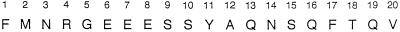Abstract
Caffeine synthase (CS), the S-adenosylmethionine-dependent N-methyltransferase involved in the last two steps of caffeine biosynthesis, was extracted from young tea (Camellia sinensis) leaves; the CS was purified 520-fold to apparent homogeneity and a final specific activity of 5.7 nkat mg−1 protein by ammonium sulfate fractionation and hydroxyapatite, anion-exchange, adenosine-agarose, and gel-filtration chromatography. The native enzyme was monomeric with an apparent molecular mass of 61 kD as estimated by gel-filtration chromatography and 41 kD as analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The enzyme displayed a sharp pH optimum of 8.5. The final preparation exhibited 3- and 1-N-methyltransferase activity with a broad substrate specificity, showing high activity toward paraxanthine, 7-methylxanthine, and theobromine and low activity with 3-methylxanthine and 1-methylxanthine. However, the enzyme had no 7-N-methyltransferase activity toward xanthosine and xanthosine 5′-monophosphate. The Km values of CS for paraxanthine, theobromine, 7-methylxanthine, and S-adenosylmethionine were 24, 186, 344, and 21 μm, respectively. The possible role and regulation of CS in purine alkaloid biosynthesis in tea leaves are discussed. The 20-amino acid N-terminal sequence for CS showed little homology with other methyltransferases.
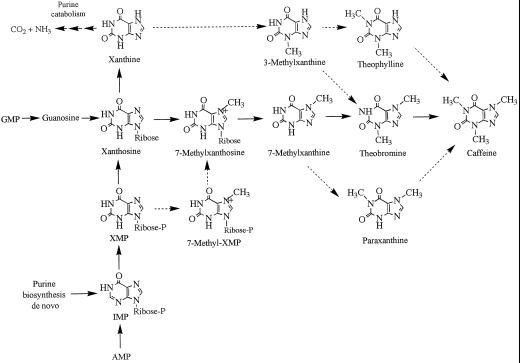
Compared with other alkaloids, such as nicotide and morphine, purine alkaloids, including theobromine (3,7-dimethylxanthine), caffeine (1,3,7-methylxanthine), and theacrine (1,3,7,9-tetramethyluric acid) are distributed widely throughout the plant kingdom (Ashihara and Crozier, 1999). Recently, extensive metabolic studies of purine alkaloids in leaves of tea (Camellia sinensis) and coffee have elucidated the caffeine biosynthesis pathway in some detail (Suzuki et al., 1992; Ashihara et al., 1996, 1997). The available data support the operation of a xanthosine → 7-methylxanthosine → 7-methylxanthine → theobromine → caffeine pathway as the major route to caffeine. In addition, a 7-methylxanthine → paraxanthine → caffeine pathway is one of a number of minor pathways operating in tea leaves (Kato et al., 1996). There is one report of an alternative entry in the caffeine biosynthesis pathway in coffee that involves conversion of XMP → 7-methyl XMP → 7-methylxanthosine (Schulthess et al., 1996). These pathways are illustrated in Figure 1.
Figure 1.
Pathways for the biosynthesis of caffeine. Solid arrows indicate major biosynthesis routes; dotted arrows indicate minor pathways. A XMP → 7-methyl XMP → 7-methylxanthosine pathway is operative in coffee leaves but not in tea leaves. Xanthine is converted to purine alkaloid via a minor route; it is also the entry point in the purine catabolism pathway (based on data reviewed by Ashihara and Crozier [1999]).
Little is known about the properties of enzymes that participate in the caffeine biosynthesis pathway; the pathway contains three SAM-dependent methylation steps, indicating that N-methyltransferases play an important role. The activities of 7-methylxanthine N-methyltransferase and theobromine N-methyltransferase, which catalyze the second and the third methylation steps in the main pathway, were first demonstrated in crude extracts from tea leaves by Suzuki and Takahashi (1975). They showed that the two enzymes have identical pH optima and are affected similarly by metal ions and inhibitors. Since then, caffeine biosynthesis N-methyltransferase activities have been detected in cell-free extracts prepared from immature fruits (Roberts and Waller, 1979) and cell-suspension cultures (Baumann et al., 1983) of coffee. The first methylation enzyme, xanthosine N-methyltransferase, which catalyzes the formation of 7-methylxanthosine from xanthosine, was demonstrated in tea-leaf extracts by Negishi et al. (1985). Fujimori et al. (1991) confirmed the presence of activities of the three N-methyltransferases in tea-leaf extracts and found that they were present at high levels in very young developing leaves but were absent in fully developed leaves.
