Abstract
The flacca tomato (Lycopersicon esculentum) mutant displays a wilty phenotype as a result of abscisic acid (ABA) deficiency. The Mo cofactor (MoCo)-containing aldehyde oxidases (AO; EC 1.2.3.1) are thought to play a role in the final oxidation step required for ABA biosynthesis. AO and related MoCo-containing enzymes xanthine dehydrogenase (XDH; EC 1.2.1.37) and nitrate reductase (EC 1.6.6.1) were examined in extracts of the flacca tomato genotype and of wild-type (WT) roots and shoots. The levels of MoCo were found to be similar in both genotypes. No significant XDH or AO (MoCo-containing hydroxylases) activities were detected in flacca leaves; however, the mutant exhibited considerable MoCo-containing hydroxylase activity in the roots, which contained notable amounts of ABA. Native western blots probed with an antibody to MoCo-containing hydroxylases revealed substantial, albeit reduced, levels of cross-reactive protein in the flacca mutant shoots and roots. The ABA xylem-loading rate was significantly lower than that in the WT, indicating that the flacca is also defective in ABA transport to the shoot. Significantly, in vitro sulfurylation with Na2S reactivated preexisting XDH and AO proteins in extracts from flacca, particularly from the shoots, and superinduced the basal-level activity in the WT extracts. The results indicate that in flacca, MoCo-sulfurylase activity is impaired in a tissue-dependent manner.
ABA is a plant growth regulator involved in various processes, including the reactions of plants to environmental stress and seed maturation (Zeevaart and Creelman, 1988). In higher plants ABA is derived from an epoxy-carotenoid precursor that is oxidatively cleaved to produce xanthoxin (Parry et al., 1988). After the cleavage reaction, xanthoxin is converted to ABA by a series of ring modifications to yield abscisic aldehyde, which is oxidized to ABA by AO (EC 1.2.3.1), a MoCo-containing enzyme (Walker-Simmons et al., 1989; Leydecker et al., 1995). In addition to AO, plant MoCo-containing enzymes include NR (EC 1.6.6.1) and XDH (EC 1.2.1.37).
XDH and AO (MoCo-containing hydroxylases) from various organisms have been characterized as homodimers of 150-kD subunits. They have a high degree of homology in their amino acid sequence and contain binding sites for two Fe-S centers and a MoCo-binding region (Ori et al., 1997; Sekimoto et al., 1997). Whereas NR requires a dioxo-Mo center (Rajagopalan and Johnson, 1992), XDH and AO incorporate mono-oxo-MoCo in which the second oxygen is replaced by an S ligand. AO belongs to a multigene family (Ori et al., 1997) and appears to display a broad range of substrate specificities (Koshiba et al., 1996; Ori et al., 1997; Sekimoto et al., 1997; Omarov et al., 1999), among them the oxidation of indole-3-acetaldehyde to IAA (Bandurski et al., 1995; Koshiba et al., 1996).
The characterization of ABA-deficient mutants has been valuable in elucidating the function of ABA and the pathway of ABA biosynthesis. A number of mutants with reduced capacity to synthesize ABA have been described. These include flacca, notabilis, and sit in tomato (Lycopersicon esculentum), dr in potato, aba1 in wild tobacco, nar2a in barley, and aba3 in Arabidopsis (Walker-Simmons et al., 1989; Taylor, 1991; Schwartz et al., 1997). The nar2a mutant in barley was shown to lack AO, XDH, and NR activities, suggesting a lesion in the synthesis of the MoCo, which all three enzyme activities require (Walker-Simmons et al., 1989). In contrast, shoots of aba1, aba3, and flacca apparently lack AO and XDH activities but not NR, suggesting an additional step in MoCo biosynthesis (Leydecker et al., 1995; Marin and Marion-Poll, 1997; Schwartz et al., 1997). The Arabidopsis aba3 mutant may have lost its ability to replace one of the oxygens of dioxo-MoCo by an S ligand required for AO and XDH activity (Schwartz et al., 1997).
To our knowledge, the molecular basis for ABA mutations in tomato has yet to be described. ABA content in flacca leaves was about 20% to 26% of that in the WT (Neill and Horgan, 1985; Linforth et al., 1987; Taylor et al., 1988; Rock et al., 1991). Plants of flacca display a marked tendency to wilt, apparently because of excessive transpiration resulting from a lack of control of stomata closure (Tal, 1966). It has been suggested that the primary reason for the wilty phenotype was the lower endogenous ABA content in the leaves (Imber and Tal, 1970). Previous studies of flacca mutants focused on changes in enzyme activity (Marin and Marion-Poll, 1997) or the determinations of ABA concentration and synthesis in shoots (Imber and Tal, 1970; Taylor et al., 1988) but not roots, although roots are the main site of ABA synthesis (Bano et al., 1993). Thus, characterization of the Mo-enzymes involved in hormone biosynthesis in roots and shoots of flacca and the WT are required. The main goal of this study was to examine the simultaneous analysis of enzymatic and immunological characteristics of the MoCo-containing hydroxylases in the whole plant, as well as to estimate the capacity of roots to produce and transport ABA to the shoots.
