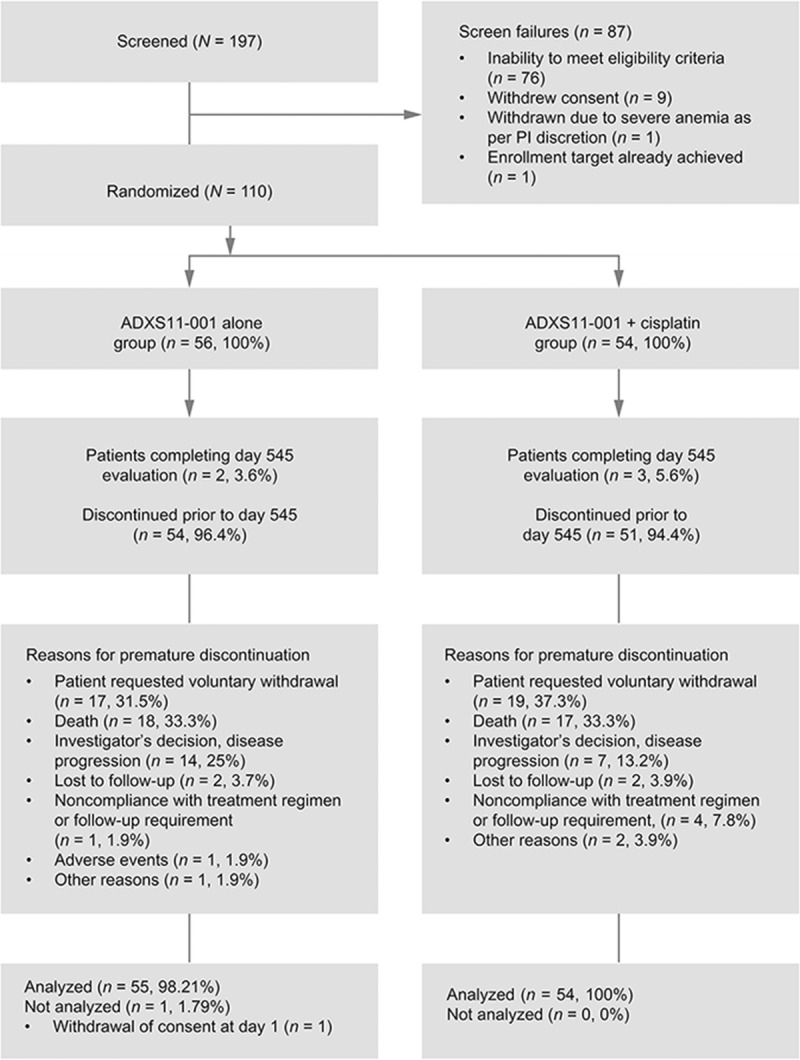
CONSORT diagram. PI, Principal investigator.
Efficacy
Median OS was comparable between treatment groups (ADXS11-001: 8.28 months; 95% confidence interval [CI], 5.85–10.5 months; ADXS11-001 + cisplatin: 8.78 months; 95% CI, 7.4–13.3 months) (Fig. 2). There was no statistically significant difference in OS between treatment groups according to ECOG PS at baseline (P = 0.5258), time to disease recurrence after primary therapy (P = 0.8156), HPV strain identified (P = 0.3492), or prior therapy (chemotherapy alone [P = 0.9823], radiotherapy alone [P = 0.8714], or chemoradiotherapy [P = 0.7066]).
FIGURE 2.
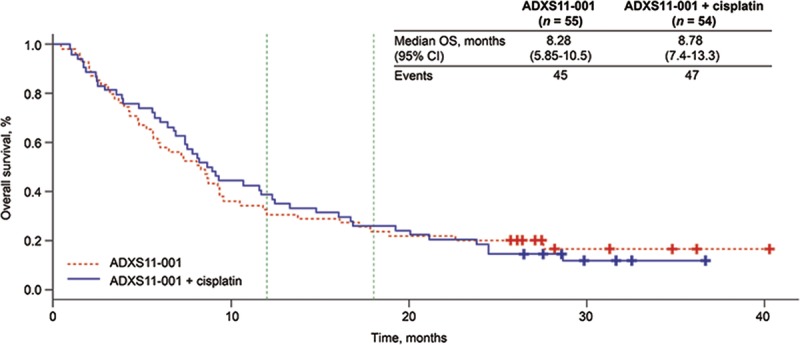
Kaplan-Meier curve for OS in the efficacy population, survival follow-up (n = 109) by treatment group. CIS, Cisplatin; LCL, lower confidence limit; UCL, upper confidence limit; n, number.
