ABSTRACT
Background:
The nitric oxide system could play an important role in the pathophysiology related to necrotizing soft tissue infection (NSTI). Accordingly, we investigated the association between plasma nitrite level at admission and the presence of septic shock in patients with NSTI. We also evaluated the association between nitrite, asymmetric dimethylarginine (ADMA), l-arginine, l-arginine/ADMA ratio, and outcome.
Methods:
We analyzed plasma from 141 NSTI patients taken upon hospital admission. The severity of NSTI was assessed by the presence of septic shock, Simplified Acute Physiology Score (SAPS) II, Sepsis-Related Organ Failure Assessment (SOFA) score, use of renal replacement therapy (RRT), amputation, and 28-day mortality.
Results:
No difference in nitrite levels was found between patients with and without septic shock (median 0.82 μmol/L [interquartile range (IQR) 0.41–1.21] vs. 0.87 μmol/L (0.62–1.24), P = 0.25). ADMA level was higher in patients in need of RRT (0.64 μmol/L (IQR 0.47–0.90) vs. (0.52 μmol/L (0.34–0.70), P = 0.028), and ADMA levels correlated positively with SAPS II (rho = 0.32, P = 0.0002) and SOFA scores (rho = 0.22, P = 0.01). In a logistic regression analysis, an l-arginine/ADMA ratio below 101.59 was independently associated with 28-day mortality, odds ratio 6.03 (95% confidence interval, 1.41–25.84), P = 0.016. None of the other analyses indicated differences in the NO system based on differences in disease severity.
Conclusions:
In patients with NSTI, we found no difference in baseline nitrite levels according to septic shock. High baseline ADMA level was associated with the use of RRT and patients with a low baseline l-arginine/ADMA ratio were at higher risk of dying within 28 days after hospital admission.
Keywords: Bacterial infection, biomarker, endothelium, necrotizing fasciitis, nitric oxide, sepsis, survival
INTRODUCTION
Necrotizing soft tissue infections (NSTIs) represent a spectrum of bacterial infections causing necrotic lesions in any layer of the soft tissue compartments. Each year, approximately 1,000 people are diagnosed with the disease in the United States (1, 2).
Nitric oxide (NO) is involved in a wide variety of regulatory mechanisms and exerts its physiological role through the cardiovascular, immune, and nervous systems (3, 4). NO is derived from the vascular wall and it is thought to contribute to hypotension in patients with sepsis, but it may also improve organ perfusion by microcirculatory vasodilation (5, 6). This could be of clinical importance as NSTI is often accompanied by septic shock and organ failure (7). However, the mechanisms linking NSTI to vascular dysfunction remain to be investigated. Interestingly, NO is also involved in the host–pathogen interactions through its function as a signaling molecule, antimicrobial agent, and downstream effector of innate immunity (8–10), thus potentially playing a role during NSTI.
NO is formed in the vascular endothelium by NO synthase (NOS) from l-arginine and oxygen. Once produced, NO (half-life <2 ms) is rapidly oxidized to nitrite (NO2−) and nitrate (NO3−) in blood. Together, these two metabolites are widely used as an indirect measure of NO plasma levels (11–13). There is a growing interest in nitrite as it reflects constitutive NO availability (14) and provides a more reliable measure of endothelial NOS (eNOS) activity compared with nitrate (15, 16), whereas inducible NOS (iNOS) is thought to release an order of magnitude more NO in response to tissue damage and infection (17, 18). Therefore, nitrite assessment may aid in the early detection of endothelial dysfunction, but its prognostic role in critically ill patients has been sparsely evaluated due to the lack of sufficiently sensitive analytical methods (19, 20).
NOS activity is inhibited endogenously by asymmetric dimethylarginine (ADMA). An imbalance of NO (nitrite/nitrate), l-arginine, and ADMA is thought to be involved in endothelial and cardiac dysfunction (21). In line with this, high ADMA levels have been associated with increased mortality in patients with sepsis (22–24) and as an independent risk factor of intensive care unit (ICU) mortality (25), but data are conflicting (26). As ADMA competes with l-arginine for the binding to NOS, the l-arginine/ADMA ratio has been suggested as a better indicator to NOS substrate availability than l-arginine alone (27). NO, ADMA, and l-arginine are potential therapeutic targets and may be used in the identification of relevant subgroups of patients at high risk of poor outcome. To our knowledge, no studies have investigated the levels of these vasoactive biomarkers in patients with NSTI, despite the pathophysiological differences compared with other patients with sepsis. In addition, the treatment of NSTI often relies on knowledge from sepsis patients with pneumonia or other frequent sites of infection even though the immunological processes likely differ because of the extensive necrosis. Moreover, the diagnosis of NSTI is clinical which makes it interesting to investigate whether these biomarkers are able to predict patient outcome.
Accordingly, we evaluated the association between the level of NO system biomarkers in plasma and disease severity and outcome in patients with NSTI. The primary analysis focused on the association between nitrite level upon hospital admission (baseline) and septic shock. In the secondary analyses, we focused on the association between nitrite, l-arginine, ADMA, and l-arginine/ADMA ratio and disease severity as well as outcome, defined as use of renal replacement therapy (RRT), rates of amputation, and 28-day mortality.