MATERIALS AND METHODS
Plant Material
WT and flacca seeds of tomato (Lycopersicon esculentum Mill. cv Rheinlands Ruhm) were germinated and allowed to establish for 14 d on wet filter paper. Uniform plants were transplanted to pots with dune sand (96% sand, 2% silt, and 2% clay, pH 8.25 and electrical conductivity 0.7 decisiemens m−1) irrigated with 2.5 mm (NH4)2SO4 as the N component of a modified one-half-strength Hoagland solution (Hoagland and Arnon, 1938). In the greenhouse average day temperatures during the growth period fluctuated from 20°C to 25°C, and average night temperatures fluctuated from 8°C to 12°C. Midday PPFD in the greenhouse was 900 to 1000 μmol m−2 s−1.
Tissue Extraction
Shoot and root samples were obtained from 6- to 8-week-old plants and extracted immediately. Crude extracts for assays of NR and MoCo were prepared as previously described (Gao et al., 1996). Crude extracts for assays of XDH and AO in native-gel electrophoresis and western analysis were prepared in a modified version of the method described previously by Sagi et al. (1998). Tissue was macerated with acid-washed sand in an ice-cold extraction medium containing 250 mm Tris-HCl (pH 8.5), 1 mm EDTA, 1 mm DTT, 5 mm l-Cys, 80 μm Na2MoO4, 10 μm antipain, 0.1 mm PMSF, 10 mm GSH, and 0.03 mm FAD. Samples of 1 g of shoot or root were extracted in 2 and 1 mL of buffer (1:2 and 1:1, w/v), respectively. The homogenized plant material was centrifuged at 30,000g in a refrigerated centrifuge (model RC-5, Sorvall) at 3°C to 5°C for 15 min. The resulting supernatant was used for subsequent assays.
Crude extracts for AO assays in vitro were prepared according to the method of Triplett et al. (1982). Samples of 1 g fresh weight of tissue were ground in liquid N2, and the resulting powder was mixed with 0.25 g of polyvinylpolypyrrolidone and then extracted in 1 mL of 50 mm potassium-phosphate buffer, pH 7.5. The homogenized plant material was centrifuged as described above. The supernatants were brought up to 60% saturation with solid ammonium sulfate. After stirring for 30 min, the mixture was centrifuged at 40,000g for 20 min. The pellet was suspended in 1 to 2 mL of 50 mm potassium-phosphate buffer, pH 7.5, and desalted on a 1.5- × 30-cm Sephadex G-25 column (Pharmacia) equilibrated with 50 mm potassium-phosphate buffer, pH 7.5. Crude extracts used for XDH assays in vitro were prepared as described for AO, using 50 mm Tris-HCl buffer, pH 8.48. All of the preceding steps were carried out at 4°C.
Enzyme Activity and Protein Analysis
NR (EC 1.6.6.1) activity was measured in crude extracts as described previously (Gao et al., 1996). MoCo activity in plant tissue was estimated using the MoCo-deficient nit-1 mutant of Neurospora crassa, complemented with tomato MoCo released by heating extracts at 80°C for 90 s, following the original procedure of Mendel et al. (1985), as recently modified by Sagi et al. (1997).
AO and XDH activities in vitro, before or after in vitro sulfurylation, were assayed, monitoring the decrease of A600 of the electron donor DCIP (Courtright, 1967; Rajagopalan and Handler, 1967; Perez-Vicente et al., 1988) in a spectrophotometer (Genesis-2, Milton Roy, Rochester, NY). The 1.5-mL AO assay reaction mixture contained 500 to 1000 μg of protein of the desalted extract, 0.002% DCIP, 0.1 mm phenazine methosulfate, and 2 mm indole-3-aldehyde in 50 mm potassium-phosphate buffer, pH 7.4. The 1.5-mL XDH reaction mixture contained 1000 μg of protein of the desalted extract, 0.002% DCIP, and 0.6 mm hypoxanthine in 50 mm Tris-HCl buffer, pH 8.48. AO and XDH activities were expressed as nanomoles DCIP reduced per milligram protein per minute. Soluble proteins in the assays were measured (Bradford, 1976) using crystalline BSA as a reference.