Milestone OS rates in the ADXS11-001 monotherapy and ADXS11-001 + cisplatin groups at 6, 9, 12, and 18 months are presented in Table 2. Overall, 34.9% (38/109) of patients achieved 12-month milestone OS, and 24.8% (27/109) achieved 18-month OS. Of the 15 patients consenting to follow-up beyond 18 months, 12 (11%) achieved 24-month OS status (range, 24–34+ months) at the time of study closure.
TABLE 2.
Summary of milestone OS rates
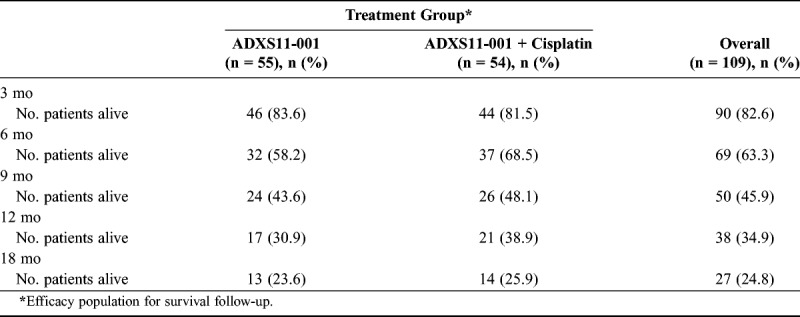
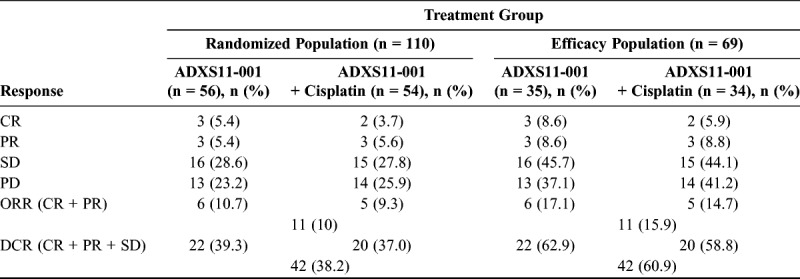
Table 3 and Supplemental Digital Content Figure 2, http://links.lww.com/IGC/A670 present investigator assessment of best OR in the efficacy population (n = 69). These data demonstrate best ORR (CR + PR) of 17.1% (n = 6) and 14.7% (n = 5) in the ADXS11-001 monotherapy and ADXS11-001 + cisplatin groups, respectively. In addition, 29 patients had at least 2 postbaseline scans. In this group, 1 patient (1/29 [3.4%]) had a confirmed PR, and 3 patients (3/29 [10.3%]) had confirmed CR (ORR, 13.8%). Combined mean duration of OR was 8.3 months (ADXS11-001 monotherapy: 7.2 months; ADXS11-001 + cisplatin: 9.4 months). The DCR (CR + PR + SD) was 62.9% (n = 22) and 58.8% (n = 20) for the ADXS11-001 monotherapy and ADXS11-001 + cisplatin groups, respectively, in the efficacy population (n = 69; 60.9% overall DCR). Mean duration of SD was 5.2 months (ADXS11-001 monotherapy: 4.8 months; ADXS11-001 + cisplatin: 5.6 months). The proportion of patients with CR, PR, SD, or PD was comparable between treatment groups (Table 3).
TABLE 3.
Summary of best overall tumor response results
Median PFS (n = 69) was similar for patients receiving ADXS11-001 monotherapy (6.08 months; 95% CI, 5.88–9.36 months) or ADXS11-001 + cisplatin (6.44 months; 95% CI, 4.17–8.94 months; P = 0.7509) (Supplemental Digital Content Figure 3, http://links.lww.com/IGC/A671). Treatment group (hazard ratio [HR], 1.126; 95% CI, 0.681–1.860; P = 0.6437), time to disease recurrence after primary therapy (HR, 0.737; 95% CI, 0.361–1.507; P = 0.4033), prior chemotherapy (HR, 0.792; 95% CI, 0.468–1.342; P = 0.3864), and baseline PS (HR, 0.932; 95% CI, 0.367–2.369; P = 0.8830) did not significantly affect PFS.