MATERIALS AND METHODS
Study design and population
In Denmark, the treatment of NSTI has been centralized at Rigshospitalet, University of Copenhagen, where this prospective, observational cohort study was conducted between February 2013 and April 2015. The study protocol has been published (28) and is available online (ClinicalTrials.gov: NCT02180906).
We included patients diagnosed with NSTI, based on surgical findings of necrosis. Inclusion criteria included admission to the ICU or surgery for NSTI at Rigshospitalet and a minimum age of 18 years. Patients were excluded if the diagnosis could not be confirmed during surgery.
Data collection
Data were obtained from patient medical records and entered into an online database that was regularly validated. The following data were retrieved from the database: demographics (age, sex), comorbidities (diabetes, liver cirrhosis, chronic kidney disease, cardiovascular disease, chronic obstructive pulmonary disease, peripheral vascular disease, immune deficiency, malignancy, rheumatoid disease), biochemistry and physiological values, and disease severity scores (Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC), Simplified Acute Physiology Score (SAPS) II, Sepsis-Related Organ Failure Assessment (SOFA) score). Data on vital status (date of death or emigration) were retrieved from the Danish Civil Registration System. The presence of septic shock was defined according to Bone et al. (29) with at least two systemic inflammatory response syndrome (SIRS) criteria, suspected/verified focus of infection and vasopressor infusion.
Arterial blood was obtained in EDTA tubes upon hospital admission and immediately put on ice. After centrifugation, plasma was collected in 1 mL aliquots and stored at −80°C until processing. In 67% (n = 94) of the cases, baseline blood samples were collected after the primary operation of NSTI.
Chemiluminescent assay
We quantified baseline plasma NO level indirectly through the measurement of nitrite using the ozone-chemiluminescence technology (Sievers Nitric Oxide Analyzer system, NOA 280i, GE Analytical Instruments, Boulder, Colo) as previously described (30). In brief, we thawed the plasma just before NO determination under the protection from light. We injected 300 μL plasma sample into the purge vessel of the NO analyzer containing glacial acetic acid and an iodide solution (NaI and KI), which converts nitrite to NO. Samples were tested in duplicates using a standard curve constructed with distilled water as blanks and sodium nitrite solutions of known concentrations (10 nM, 50 nM, 100 nM, 1 μM, 5 μM, 10 μM, 50 μM, and 100 μM), which yielded a linear relationship. The lower detection limit in the samples was ∼15 nM due to the nitrite content in the empty EDTA sampling tubes.
Enzyme-linked immunosorbent assay
We measured baseline plasma ADMA and l-arginine using a competitive enzyme-linked immunosorbent assay (ELISA) (EA207/92; DLD Diagnostica GmbH, Hamburg, Germany) according to the manufacturer's instructions. Samples were tested in duplicate against a standard pool with known concentration. The standard range of ADMA was 0.2 to 3.0 μmol/L (normal values 0.40–0.75 μmol/L) with an intra-assay variation of 6% and interassay of 10%. The standard range of l-arginine was 5 to 300 μmol/L (normal values 20–80 μmol/L) with an intra-assay variation of 4% and interassay variation of 10%.
Statistical analysis
Categorical data are presented as numbers with percentage (%) and compared using the chi-square test. Continuous data are presented as medians with interquartile range (IQR). We used the unpaired t test for the primary analysis to quantify potential differences between the groups and elaborate on a potential risk of overlooking a clinical relevant difference. As data were not normally distributed, we used the Mann–Whitney U test for significance testing of differences between groups (28). The association between 28-day mortality and biomarker levels are illustrated with Kaplan–Meier plots and tested using a logistic regression analysis and expressed with odds ratio (OR) and 95% confidence interval (CI). In the prediction of 28-day mortality, we analyzed both median values and optimal cutoff (maximum sum of sensitivity and specificity) (28). In the multivariate analysis, we adjusted for age, sex, comorbidities, and SAPS II. Patients with missing data on covariates were excluded in the multivariate analyses. No data were missing on the outcome variable, thus no multiple imputations were performed. We analyzed receiver operating characteristic (ROC) curves for 28-day mortality and Spearman rank test for correlation between biomarker level and disease severity.
P < 0.05 was considered to be statistically significant. Analyses were performed using GraphPad Prism 6.0 software (Graphpad Inc., Calif) and SPSS 22.0 software (SPSS Inc., Ill).
Initially, we planned to include 110 patients based on a sample size calculation using total levels of nitrate and nitrite (28). As we aimed to measure only nitrite levels in the samples, this estimate is subject to great uncertainty, especially as no studies have investigated nitrite levels exclusively in infected patients using chemiluminescent. We therefore decided to analyze all samples obtained from patients with NSTI, who were included during the study period, as previously described (7). Baseline characteristics of the majority of the study cohort (n = 135) have been previously described in two studies elaborating on pattern recognition molecules (7, 31).
Ethics
The regional ethics committee (H-2-2014-071) and the Danish Data Protection Agency (30-1282) approved the study. All patients or their next of kin gave oral and written informed consent.