Gel Electrophoresis and Analysis of Enzyme Activity
Enzyme electrophoresis and staining were carried out using 1.5-mm-thick slabs of 7.5% native-polyacrylamide gels loaded with 300 mg of shoot proteins or 100 mg of root proteins. Enzyme activities of AO and XDH were estimated in gels by staining, after native electrophoresis, using 3(4,5-dimethylthiazolyl-2)2,5-diphenyltetrazolium-bromide, which resulted in the development of specific formazan bands. The quantity of formazan was directly proportional to enzyme activity during a given incubation time and in the presence of excess substrate and tetrazolium salt (Rothe, 1974). Quantitative analyses were made by scanning the formazan bands with a computing laser densitometer using Image Quant version 3.19.4 software (Molecular Dynamics, Sunnyvale, CA). XDH activity was determined using hypoxanthine as a specific substrate (Mendel and Muller, 1976), and specificity was confirmed with allopurinol, an XDH inhibitor (Leydecker et al., 1995). AO activity was detected after immersing gels in 0.2 m phosphate buffer, pH 8.0, for 10 min followed by gentle shaking in a reaction mixture containing 0.1 mm phenazine methosulfate and 1 mm 3(4,5-dimethylthiazolyl-2)2,5diphenyltetrazolium-bromide in the presence of 1 mm indole-3-aldehyde or 1 mm acetaldehyde in 0.1 m Tris-HCl buffer, pH 8.48, at 25°C.
Western Analysis of MoCo-Containing Hydroxylases
The MoCo-containing hydroxylase proteins in shoots and roots were detected by western blotting. Native-PAGE loaded with flacca and WT crude extracts, with and without treatments of sulfurylation or heating to 80°C for 90 s, were carried out as described above. SDS-PAGE was performed in 10% polyacrylamide gels (Laemmli, 1970). The resulting gels with the separated proteins were then blotted onto a nitrocellulose membrane (0.2 μm pore size; Schleicher & Schüell). Blotting time was 1.5 h at 2 mA cm−2. Immunodetection of MoCo-containing hydroxylases was carried out with polyclonal guinea pig antibodies raised against recombinant TAO1 (tomato aldehyde oxidase 1) polypeptides (Ori et al., 1997). The TAO1 sequence contains binding sites for two Fe-S centers and the Mo-binding regions of XDH and AO of various organisms (Ori et al., 1997). Primary antibodies were diluted 500-fold in TBS and secondary antibodies (anti-guinea pig IgG; Sigma) were diluted 1000-fold in TBS. Phosphatase activity was developed by staining with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium.
In Vitro Sulfurylation of Crude Extracts
This procedure was carried out following a modification of the method described previously by Wahl and Rajagopalan (1982). Detection of AO and XDH in vitro or on polyacrylamide gels was carried out with desalted WT and flacca extracts. One-half milliliter of root or shoot extracts was desalted on G-25 Sephadex and incubated at 32°C for 40 min with 10 μL of 0.1 m dithionite and 4 μL of 0.5 m Na2S; 5 μL of 1.25 mm methyl viologen was used as an indicator of reducing conditions, while gently flushing N2 through the mixture to maintain anaerobic conditions. After sulfurylation, the extracts were desalted through Sephadex G-25 (Sigma). XDH and AO activities were determined in gels after electrophoresis and also by in vitro assays.
Collection of Xylem Sap and ABA Determination
Xylem exudate was collected 1 h after sunrise for a period of 2 h. Shoots were removed with a smooth horizontal cut with a razor blade 1 cm below the first leaves. Exudate was collected at 15-min intervals followed immediately by ABA determination. ABA in shoots, roots, and exudate of xylem sap was analyzed by ELISA with monoclonal antibodies as described by Mertens et al. (1985).
RESULTS
Activities of MoCo-Containing Enzymes
MoCo-containing enzymes and MoCo levels were measured as described in Methods. The WT and flacca exhibited significant NR activity and MoCo levels in shoots and roots. Root NR activity in WT was 3-fold higher than in flacca. The MoCo levels in the roots and shoots of both genotypes was similar, with higher levels in roots than in shoots (Table I).
Table I.
NR and MoCo activities in WT and flacca plants
| Genotype | NR Activitya
|
MoCo Levelb
|
||
|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |
| units mg−1 protein | ||||
| WT | 0.63a | 0.48a | 6.5a | 27.2a |
| flacca | 0.44b | 0.16b | 5.8a | 28.0a |
Letters following values indicate statistical significance of separation between the varieties Duncan test; (P < 0.05, n = 3 different experiments with eight replications each).
One unit of NR activity is expressed as the amount of enzyme that catalyzes the production of 1 μmol NO2− h−1.
One unit of MoCo is defined as the amount of plant MoCo that yields 1 unit of reconstituted NR activity after in vitro complementation of apoNR from the MoCo-deficient N. crassa nit-1 mutant (Sagi et al., 1997).