Safety—Adverse and Serious Adverse Events
All AEs were analyzed in the safety population (randomized patients who received at least 1 dose of ADXS11-001; n = 109). A greater number of AEs were reported in the ADXS11-001 + cisplatin group compared with the ADXS11-001 monotherapy group (429 vs 275 AEs, respectively; Table 4). This difference may be attributable to the number of cisplatin-related events experienced by patients in the combination group. However, the same number of patients in each group reported at least 1 AE (48 patients). Most AEs were mild to moderate in severity (566/704 reported AEs [80.4%]) and were not study drug related (539/704 reported AEs [76.6%]). The severity of the AEs and their relationship to study drug were similar between the 2 treatments (Table 4).
TABLE 4.
Summary of AEs
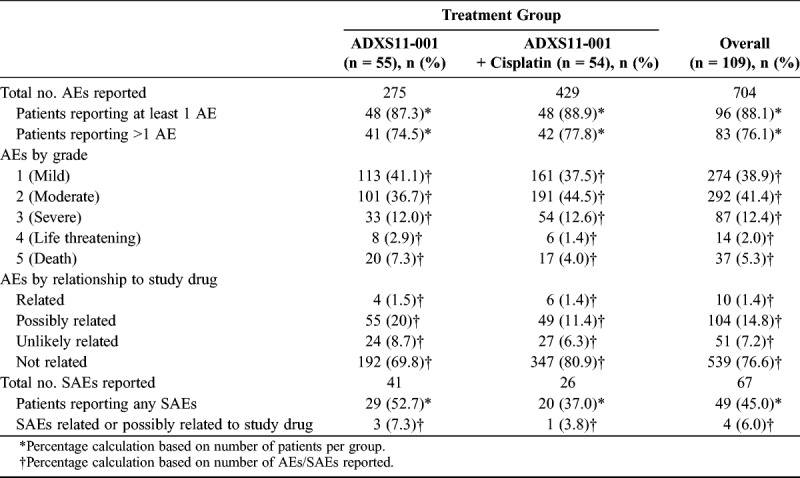
Of the AEs considered related/possibly related to study drug, the majority were reported in patients receiving ADXS11-001 + cisplatin (25 [46.3%] vs 20 [36.4%] patients). Chills and pyrexia were the most commonly reported related/possibly related AEs in both treatment groups (Supplemental Digital Content Table 3, http://links.lww.com/IGC/A676).
Sixty-seven serious AEs (SAEs; 10%) occurred in 49 patients (45%). One patient (1.9%) treated with ADXS11-001 + cisplatin reported an SAE of pyrexia that was considered related to study drug by the investigator. Two patients (3.6%) treated with ADXS11-001 monotherapy reported 3 SAEs that were considered possibly related to study drug by the investigator: cytokine release syndrome in 1 patient and abdominal pain and bacterial peritonitis with septicemia in 1 patient (fatal). In this patient, Escherichia coli was determined to be the causative pathogen, not Lm; therefore, the event was not considered study drug related by the sponsor. The remaining 63 SAEs were ruled either not related or unlikely related to study drug (Table 4).
DISCUSSION
Patients with PRmCC have low 5-year survival rates for stage III and stage IV disease of approximately 33% and 15%, respectively, following first-line treatment with platinum-based chemotherapy.16 Bevacizumab demonstrated a 3.7-month improvement in median OS compared with chemotherapy alone.6 However, widespread use may be limited because of safety concerns (eg, fistula rate).
Recent advancements in understanding activity profiles of immunotherapies suggest that tumor response may not be the best primary outcome measure for an immunotherapy in advanced stages of cancer. As demonstrated by the 10-year survival data from phases 2 and 3 ipilimumab clinical studies in metastatic melanoma,17 a proportion of patients remain alive after long-term follow-up. This observed “plateauing effect” demonstrates the delayed onset of a prolonged survival benefit (Fig. 2).
While OS is a well-accepted clinical end point, given the potential of immunotherapies to extend long-term survival, other ICEs may demonstrate a meaningful clinical benefit sooner. Milestone OS rate, in which OS is dichotomized at a prespecified time point, has been proposed as one such ICE. Petrelli et al14 assessed whether 1- and 2-year milestone OS rates are reliable ICEs for median OS through a meta-analysis of 13 published trials of immunotherapies for metastatic melanoma. The correlation between 1-year OS and median OS (R = 0.93; 95% CI, 0.84–0.96; P < .00001) and between 2-year OS and median OS (R = 0.79; 95% CI, 0.51–0.91; P = 0.0001) was very strong. In addition, the correlation between treatment effects on 1-year OS and median OS was significant (R = −0.86; 95% CI, −0.3 to 0.97; P = 0.01; R2 = 0.75), with similar results obtained for 2-year OS.