RESULTS
We enrolled 171 patients with suspected NSTI between February 2013 and April 2015. Of those, 30 patients did not fulfill the inclusion criteria or fulfilled the exclusion criterion. Thus, we analyzed 141 baseline samples from patients with NSTI. The patients had a median age of 62 years (53–69) and 60% were men. The majority of patients presented with septic shock (72%) and 28-day mortality was 17% (n = 24 (95% CI, 12%–24%)). The median SAPS II was 45 (35–54) and could not be calculated in five patients due to missing data.
Disease severity
Patients with NSTI and septic shock had a mean baseline nitrite level of 0.88 μmol/L compared with 1.00 μmol/L in those without septic shock (mean difference 0.12 μmol/L (95% CI, –0.10 to 0.34), P = 0.83) and we found no significant difference between the groups using the Mann–Whitney U test (median 0.82 μmol/L, IQR 0.41–1.21 vs. 0.87 μmol/L, IQR 0.62–1.24, P = 0.25) (Fig. 1). Only baseline ADMA level was significantly higher in patients in need of RRT within the first 7 days of admission (Figs. 1 and 2). This was in line with the correlation analyses showing that ADMA correlated significantly with the SAPS II and the SOFA score (Table 1). In addition, nitrite, ADMA, and l-arginine/ADMA ratio correlated with creatinine levels.
Fig. 1.
Plasma levels of nitrite, l-arginine, ADMA, and l-arginine/ADMA ratio on admission according to septic shock in patients with necrotizing soft tissue infection.
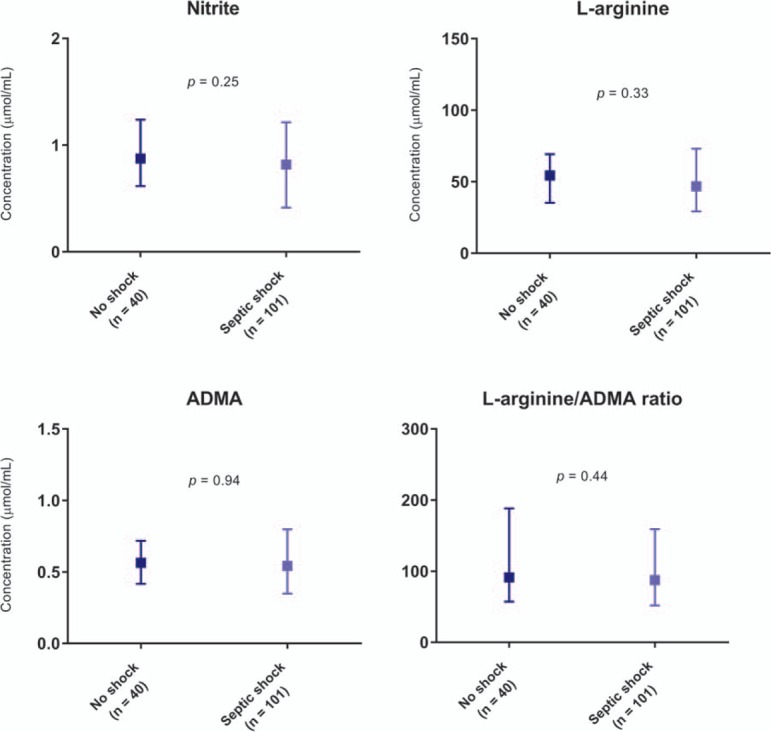
ADMA indicates asymmetric dimethylarginine. Median with interquartile range is illustrated. Comparisons were performed using the Mann–Whitney U test.
Fig. 2.
Plasma levels of nitrite, l-arginine, ADMA indicates and l-arginine/ADMA ratio on admission according to amputation and RRT within the first 7 days in patients with necrotizing soft tissue infection.
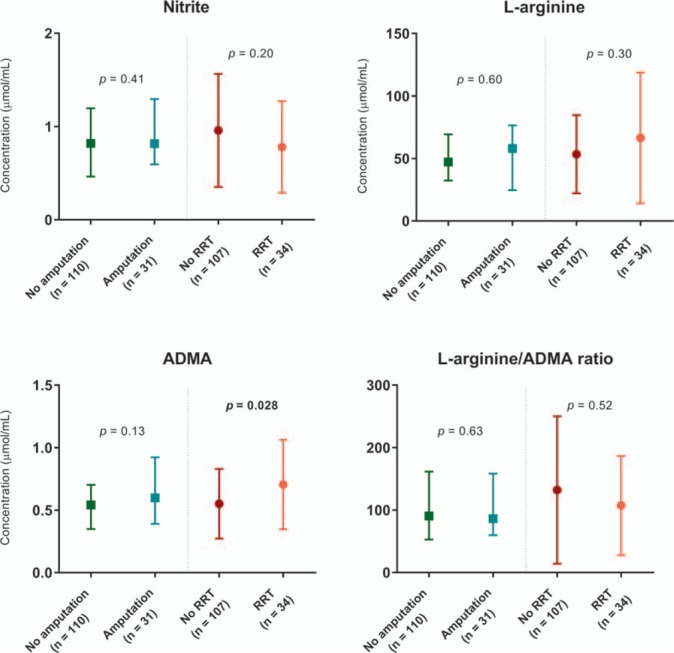
ADMA indicates asymmetric dimethylarginine; RRT, renal replacement therapy. Median with interquartile range is illustrated. Comparisons were performed using the Mann–Whitney U test.