Qualitative analysis of the MoCo-containing hydroxylases was carried out in nondenaturating-PAGE. The substrates indole-3-aldehyde, acetaldehyde, and hypoxanthine (a substrate for XDH) were used as indicators of AO activity. WT extracts exhibited at least three XDH and AO activity bands in roots and two bands in shoots with hypoxanthine, indole-3-aldehyde, or acetaldehyde (Fig. 1). Shoot extracts of flacca did not exhibit detectable XDH or AO in activity gels. However, root extracts of flacca revealed one band of MoCo-containing hydroxylase activity that appeared to comigrate with the upper band detected in WT extracts.
Figure 1.
Native-PAGE of shoot and root extracts of WT and flacca (flc) tomato genotypes showing AO and XDH activities. Activity gels were loaded with 300 μg of soluble protein of the crude extract of shoots or 100 μg of soluble protein of roots and stained with the appropriate substrate as described in Methods.
Immunoblot Analysis of MoCo-Containing Hydroxylases
Native-PAGE fractionation of WT and flacca root and shoot extracts followed by immunoblot analysis with antibodies raised against recombinant TAO1 was carried out to detect MoCo-containing hydroxylase cross-reacting proteins. The blots revealed a few slowly migrating major bands in the WT and flacca extracts. Their mobility was similar to the activity bands detected in MoCo-containing hydroxylase substrate staining gels (compare Figs. 1 and 2). The relative amounts (density) of protein detected in flacca were approximately 40% to 46% and 10% to 17% of that in the WT in roots and shoots, respectively (Fig. 2). The activity of MoCo-containing hydroxylase proteins in crude extracts of the WT and flacca was relatively stable during heat treatment at 80°C for 90 s (data not shown). Heat stability of MoCo-containing hydroxylases in plant shoots was reported earlier (Bower et al., 1978, Koshiba and Matsuyama, 1993; Montalbini, 1998). Rapidly migrating MoCo-containing hydroxylase cross-reacting proteins, which did not correlate with positions of substrate activity staining in the gel, exhibited lower thermostability than the active proteins in the roots and shoots of both genotypes (Fig. 2, lower arrows).
Figure 2.
Immunoblot analysis of MoCo-containing hydroxylases. Approximately 200 μg of total soluble protein from root and shoot extracts were fractionated on native-PAGE and examined with anti-TAO1 antibodies. Untreated (Control) and preheated (80°C for 90 s) treatments were used. flc, flacca tomato genotype.
SDS-PAGE of plant extracts followed by immunoblotting analysis revealed in the WT shoots the expected polypeptide with a molecular mass of 150 kD and three polypeptides with molecular masses of 78, 76, and 72 kD. It is interesting that the 150-and 78-kD bands were conspicuously absent from the flacca shoot extracts (Fig. 3).
Figure 3.
Immunoblot analysis of MoCo-containing hydroxylases. Crude extracts of roots and shoots of WT and flacca (flc) tomato plants were fractionated on SDS-PAGE. The gel was loaded with approximately 50 μg of total soluble protein and examined with anti-TAO1 antibodies. Relative density measurements of the bands are indicated at the bottom of the panels.
Polypeptides with molecular masses of 150, 102, 78, 76, and 72 kD were detected in root extracts of both genotypes. The relative amounts of protein detected in roots and shoots of flacca were significantly lower than in the WT (Fig. 3, relative densities of bands a–e). The multiplicity of bands detected may represent proteolytic products resulting from physiological processes in the plant or degradation during the extraction process (Ichida et al., 1993; Koshiba et al., 1996; Ori et al., 1997).
Sulfurylation of MoCo-Containing Hydroxylases
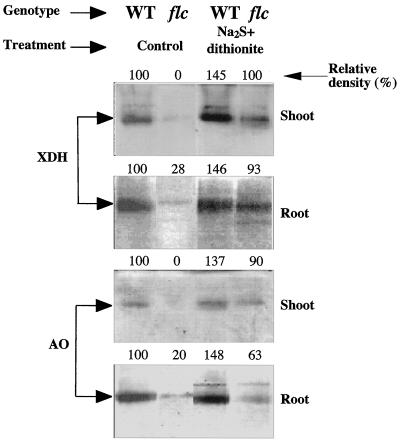
Na2S and dithionite can directly sulfurylate the dioxo-MoCo moiety (Wahl et al., 1982; Schwarz et al., 1997). This procedure was applied to extracts of the WT and flacca. In vitro sulfurylation of WT extracts under anaerobic conditions increased enzyme activities in shoot and root extracts by nearly 2-fold, as measured by the DCIP reduction assay (Fig. 4). The effect of in vitro sulfurylation in flacca shoot extracts was an enhancement of 10- to 100-fold for XDH and AO activities, whereas in flacca roots it was proportional to the changes observed in the WT root extracts. Qualitative analysis of XDH and AO levels using activity gels revealed a similar pattern of increased activity after sulfurylation, as observed in in vitro assays (Fig. 5). A modest increase in XDH and AO bands was observed in the WT and flacca roots, whereas a larger increase was observed in flacca shoots. In vitro sulfurylation under anaerobic conditions of flacca and WT desalted shoot crude extracts preheated to 80°C for 90 s revealed a similar pattern of activity as that for the unheated extract (data not shown).