A meta-analysis of 10 published randomized controlled studies conducted by the Gynecologic Oncology Group (GOG) and Japan Clinical Oncology Group in more than 3500 women with PRmCC6,7,18–25 also showed a very strong correlation between 1-year OS and median OS (R = −0.89; 95% CI, −0.93 to −0.82; P < .001; R2 = 0.79) (Supplemental Digital Content Figure 4, http://links.lww.com/IGC/A672). These results indicate that 1-year OS rate is a viable ICE for median OS in immunotherapy trials of cervical cancer.
In this phase 2 study, the 12-month (34.9%) and 18-month (24.8%) milestone OS rates are important landmarks because they reflect an approximate 1.5- to 2-fold increase in median OS rates observed in this population that received at least 1 prior therapy following chemoradiotherapy. The 12-month milestone OS rate in this study exceeds historical GOG series data and represents the highest rates achieved (Supplemental Digital Content Figure 5, http://links.lww.com/IGC/A673).
Overall responses are indicative of meaningful clinical benefit in PRmCC patients. However, ORRs are generally low, as might be expected in this advanced population (Supplemental Digital Content Table 4, http://links.lww.com/IGC/A677). Recent data from a study using pembrolizumab demonstrated an ORR of 13% (n = 3/24 patients). All responses were PR.26 In the present study, a best ORR of 17.1% and 14.7% was observed in the ADXS11-001 monotherapy and ADXS11-001 + cisplatin groups, respectively, demonstrating the antitumor activity of ADXS11-001. Possible explanations for the slightly lower OS rate in the ADXS11-001 + cisplatin arm are (i) the interrupted schedule of ADXS11-001 administration, which might not have allowed for an optimal immunologic prime-boosting effect; (ii) the compounding immunosuppressive effect of chemotherapy and nonsteroidal anti-inflammatory drugs used to reduce the incidence of ADXS11-001–related AEs; and (iii) the fact that, in the population with at least 1 postbaseline scan (n = 69), 50% of patients in the ADXS11-001 + cisplatin arm did not receive all ADXS11-001 doses.
This study presents with some limitations. One is the lack of a cisplatin-alone treatment arm, which would have allowed for a more accurate explanation of the difference in AEs in the ADXS11-001–alone versus cisplatin-containing arms. Furthermore, evaluating the efficacy in the cisplatin-alone arm would have possibly helped explain the differences in ORR observed between treatment arms. In addition, data on postprogression therapy, which may have influenced survival in some patients, were not collected. Another limitation is the relatively high rate of voluntary withdrawal of patients from the study, possibly due to their receiving mostly palliative advice following disease progression and their poor general condition precluding travel to study centers in preference for general local care. Nevertheless, this did not hinder assessment of study end points in either of the 2 treatment arms. The results of this initial study of ADXS11-001 in a RRCC population indicated there was no added benefit in survival with the addition of cisplatin in this setting. However, the OS, 12-month OS rate, and tumor response were compelling and formed the basis for the phase 2 GOG/NRG 0265 monotherapy trial in a similar population, in which the 12-month OS rate was 38%,27 consistent with the findings of this study.
In conclusion, ADXS11-001 is well tolerated in patients with RRCC, as monotherapy and in combination with cisplatin. Median OS in both treatment groups was comparable to historical data. Both treatment groups raised “the tail” of the survival curve, demonstrating the potential for long-term clinical benefit. Based on its favorable safety profile and the potential long-term clinical benefit, further investigation of ADXS11-001 is warranted.
Supplementary Material
Footnotes
P.B. has received research funding from Advaxis, Inc, while working for Chittaranjan National Cancer Institute; S.G. has received research funding from Sanofi-Aventis; R.P. is employed by and owns stock from Advaxis, Inc. The other authors declare no conflicts of interest.
Lm-LLO-E7-15 (Clinical Trials Registry–India #CTRI/2010/091/001232) was funded by Advaxis, Inc, Princeton, NJ. The sponsor was involved in data gathering, analysis, review, interpretation, and writing of the report. Editorial support was provided by Tom Hare and Fatima Ahmad, Advaxis Inc; and Mary Smith and Oana Draghiciu, TRM Oncology, funded by Advaxis, Inc, Princeton, NJ.