Table 1.
Spearman rank correlation between disease severity scores and baseline biomarker levels in patients with necrotizing soft tissue infection
| LRINEC | SAPS II | SOFA score* | Creatinine** | |||||
| Rho | P | Rho | P | Rho | P | Rho | P | |
| Nitrite | −0.11 | 0.25 | −0.13 | 0.15 | −0.30 | 0.001 | −0.23 | 0.009 |
| l-Arginine | −0.13 | 0.17 | 0.09 | 0.33 | 0.02 | 0.87 | −0.12 | 0.17 |
| ADMA | −0.05 | 0.59 | 0.32 | 0.0002 | 0.22 | 0.01 | 0.17 | 0.048 |
| l-Arginine/ADMA ratio | −0.07 | 0.43 | −0.14 | 0.10 | −0.12 | 0.18 | −0.22 | 0.01 |
*SOFA score day 1.
**Highest value measured during the first 24 h of admission.
ADMA indicates asymmetric dimethylarginine; LRINEC, Laboratory Risk Indicator For Necrotizing Fasciitis; SAPS II, Simplified Acute Physiology Score II; SOFA, Sepsis-Related Organ Failure Assessment.
Mortality
Survival according to median biomarker levels and 28-day mortality is illustrated in Figure 3 and the diagnostic accuracy is shown in Table 2. Only a l-arginine/ADMA ratio below the median correlated significantly with the mortality, but it was not an independent predictor (Table 3). However, when the optimal cutoff was used (maximum sum of sensitivity and specificity) instead of the median, the l-arginine/ADMA ratio independently predicted the 28-day mortality (Table 4).
Fig. 3.
Kaplan–Meier curves with related 95% CIs of 28-day mortality in patients with necrotizing soft tissue infection according to levels above or below median biomarker levels.
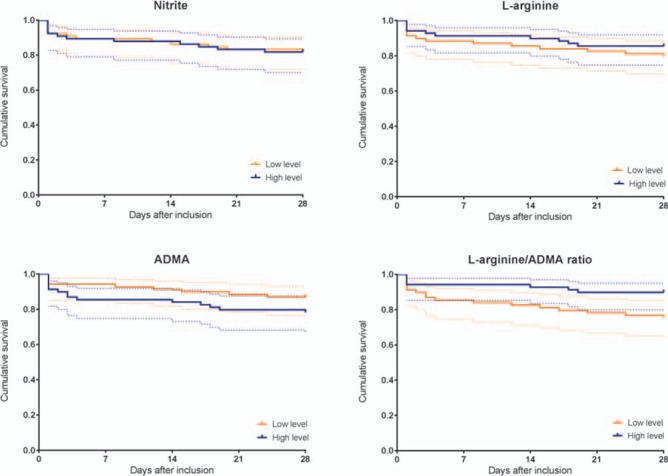
ADMA indicates asymmetric dimethylarginine.
Table 2.
Diagnostic accuracy of high baseline nitrite and ADMA levels and low baseline l-arginine level and l-arginine/ADMA ratio in predicting 28-day mortality in patients with necrotizing soft tissue infection
| Nitrite | l-Arginine | ADMA | l-Arginine/ADMA | |
| Sensitivity | 0.50 (0.31–0.69) | 0.58 (0.39–0.76) | 0.63 (0.42–0.80) | 0.71 (0.51–0.86) |
| Specificity | 0.50 (0.46–0.54) | 0.52 (0.48–0.56) | 0.53 (0.48–0.56) | 0.54 (0.50–0.58) |
| PPV | 0.18 (0.11–0.25) | 0.20 (0.13–0.27) | 0.22 (0.15–0.28) | 0.25 (0.18–0.30) |
| NPV | 0.82 (0.75–0.89) | 0.86 (0.79–0.92) | 0.87 (0.80–0.93) | 0.90 (0.83–0.95) |
| Area under ROC curve | 0.48 (0.34–0.62) | 0.52 (0.38–0.65) | 0.63 (0.51–0.75) | 0.63 (0.53–0.73) |
ADMA indicates asymmetric dimethylarginine; NPV, negative predictive value; PPV, positive predictive value. Data are presented as fractions (95% CI). The prevalence of 28-day mortality was 17%. High and low baseline levels were defined by the median.
Table 3.