Figure 4.
AO and XDH activities of shoot and root extracts of the WT and flacca (flc) genotypes. Extracts were assayed in vitro with hypoxanthine and indole-3-aldehyde as the substrates. The assays were carried out with desalted extracts, with or without preincubation with Na2S and dithionite. Enzyme activities are expressed as nmol DCIP mg−1 min−1. Lowercase letters indicate statistical significance of the separation between treatments carried out by the multiple range test (Duncan test; P < 0.05, n = 5). The data represent one of three different experiments that yielded essentially identical results.
Figure 5.
Native-PAGE of XDH and AO from shoot and root extracts of the WT and flacca (flc) genotypes. XDH and AO activities were detected with hypoxanthine and indole-3-aldehyde as the substrates. The assays were carried out with desalted extracts, with or without preincubation with Na2S and dithionite, under anaerobic conditions. The zymogram is one of three different experiments that yielded essentially identical results.
ABA Determination
The considerable AO activity measured in the roots, as determined by aldehyde-containing substrates, suggests that flacca roots may have the ability to synthesize ABA via ABA-aldehyde. The levels of ABA measured in the shoots, roots, and xylem sap of flacca were 23%, 67%, and 67%, respectively, of those of the WT (Table II). The ABA xylem-loading rate was estimated on the basis of [ABA] in sap exudate and the exudate flow rate. A significantly lower exudate flow rate was detected in flacca than in WT plants, implying a significantly reduced ABA xylem loading rate (Table II).
Table II.
ABA accumulation in plant organs and measured exudate [ABA] and flow rate of WT and flacca
| Genotype | ABA
|
[ABA] in Xylem Exudate | Exudate Flow Rate | ABA Xylem Loading Rate | |
|---|---|---|---|---|---|
| Shoot | Root | ||||
| ng g−1 fresh wt | pmol mL−1 | μL g−1 fresh wt root h−1 | pmol g−1 fresh wt root−1 h−1 | ||
| WT | 270.8a | 27.1a | 150a | 69a | 10.4a |
| flacca | 63.2b | 18.1b | 100b | 15b | 1.5b |
| flacca of WT (%) | 23 | 67 | 67 | 22 | 14 |
The letters following values in a column indicate statistical significance of separation between the varieties (Duncan test; P < 0.05, n = 4). The data represent one of two experiments. ABA was extracted, collected, and analyzed as described in Methods and by Walker-Simmons et al. (1988) using monoclonal antibodies (Mertens et al., 1985).
DISCUSSION
In an attempt to elucidate the molecular lesion that causes reduced ABA content in flacca mutants, we measured parameters that are relevant to MoCo production, and we measured the activity of MoCo-dependent hydroxylases. The levels of MoCo were found to be similar in flacca and WT plants and cannot be responsible for the flacca phenotype. flacca lacked significant XDH and AO activities in its leaves, although considerable MoCo levels and NR activity were observed in the tissue (Table I). However, in the root, XDH and AO activities were readily detected by native-PAGE activity gels and by DCIP reduction assays. Sulfurylation of flacca leaf extracts resulted in recovery of XDH and AO activities. The results suggest that flacca is defective in the sulfurylation of MoCo in the MoCo-containing hydroxylases and that the expression of this mutation has a tissue-specific determinant. The flacca mutation appears to be most similar to the Arabidopsis aba3 mutant (Schwartz et al., 1997). Tissue differences in the activity of MoCo have been observed in the Cnx2 and Cnx3 genes. These genes, responsible for defined steps in MoCo biosynthesis, were expressed mainly in the roots of Arabidopsis and are related to MoCo enzymes other than NR (Hoff et al., 1995).
Our observations show that the dioxo type of MoCo should not be rate limiting in flacca. Unexpectedly, however, in flacca roots the NR activity that is dependent on the dioxo-type of MoCo was only 33% of the corresponding level in WT roots. The lower NR activity may reflect modifications of NR in the root because of the lower water potential in flacca plants (Bradford, 1983). Reduced water potential impairs the driving force for flow of phloem sap, which in turn compromises the level of total measured soluble sugar in flacca roots (Johnson et al., 1992; Guerrier and Bourgeais-Chaillou, 1994). Under conditions of carbohydrate restriction, nitrate reduction is reduced (Radin et al., 1978; Oaks, 1986).