Results of this trial have partially been presented at the ASCO, Chicago, IL, June 2012; ASCO, Chicago, IL, June 2013; SITC, North Bethesda, MD, October 2012; SITC, National Harbor, MD, November 2013; and UICC World Cancer Congress, Melbourne, Australia, December 2014.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
REFERENCES
- 1.World Health Organization. International Agency for Research on Cancer. In: Latest World Cancer Statistics. Global Cancer Burden Rises to 14.1 Million New Cases in 2012: Marked Increase in Breast Cancers Must Be Addressed [press release]. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Bruni L, Barrionuevo-Rosas L, Albero G, et al. Human Papillomavirus and Related Diseases in the World. Summary Report. Barcelona, Spain: ICO Information Centre on HPV and Cancer (HPV Information Centre); 2017. [Google Scholar]
- 3.World Health Organization/Institut Català d'Oncologia Information Centre on HPV and Cervical Cancer (HPV Information Centre). Human Papillomavirus and Related Cancers. Summary Report Update. Barcelona, Spain: WHO/ICO HPV Information Centre; 2010. [Google Scholar]
- 4.Wheeler CM, Hunt WC, Joste NE, et al. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst. 2009;101:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford GM, Rana RK, Franceschi S, et al. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1157–1164. [DOI] [PubMed] [Google Scholar]
- 6.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3113–3119. [DOI] [PubMed] [Google Scholar]
- 8.Bookman MA, Blessing JA, Hanjani P, et al. Topotecan in squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2000;77:446–449. [DOI] [PubMed] [Google Scholar]
- 9.Garcia AA, Blessing JA, Vaccarello L, et al. Phase II clinical trial of docetaxel in refractory squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Am J Clin Oncol. 2007;30:428–431. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Cervical Cancer. Version 2.2015. National Comprehensive Cancer Network; 2015.
- 11.Campisi L, Soudja SM, Cazareth J, et al. Splenic CD8α+ dendritic cells undergo rapid programming by cytosolic bacteria and inflammation to induce protective CD8+ T-cell memory. Eur J Immunol. 2011;41:1594–1605. [DOI] [PubMed] [Google Scholar]
- 12.Wallecha A, French C, Petit R, et al. Lm-LLO–based immunotherapies and HPV-associated disease. J Oncol. 2012;2012:542851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krupar R, Imai N, Miles B, et al. HPV E7 antigen-expressing Listeria-based immunotherapy (ADXS11-001) prior to robotic surgery for HPV-positive oropharyngeal cancer enhances HPV-specific T cell immunity. Presented at the American Association for Cancer Research Annual Meeting; April 16–20, 2016; New Orleans, Louisiana. Abstract LB-095.
- 14.Petrelli F, Coinu A, Cabiddu M, et al. Early analysis of surrogate endpoints for metastatic melanoma in immune checkpoint inhibitor trials. Medicine (Baltimore). 2016;95:e3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–3983. [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society. Survival rates for cervical cancer, by stage. December 5, 2016. Available at: https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/survival.html. Accessed August 10, 2017.
- 17.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonomi P, Blessing JA, Stehman FB, et al. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1985;3:1079–1085. [DOI] [PubMed] [Google Scholar]
- 19.Thigpen JT, Blessing JA, DiSaia PJ, et al. A randomized comparison of a rapid versus prolonged (24 hr) infusion of cisplatin in therapy of squamous cell carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1989;32:198–202. [DOI] [PubMed] [Google Scholar]
- 20.McGuire WP, 3rd, Arseneau J, Blessing JA, et al. A randomized comparative trial of carboplatin and iproplatin in advanced squamous carcinoma of the uterine cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1989;7:1462–1468. [DOI] [PubMed] [Google Scholar]
- 21.Omura GA, Blessing JA, Vaccarello L, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1997;15:165–171. [DOI] [PubMed] [Google Scholar]
- 22.Bloss JD, Blessing JA, Behrens BC, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:1832–1837. [DOI] [PubMed] [Google Scholar]
- 23.Long HJ, 3rd, Bundy BN, Grendys EC, Jr, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2005;23:4626–4633. [DOI] [PubMed] [Google Scholar]
- 24.Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized phase III trial JCOG0505. J Clin Oncol. 2015;33:2129–2135. [DOI] [PubMed] [Google Scholar]
- 26.Ott PA, Bang YJ, Berton-Rigaud D, et al. Pembrolizumab in advanced endometrial cancer: preliminary results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2016;34: abstract 5581. [Google Scholar]
- 27.Huh W, Brady WE, Dizon DS, et al. A prospective phase II trial of the Listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second- and third-line metastatic cervical cancer: a NRG Oncology Group trial. Presented at the 48th Annual Meeting of the Society of Gynecologic Oncology; March 12–15, 2017; National Harbor, MD. Late Breaking Abstract 3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