Univariate and multivariate logistic regression analyses of mortality up to day 28 (time of censoring) in patients with necrotizing soft tissue infection based on high versus low concentrations of the biomarkers according to median values
| Unadjusted | Adjusted for age, sex, chronic disease | Adjusted for sex, chronic disease, SAPS II* | |||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Nitrite | |||||||||
| Low (≤0.82 μmol/L) | 1 | 1 | 1 | ||||||
| High (>0.82 μmol/L) | 1.00 | 0.41–2.42 | 1.00 | 0.84 | 0.34–2.11 | 0.71 | 1.3 | 0.41–4.11 | 0.66 |
| l-Arginine | |||||||||
| High (>50.28 μmol/L) | 1 | 1 | 1 | ||||||
| Low (≤50.28 μmol/L) | 1.50 | 0.62–3.66 | 0.37 | 1.44 | 0.58–2.56 | 0.43 | 2.82 | 0.83–9.66 | 0.10 |
| ADMA | |||||||||
| Low (≤0.54 μmol/L) | 1 | 1 | 1 | ||||||
| High (>0.54 μmol/L) | 1.85 | 0.75–4.58 | 0.18 | 1.84 | 0.73–4.63 | 0.20 | 1.2 | 0.38–3.86 | 0.76 |
| l-Arginine/ADMA ratio | |||||||||
| High (>89.38) | 1 | 1 | 1 | ||||||
| Low (≤89.38) | 2.90 | 1.12–7.52 | 0.029 | 2.59 | 0.98–6.85 | 0.06 | 3.07 | 0.90–10.48 | 0.07 |
*Five patients were not included in the analysis due to missing data of SAPS II (one of whom died the first day of admission). When the missing variables were replaced with the minimal value and maximum value, the median SAPS II was 27 (18–44) and 37 (30–54), respectively, compared with 45 (35–54) for the entire cohort. Age is included in SAPS II.
ADMA indicates asymmetric dimethylarginine; CI, confidence interval; OR, odds ratio; SAPS II, Simplified Acute Physiology Score II.
Table 4.
Univariate and multivariate logistic regression analyses of mortality up to day 28 (time of censoring) in patients with necrotizing soft tissue infection based on high versus low concentrations of the biomarkers according to the optimal cutoff
| Unadjusted | Adjusted for age, sex, chronic disease | Adjusted for sex, chronic disease, SAPS II* | |||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Nitrite | |||||||||
| Low (≤1.49 μmol/L) | 1 | 1 | 1 | ||||||
| High (>1.49 μmol/L) | 2.20 | 0.62–7.85 | 0.22 | 1.87 | 0.50–6.93 | 0.35 | 4.60 | 0.92–23.03 | 0.06 |
| l-Arginine | |||||||||
| High (>71.98 μmol/L) | 1 | 1 | 1 | ||||||
| Low (≤71.98 μmol/L) | 0.59 | 0.23–1.54 | 0.28 | 0.56 | 0.21–1.48 | 0.24 | 0.61 | 0.18–2.06 | 0.43 |
| ADMA | |||||||||
| Low (≤0.40 μmol/L) | 1 | 1 | 1 | ||||||
| High (>0.40 μmol/L) | 11.5 | 1.50–88.41 | 0.02 | 10.23 | 1.32–79.38 | 0.03 | 13.17 | 0.91–191 | 0.06 |
| l-Arginine/ADMA ratio | |||||||||
| High (>101.59) | 1 | 1 | 1 | ||||||
| Low (≤101.59) | 5.76 | 1.85–17.90 | 0.003 | 5.02 | 1.59–15.91 | 0.006 | 6.03 | 1.41–25.84 | 0.016 |
*Five patients were not included in the analysis due to missing data of SAPS II (one of whom died the first day of admission). When the missing variables were replaced with the minimal value and maximum value, the median SAPS II was 27 (18–44) and 37 (30–54), respectively. Age is included in SAPS II. Optimal cutoff was found by the maximum sum of sensitivity and specificity.
ADMA indicates asymmetric dimethylarginine; CI, confidence interval; OR, odds ratio; SAPS II, Simplified Acute Physiology Score II.
DISCUSSION
We found no difference in admission (baseline) plasma nitrite level according to septic shock in patients who had NSTI. In contrast, baseline ADMA level was associated with the use of RRT and it correlated with the SAPS II and the SOFA score. In line with this, patients with an l-arginine/ADMA ratio below 101.59 (optimal cutoff) upon hospital admission had 5 times the odds of dying within the first 28 days.
A main strength of this study is the full follow-up of the patients due to the individually assigned person number that is coupled to the national health registries. Furthermore, treatment of NSTI has been centralized at a national level at Rigshospitalet, University of Copenhagen, providing us with the opportunity to investigate the disease in a large but well-defined geographical area, increasing the external validity of the study.
A number of limitations need to be taken into consideration. First, the study might be subject to bias because of the inability to control for unknown confounders. We decided a priori to adjust for potential confounders (age, sex, comorbidities, and SAPS II) that most likely affect disease severity and mortality. It has previously been shown that kidney function has an impact on the nitrite, l-arginine, and ADMA levels in plasma (32), and this is confirmed in this study because there was a significant correlation between the biomarker levels and the creatinine levels. An adjustment for this was done as urine output and blood urea nitrogen levels are included in the SAPS II. Hemodilution may also be an important factor to consider. One would expect patients with severe illness (i.e., septic shock) to receive more fluids, thus influencing biomarker levels. Unfortunately, we do not have data on the amount on fluid resuscitation before the blood samples were obtained. However, in a previous study constituting the same cohort as this, we found no difference in hemoglobin levels in patients with and without septic shock (7), indicating that a potential diluting factor may not affect data significantly.