Despite the lack of AO activity in shoots, considerable [ABA]s were measured in flacca organs. They were 23%, 67%, and 67% of the concentration found in WT shoot, root, and xylem exudate, respectively (Table II). ABA detected in flacca leaves may have originated in the roots or may be the product of a minor shunt pathway converting ABA-aldehyde via ABA-alcohol to ABA in the shoots (Taylor et al., 1988; Rock et al., 1991). Impaired stomatal closing of flacca was corrected by foliar applications of ABA (Imber and Tal, 1970) or by dipping leaf petioles into solutions containing ABA (Neill and Horgan, 1985). This suggests that the reduced levels of ABA and/or its reduced mobilization rate were not enough to initiate correct stomatal closure. Our results indicate that the flacca mutation affects ABA transport from the root to the shoot. The reduced root pressure, resulting in a low ABA xylem-loading rate in addition to lower levels of root ABA synthesis, probably further exacerbates the ability of the plant to transport ABA to the shoot (Table II).
Sulfurylation of shoot and root extracts in vitro using dithionite and Na2S under anaerobic conditions activated preexisting inactive XDH and AO proteins in flacca mutant extracts (Figs. 4 and 5). The sulfurylation assay used desalted extracts that probably removed free MoCo. This suggests that dioxo-MoCo may be bound to the inactive MoCo-containing hydroxylase apoproteins and are rendered active by in vitro sulfurylation under reducing conditions.
The activity of the AO and XDH enzymes that were recovered after in vitro sulfurylation was lower in flacca than in the WT extracts, presumably because flacca contains fewer total (active and inactive) AO and XDH proteins. The lower number of these MoCo-containing hydroxylase proteins in flacca may be due to the decreased stability of the inactive enzyme molecules. Only small amounts of the expected full-sized 150-kD monomer were observed in flacca extracts after SDS-PAGE, although lower molecular mass cross-reacting polypeptides were evident. Alternatively, the biosynthesis of MoCo-containing hydroxylase proteins in flacca may be restricted under conditions in which enzymatic sulfurylation is either limited or absent. We note that the amount of cross-reactive protein detected by immunoblot procedures does not always correlate with the activity recovered. For example, in shoots, flacca AO and XDH activity after sulfurylation was approximately 70% of that in the WT, whereas the amount of cross-reacting protein was only approximately 17% of that in the WT. The antibodies used in the present work were prepared from areas conserved among the multigene AO and XDH families. Therefore, cross-reacting material should be taken as a global indication of protein amounts that do not necessarily correlate with enzymatic activity measured with a specific substrate.
Significant increases in MoCo-containing hydroxylase activities were detected after the in vitro sulfurylation of the WT extracts, indicating that a considerable amount of inactive enzyme was present in the flacca and WT extracts. The inactive state may be a normal feature of MoCo-containing hydroxylase metabolism or may be a result of isolation procedures. Thus, MoCo-containing hydroxylase sulfurylase may play a regulatory role in plants, modulating the levels of active AO and XDH during stress or development. One can speculate that sulfurylation of MoCo-containing hydroxylases may constitute the site stimulated by stress and the addition of NH4+ in barley (Omarov et al., 1998) and ryegrass (Sagi et al., 1998). In this respect, we note that the sulfurylation step is reversible in vitro (Wahl and Rajagopalan, 1982; Schwartz et al., 1997). The question remains, however, whether this reversibility has biological significance.
ACKNOWLEDGMENTS
The authors are grateful to Rustem Omarov for suggestions and helpful discussions and to Genia Shichman for excellent technical assistance.
Abbreviations:
- AO
aldehyde oxidase
- DCIP
2,6-dichloroindophenol
- MoCo
Mo cofactor(s)
- NR
nitrate reductase
- XDH
xanthine dehydrogenase
- WT
wild type
Footnotes
This work was supported by the U.S. Agency for International Development/Cooperative Development Research (project nos. C12-157 and CA15-024), by the Fohs Foundation, Israel Charitable Association, and by the Jewish National Foundation (the Ramat Negev Research and Development Project).
LITERATURE CITED
- Bandurski RS, Cohen JD, Slovin J, Reinecke DM. Auxin biosynthesis and metabolism. In: Davies PJ, editor. Plant Hormones. Physiology, Biochemistry and Molecular Biology, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 39–65. [Google Scholar]
- Bano A, Dorffing K, Bettin D, Hahn H. Abscisic acid and cytokinins as possible root-shoot signals in xylem sap of rice plants in drying soil. Aust J Plant Physiol. 1993;20:109–115. [Google Scholar]
- Bower PJ, Brown HM, Purves WK. Cucumber seedlings indoleacetaldehyde oxidase. Plant Physiol. 1978;61:107–110. doi: 10.1104/pp.61.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford KJ. Water relations and growth of flacca tomato mutant in relation to abscisic acid. Plant Physiol. 1983;72:251–255. doi: 10.1104/pp.72.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Courtright JB. Polygenic control of aldehyde oxidase in Drosophila. Genetics. 1967;57:25–39. doi: 10.1093/genetics/57.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZF, Sagi M, Lips SH. Assimilate allocation priority as affected by nitrogen compounds in the xylem sap. Plant Physiol Biochem. 1996;34:807–815. [Google Scholar]
- Guerrier G, Bourgeais-Chaillou P. Solute contents in roots and root calli of NaCl-sensitive tissues of Lycopersicon. Biol Plant (Prague) 1994;36:321–328. [Google Scholar]
- Hoagland DR, Arnon DI. The water culture method for growing plants without soil. Calif Agric Exp Stn Circ. 1938;347:1–39. [Google Scholar]
- Hoff T, Schnorr KM, Meyer C, Caboch M. Isolation of two Arabidopsis cDNAs involved in early steps of molybdenum cofactor biosynthesis by functional complementation of Escherichia coli mutants. J Biol Chem. 1995;270:6100–6107. doi: 10.1074/jbc.270.11.6100. [DOI] [PubMed] [Google Scholar]
- Ichida K, Amaya Y, Noda K, Minoshima S, Hosoya T, Sakai O, Shimizu N, Nishimo T. Cloning of the cDNA encoding human xanthine dehydrogenase (oxidase): structural analysis of the protein and chromosomal location of the gene. Gene. 1993;133:279–284. doi: 10.1016/0378-1119(93)90652-j. [DOI] [PubMed] [Google Scholar]
- Imber D, Tal M. Phenotypic reversion of flacca, a wilty mutant of tomato, by abscisic acid. Science. 1970;169:592–593. doi: 10.1126/science.169.3945.592. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Dixon NA, Lee DR. Water relations of the tomato during fruit growth. Plant Cell Environ. 1992;15:947–953. [Google Scholar]
- Koshiba T, Matsuyama H. An in vitro system of indole-3-acetic acid formation from tryptophan in maize (Zea mays) coleoptile extracts. Plant Physiol. 1993;102:1319–1324. doi: 10.1104/pp.102.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Saito E, Ono N, Yamamoto N, Sato M. Purification and properties of flavin- and molybdenum-containing aldehyde oxidase from coleoptiles of maize. Plant Physiol. 1996;110:781–789. doi: 10.1104/pp.110.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage and structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leydecker MT, Moureaux T, Kraepiel Y, Schnorr K, Caboche M. Molybdenum cofactor mutants, specifically impaired in xanthine dehydrogenase activity and abscisic acid biosynthesis, simultaneously overexpress nitrate reductase. Plant Physiol. 1995;107:1427–1431. doi: 10.1104/pp.107.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linforth RS, Taylor IB, Hedden P. Abscisic acid and C10 dicarboxylic acids in wilty tomato mutants. J Exp Bot. 1987;195:1734–1740. [Google Scholar]
- Marin A, Marion-Poll A. Tomato flacca mutant is impaired in ABA aldehyde oxidase and xanthine dehydrogenase activities. Plant Physiol Biochem. 1997;35:369–372. [Google Scholar]
- Mendel RR, Kirk DW, Wray JL. Assay of molybdenum cofactor of barley. Phytochemistry. 1985;24:1631–1634. [Google Scholar]
- Mendel RR, Muller A. Biochem Physiol Pflanz. 1976;170:538–541. [Google Scholar]
- Mertens RJ, Deus-Neumann B, Weiler EW. Monoclonal antibodies for the detection and quantitation of the endogenous plant growth regulator, abscisic acid. FEBS Lett. 1985;160:269–272. [Google Scholar]
- Montalbini P. Purification and some properties of xanthine dehydrogenase from wheat leaves. Plant Sci. 1998;134:89–102. [Google Scholar]
- Neill SJ, Horgan R. Abscisic acid production and water relations in wilty tomato mutants subjected to water deficiency. J Exp Bot. 1985;36:1222–1231. [Google Scholar]