Second, five patients (3.5%) had missing SAPS II data and were not included in the multivariate analyses. No major changes were seen in OR when SAPS II was included in the final step of adjustment, thus making it unlikely for the missing values of the five patients to have a major impact. Moreover, we conducted multiple analyses, and the analyses regarding l-arginine, ADMA, l-arginine/ADMA ratio, and outcome were secondary in nature, thus increasing the risk of chance findings. Therefore, the diagnostic accuracy of the biomarkers should be tested in another cohort. It is also relevant to investigate these biomarkers in future cohorts defined by the Sepsis-3 Septic Shock definitions (33). In this study, most of the patients in the septic shock group (hypotension and receiving vasopressors) will probably also meet the Sepsis-3 definition as they also exhibited a lactate increase. However, the precise impact of the Sepsis-3 definition needs to be investigated in future studies. Importantly, we used the same criteria as previous studies investigating vasoactive biomarkers, which allow us to make relevant comparisons of the results and conclusions.
Third, the results from the logistic regression analyses had rather wide 95% CIs, thus complicating the interpretation. However, a low l-arginine/ADMA ratio upon hospital admission was associated with increased mortality and OR for death at Day 28 was approximately 5 as compared with patients with a high l-arginine/ADMA ratio. The l-arginine/ADMA ratio has been suggested as a superior indicator of NO dysfunction as the production of NO might be affected by abnormal levels of both l-arginine and ADMA (21, 27). There are conflicting data on the association between l-arginine, ADMA, and mortality in patients with sepsis and septic shock (21, 22, 24, 26, 34–36), but there seems to be a relatively consistent association between low l-arginine/ADMA ratio and mortality in infected patients (21, 22, 34, 37). However, the studies are limited by a small number of patients and the use of different time points of mortality assessment and different cutoff levels. A recent study investigating 267 patients with severe sepsis or septic shock found a significant association between high baseline ADMA levels and 90-day mortality, but no association between l-arginine/ADMA ratio and mortality (38). The results of that study may not be directly comparable to those of our study as the most frequent sources of infection in the former were the lungs and abdomen. In our study, all patients were severely infected in the soft tissues and/or muscles with associated tissue necrosis. It may be that the pathophysiology of these infections differs and that the response pattern from the NO system and the associated biomarkers varies.
In line with this, we are not able to establish a mechanistic explanation for our findings. However, the association between l-arginine/ADMA ratio and mortality could be attributed to the NO system. Endothelial cells regulate blood flow in microvessels by producing NO. Interestingly, the microcirculation is impaired in patients with septic shock and this is associated with higher mortality (39, 40). Moreover, NO production is stimulated by TNF-α and IL-1, and the level of both cytokines is increased in this group of patients and also associated with disease severity and mortality (41). It would have been interesting to investigate the NOS expression in these patients as the pronounced inflammation may be a significant inducer of inducible NOS, contributing to circulating NO metabolites (42).
We used nitrite level as a surrogate measure of NO production because the half-life of NO in blood is short making it difficult to measure (43, 44). In plasma, NO reacts with oxygen species to form nitrate and nitrite. Nitrite is the major oxidation product of NO in the absence of oxyhemoglobin or superoxide anion and may be a more reliable measure of eNOS activity than nitrate (15, 16). NO synthesis in vascular endothelial cells is of special interest as it reflects the microcirculation, thus making nitrite measurements relevant to our patients. Nitrate, on the contrary, is influenced by numerous factors, such as diet, the intestinal bacterial flora, and renal function. In addition, there are high concentrations of nitrate in plasma that makes it difficult to detect acute changes in accordance to eNOS (45). We are aware that the accuracy of the measurements are limited, but the purpose with this study was to determine whether clinically relevant differences exist between subgroups of patients with NSTI according to disease severity and mortality. Importantly, there seem to be an association between low substrate availability (l-arginine), high levels of NO inhibitor (ADMA), and mortality.
We speculate that this imbalance in the NO system contributes to an unfavorable impairment of the microcirculation of patients with NSTI causing organ failure and increased mortality. Further studies are needed to elucidate the precise mechanisms, but if endothelial dysfunction turns out to play a crucial role for clinical outcome, l-arginine and ADMA will be interesting biomarkers as well as the modulation of the NO pathway for this group of critically ill patients.
CONCLUSIONS
In patients with NSTI, we found no difference in baseline nitrite level according to presence of septic shock. High baseline ADMA level was associated with use of RRT, and patients with a low baseline l-arginine/ADMA ratio were in higher risk of dying within first 28 days of hospital admission. Our findings support the hypothesis that the NO system plays an important role in the pathophysiology related to NSTI. However, this needs to be validated in other cohorts.
Acknowledgments
The authors thank to the INFECT project team in Denmark for including patients and obtaining blood samples, and also thank Heidi Schou Knudsen and Anne Christine Vigh Wandall-Frostholm for help with the laboratory analyses.
Footnotes
AP and MBM are investigators of a trial on IVIG for NSTI patients, partly funded by CSL Behring. The remaining authors have no conflicts of interest.
This work was supported by European Union's Seventh Framework Programme (305340), Rigshospitalet Research Foundation (E-22514-02), and Aase and Ejnar Danielsen Foundation (10-001274). LSR has received funding from the TrygFonden (109662). PG and KP have received funding from the Danish Heart Association (16-R107-A6650-22966) and the Danish Council of Independent Research (DFF—6110-00489). US has received funding from the Danish Heart Association (14-R97-A5294-22846). OHY has received funding from the European Union's Seventh Framework Programme through the INFECT (305340; http://www.fp7infect.eu/).