- Oaks A. Biochemical aspects of nitrogen metabolism in a whole plant context. In: Lambars H, Neeteson H, Stulen I, Nijhoff M, editors. Fundamental, Ecological, and Agricultural Aspects of Nitrogen Metabolism in Higher Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1986. pp. 133–151. [Google Scholar]
- Omarov RT, Akaba S, Koshiba T, Lips SH. Aldehyde oxidase in roots, leaves and seeds of barley (Hordeum vulgare L.) J Exp Bot. 1999;50:63–69. [Google Scholar]
- Omarov RT, Sagi M, Lips SH. Regulation of aldehyde oxidase and nitrate reductase in roots of barley (Hordeum vulgare L.) by nitrogen source and salinity. J Exp Bot. 1998;49:897–902. [Google Scholar]
- Ori N, Eshed Y, Pinto P, Paran I, Zamir D, Fluhr R. TAO1, a representative of molybdenum cofactor containing hydroxylases from tomato. J Biol Chem. 1997;272:1019–1025. doi: 10.1074/jbc.272.2.1019. [DOI] [PubMed] [Google Scholar]
- Parry AD, Neill SJ, Horgan R. Xantoxin levels and metabolism in the wild-type and wilty mutants of tomato. Planta. 1988;173:397–404. doi: 10.1007/BF00401027. [DOI] [PubMed] [Google Scholar]
- Pérez-Vicente R, Pineda M, Cárdenas J. Isolation and characterization of xanthine dehydrogenase from Chlamydomonas reinhardtii. Physiol Plant. 1988;72:101–107. [Google Scholar]
- Radin JW, Parker LL, Sell CR. Partitioning of sugar between growth and nitrate reduction in cotton roots. Plant Physiol. 1978;62:550–553. doi: 10.1104/pp.62.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan KV, Handler P. Purification and properties of chicken liver xanthine dehydrogenase. J Biol Chem. 1967;267:4097–4107. [PubMed] [Google Scholar]
- Rajagopalan KV, Johnson JL. The pterin molybdenum cofactor. J Biol Chem. 1992;267:10199–10202. [PubMed] [Google Scholar]
- Rock CD, Heath TG, Gage DA, Zeevaart AD. Abscisic alcohol is an intermediate in abscisic acid biosynthesis in a shunt pathway from abscisic aldehyde. Plant Physiol. 1991;97:670–676. doi: 10.1104/pp.97.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe G. Aldehyde oxidase isoenzymes (EC 1.2.3.1) in potato tubers (Solanum tuberosum) Plant Cell Physiol. 1974;15:493–499. [Google Scholar]
- Sagi M, Omarov RT, Lips SH. The Mo-hydroxylases xanthine dehydrogenase and aldehyde oxidase in ryegrass as affected by nitrogen and salinity. Plant Sci. 1998;135:125–135. [Google Scholar]
- Sagi M, Savidov NA, L'vov NP, Lips SH. Nitrate reductase and molybdenum cofactor in annual ryegrass as affected by salinity and nitrogen source. Physiol Plant. 1997;99:546–553. [Google Scholar]
- Schwartz SS, Leon-Kloosterzeil KM, Koornneef M, Zeevaart AD. Biochemical characterization of the ab2 and aba3 mutants in Arabidopsis thaliana. Plant Physiol. 1997;114:161–166. doi: 10.1104/pp.114.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimoto H, Seo M, Dohmae N, Takio K, Kamiya Y, Koshiba T. Cloning and molecular characterization of plant aldehyde oxidase. J Biol Chem. 1997;272:15280–15285. doi: 10.1074/jbc.272.24.15280. [DOI] [PubMed] [Google Scholar]
- Tal M. Abnormal stomatal behavior in wilty mutants of tomato. Plant Physiol. 1966;41:1387–1391. doi: 10.1104/pp.41.8.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor IB. Genetics of ABA synthesis. In: Davis WJ, Jones HG, editors. Abscisic Acid, Physiology and Biochemistry. Oxford, UK: Bios Scientific; 1991. pp. 23–37. [Google Scholar]
- Taylor IB, Linforth RST, Al-Nieb RJ, Bowman WR, Marples BA. The wilty tomato mutants flacca and sitiens are impaired in the oxidation of ABA-aldehyde to ABA. Plant Cell Environ. 1988;11:739–745. [Google Scholar]
- Triplett EW, Blevins DG, Randall DD. Purification and properties of soybean nodule xanthine dehydrogenase. Arch Biochem Biophys. 1982;219:39–46. doi: 10.1016/0003-9861(82)90131-x. [DOI] [PubMed] [Google Scholar]
- Wahl RC, Rajagopalan KV. Evidence for the inorganic nature of the cyanolyzable sulfur of molybdenum hydroxylases. J Biol Chem. 1982;257:1354–1359. [PubMed] [Google Scholar]
- Wahl RC, Warner CK, Finnerty V, Rajagopalan KV. Drosophila melanogaster ma-1 mutants are defective in the sulfurylation of desulfo Mo-hydroxylases. J Biol Chem. 1982;257:3958–3962. [PubMed] [Google Scholar]
- Walker-Simmons M, Kudrna DA, Warner RL. Reduced accumulation of ABA during water stress in a molybdenum cofactor mutant of barley. Plant Physiol. 1989;90:728–733. doi: 10.1104/pp.90.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD, Creelman RA. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:439–473. [Google Scholar]