The authors report no conflicts of interest.
REFERENCES
- 1.Young MH, Aronoff DM, Engleberg NC. Necrotizing fasciitis: pathogenesis and treatment. Expert Rev Anti Infect Ther 2005; 3:279–294. [DOI] [PubMed] [Google Scholar]
- 2.Ellis Simonsen SM, van Orman ER, Hatch BE, Jones SS, Gren LH, Hegmann KT, Lyon JL. Cellulitis incidence in a defined population. Epidemiol Infect 2006; 134:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991; 43:109–142. [PubMed] [Google Scholar]
- 4.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med 1993; 329:2002–2012. [DOI] [PubMed] [Google Scholar]
- 5.Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med 2001; 345:588–595. [DOI] [PubMed] [Google Scholar]
- 6.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 2002; 360:1395–1396. [DOI] [PubMed] [Google Scholar]
- 7.Hansen MB, Rasmussen LS, Garred P, Bidstrup D, Madsen MB, Hyldegaard O. Pentraxin-3 as a marker of disease severity and risk of death in patients with necrotizing soft tissue infections: a nationwide, prospective, observational study. Crit Care 2016; 20:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 1997; 99:2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 2008; 45:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortin CF, McDonald PP, Fülöp T, Lesur O. Sepsis, leukocytes, and nitric oxide (NO): an intricate affair. Shock 2010; 33:344–352. [DOI] [PubMed] [Google Scholar]
- 11.Spack L, Havens PL, Griffith OW. Measurements of total plasma nitrite and nitrate in pediatric patients with the systemic inflammatory response syndrome. Crit Care Med 1997; 25:1071–1078. [DOI] [PubMed] [Google Scholar]
- 12.de Werra I, Jaccard C, Corradin SB, Chioléro R, Yersin B, Gallati H, Assicot M, Bohuon C, Baumgartner JD, Glauser MP, et al. Cytokines, nitrite/nitrate, soluble tumor necrosis factor receptors, and procalcitonin concentrations: comparisons in patients with septic shock, cardiogenic shock, and bacterial pneumonia. Crit Care Med 1997; 25:607–613. [DOI] [PubMed] [Google Scholar]
- 13.Doughty L, Carcillo JA, Kaplan S, Janosky J. Plasma nitrite and nitrate concentrations and multiple organ failure in pediatric sepsis. Crit Care Med 1998; 26:157–162. [DOI] [PubMed] [Google Scholar]
- 14.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Gödecke A, Schrader J, Schulz R, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 2003; 35:790–796. [DOI] [PubMed] [Google Scholar]
- 15.Kelm M, Preik-Steinhoff H, Preik M, Strauer BE. Serum nitrite sensitively reflects endothelial NO formation in human forearm vasculature: evidence for biochemical assessment of the endothelial l-arginine-NO pathway. Cardiovasc Res 1999; 41:765–772. [DOI] [PubMed] [Google Scholar]
- 16.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 2001; 98:12814–12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin G, Asensi V, Montes AH, Collazos J, Alvarez V, Pérez-Is L, Carton JA, Taboada F, Valle-Garay E. Endothelial (NOS3 E298D) and inducible (NOS2 exon 22) nitric oxide synthase polymorphisms, as well as plasma NOx, influence sepsis development. Nitric Oxide 2014; 42:79–86. [DOI] [PubMed] [Google Scholar]
- 18.Annane D, Sanquer S, Sébille V, Faye A, Djuranovic D, Raphaël JC, Gajdos P, Bellissant E. Compartmentalised inducible nitric-oxide synthase activity in septic shock. Lancet 2000; 355:1143–1148. [DOI] [PubMed] [Google Scholar]
- 19.Kehmeier ES, Kropp M, Kleinbongard P, Lauer T, Balzer J, Merx MW, Heusch G, Kelm M, Lepper W, Rassaf T. Serial measurements of whole blood nitrite in an intensive care setting. Free Radic Biol Med 2008; 44:1945–1950. [DOI] [PubMed] [Google Scholar]
- 20.Mian AI, Aranke M, Bryan NS. Nitric oxide and its metabolites in the critical phase of illness: rapid biomarkers in the making. Open Biochem J 2013; 7:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visser M, Vermeulen MAR, Richir MC, Teerlink T, Houdijk APJ, Kostense PJ, Wisselink W, de Mol BAJM, van Leeuwen PAM, Oudemans-van Straaten HM. Imbalance of arginine and asymmetric dimethylarginine is associated with markers of circulatory failure, organ failure and mortality in shock patients. Br J Nutr 2012; 107:1458–1465. [DOI] [PubMed] [Google Scholar]
- 22.Davis JS, Darcy CJ, Yeo TW, Jones C, McNeil YR, Stephens DP, Celermajer DS, Anstey NM. Asymmetric dimethylarginine, endothelial nitric oxide bioavailability and mortality in sepsis. PLoS One 2011; 6:e17260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss SL, Haymond S, Ralay Ranaivo H, Wang D, De Jesus VR, Chace DH, Wainwright MS. Evaluation of asymmetric dimethylarginine, arginine, and carnitine metabolism in pediatric sepsis. Pediatr Crit Care Med 2012; 13:e210–e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch A, Weiskirchen R, Kunze J, Dückers H, Bruensing J, Buendgens L, Matthes M, Luedde T, Trautwein C, Tacke F. Elevated asymmetric dimethylarginine levels predict short- and long-term mortality risk in critically ill patients. J Crit Care 2013; 28:947–953. [DOI] [PubMed] [Google Scholar]
- 25.Nijveldt RJ, Teerlink T, Van Der Hoven B, Siroen MPC, Kuik DJ, Rauwerda JA, van Leeuwen PAM. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin Nutr 2003; 22:23–30. [DOI] [PubMed] [Google Scholar]
- 26.Iapichino G, Umbrello M, Albicini M, Spanu P, Bellani G, Polli F, Pavlovic R, Cugno M, Fermo I, Paroni R. Time course of endogenous nitric oxide inhibitors in severe sepsis in humans. Minerva Anestesiol 2010; 76:325–333. [PubMed] [Google Scholar]
- 27.Bode-Böger SM, Scalera F, Ignarro LJ. The l-arginine paradox: importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther 2007; 114:295–306. [DOI] [PubMed] [Google Scholar]
- 28.Hansen MB, Simonsen U, Garred P, Hyldegaard O. Biomarkers of necrotising soft tissue infections: aspects of the innate immune response and effects of hyperbaric oxygenation—the protocol of the prospective cohort BIONEC study. BMJ Open 2015; 5:e006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 30.Aamand R, Dalsgaard T, Ho Y-CL, Møller A, Roepstorff A, Lund TE. A NO way to BOLD? Dietary nitrate alters the hemodynamic response to visual stimulation. Neuroimage 2013; 83:397–407. [DOI] [PubMed] [Google Scholar]
- 31.Hansen MB, Rasmussen LS, Pilely K, Hellemann D, Hein E, Madsen MB, Hyldegaard O, Garred P. The lectin complement pathway in patients with necrotizing soft tissue infection. J Innate Immun 2016; 8 5:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Hamm LL, Mohler ER, Hudaihed A, Arora R, Chen C-S, Liu Y, Browne G, Mills KT, Kleinpeter MA, et al. Interrelationship of multiple endothelial dysfunction biomarkers with chronic kidney disease. PLoS One 2015; 10:e0132047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gough MS, Morgan MAM, Mack CM, Darling DC, Frasier LM, Doolin KP, Apostolakos MJ, Stewart JC, Graves BT, Arning E, et al. The ratio of arginine to dimethylarginines is reduced and predicts outcomes in patients with severe sepsis. Crit Care Med 2011; 39:1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aydemir O, Ozcan B, Yucel H, Bas AY, Demirel N. Asymmetric dimethylarginine and l-arginine levels in neonatal sepsis and septic shock. J Matern Fetal Neonatal Med 2015; 28:977–982. [DOI] [PubMed] [Google Scholar]
- 36.O’Dwyer MJ, Dempsey F, Crowley V, Kelleher DP, McManus R, Ryan T. Septic shock is correlated with asymmetrical dimethyl arginine levels, which may be influenced by a polymorphism in the dimethylarginine dimethylaminohydrolase II gene: a prospective observational study. Crit Care 2006; 10:R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner T, Fleming TH, Rosenhagen C, Krauser U, Mieth M, Bruckner T, Martin E, Nawroth PP, Weigand MA, Bierhaus A, et al. l-arginine and asymmetric dimethylarginine are early predictors for survival in septic patients with acute liver failure. Mediators Inflamm 2012; 2012:210454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mortensen KM, Itenov TS, Haase N, Müller RB, Ostrowski SR, Johansson PI, Olsen NV, Perner A, Søe-Jensen P, Bestle MH. High levels of methylarginines were associated with increased mortality in patients with severe sepsis. Shock 2016; 46:365–372. [DOI] [PubMed] [Google Scholar]
- 39.De Backer D, Creteur J, Preiser J-C, Dubois M-J, Vincent J-L. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002; 166:98–104. [DOI] [PubMed] [Google Scholar]
- 40.De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, Vincent J-L. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med 2013; 41:791–799. [DOI] [PubMed] [Google Scholar]
- 41.Hansen MB, Rasmussen LS, Svensson M, Chakrakodi B, Bruun T, Madsen MB, Perner A, Garred P, Hyldegaard O, Norrby-Teglund A. Association between cytokine response, the LRINEC score and outcome in patients with necrotising soft tissue infection: a multicentre, prospective study. Sci Rep 2017; 7:42179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernanz R, Alonso MJ, Zibrandtsen H, Alvarez Y, Salaices M, Simonsen U. Measurements of nitric oxide concentration and hyporeactivity in rat superior mesenteric artery exposed to endotoxin. Cardiovasc Res 2004; 62:202–211. [DOI] [PubMed] [Google Scholar]
- 43.Kelm M, Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res 1990; 66:1561–1575. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR. Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem 1998; 273:18709–18713. [DOI] [PubMed] [Google Scholar]
- 45.Nagababu E, Rifkind JM. Measurement of plasma nitrite by chemiluminescence. Methods Mol Biol 2010; 610:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]