The purification of the N-methyltransferase(s) involved in caffeine biosynthesis was attempted by several investigators. Mazzafera et al. (1994) first reported the purification of a N-methyltransferase from the endosperm and leaves of coffee and showed the presence of 7-methyl xanthine and theobromine N-methyltransferase activity. However, the activity of the cell-free preparations was extremely labile, and the specific activity of the enzyme diminished with each step in a sequential purification procedure. The specific activity of the final preparation was less than 1 fkat mg−1 protein. Gillies et al. (1995) purified N-methyltransferase from liquid endosperm of coffee using Q-Sepharose in the presence of 20% glycerol. The final specific activity was 420 fkat mg−1 protein. Kato et al. (1996) partially purified N-methyltransferase from tea leaves by ion-exchange and gel-filtration chromatography. The partially purified enzyme preparation had three activities, suggesting that the N-methyltransferases for caffeine biosynthesis make up a single enzyme. Alternatively, two or more enzymes composed of proteins with similar molecular weights and comparable charges may participate in the three methylation steps. Mosli Waldhauser et al. (1997a) partially purified (to 39-fold) N-methyltransferases from coffee leaves using ion-exchange chromatography and chromatofocusing, showing that XMP N-methyltransferase was a different protein than the other two N-methyltransferases.
In the present study an N-methyltransferase from young tea leaves, which catalyzes the SAM-dependent methylation of methylxanthines, was purified to apparent electrophoretic homogeneity. This is the first report, to our knowledge, of the isolation of the N-methyltransferase protein for caffeine biosynthesis with high specific activity. The protein exhibited broad substrate specificity and catalyzed the conversion of 7-methylxanthine to caffeine via theobromine. Therefore, the single N-methyltransferase obtained is referred to as CS.
MATERIALS AND METHODS
Plant Material
The young and most recently emerged developing leaves (<100 mg fresh weight per leaf) from flush shoots of tea (Camellia sinensis L.) plants growing at the experimental farm of the National Research Institute of Vegetables, Ornamental Plants, and Tea (Makurazaki, Kagoshima, Japan) were collected in April and May for 3 years, from 1996 to 1998. Upon harvest, the leaves were frozen in liquid N2 and stored at −80°C until enzyme extraction.
Chemicals
S-Adenosyl-l-[methyl-14C]Met (1.96 GBq mmol−1) and ACS-II scintillant were purchased from Amersham. Sephadex G-25 (PD-10) and HiLoad Superdex 200 gel were obtained from Pharmacia Japan (Tokyo), and a Shodex IEC QA-824 column was purchased from the Showa Denko Corp. (Tokyo). Hydroxyapatite was obtained from the Seikagaku Corp. (Tokyo). 5′-AMP-agarose (product no. A1271), alkaline phosphatase from bovine intestinal mucosa (product no. P0280), aprotinin, and xanthine derivatives were purchased from Sigma.
Preparation of Adenosine-Agarose Affinity Support
Adenosine-agarose was prepared from 5′-AMP-agarose by dephosphorylation with bovine alkaline phosphatase (James et al., 1995). 5′-AMP-agarose (C-8 attachment) was hydrated in water for 1 h at room temperature. The 5 mL of gel was then washed with 10 × 0.5 mL of 0.5 m NaCl, followed by 10 × 0.5 mL of water, and equilibrated with 50 mm Tris-HCl, pH 8.5. It was dephosphorylated at 25°C in 10 mL of 100 mm Tris-HCl, pH 8.5, containing 1000 diethanolamine units of the alkaline phosphatase that was previously desalted using a PD-10 column. After transfer to a column (8 mm i.d. × 120 mm), the gel was washed with 100 mL of 0.5 m NaCl, followed by 100 mL of distilled water.
Determination of CS Activity
Determination of CS activity was based on the transfer of a 14C-labeled methyl group from [methyl-14C]SAM to an unlabeled substrate, the methyl acceptor. The reaction mixture for standard assays contained 100 mm Tris-HCl buffer, pH 8.5, 0.2 mm MgCl2, 0.2 mm paraxanthine, 4 μm [methyl-14C]SAM (0.9 kBq), and 5 − 20 μL of enzyme preparation in a total volume of 100 μL. The reaction mixture without paraxanthine was used as a blank control. Other procedures for the assay were carried out as described previously by Kato et al. (1996).
For the kinetics analysis, 50 μm [methyl-14C]SAM (1.8 kBq) was used; the linearity of the reaction velocity to the time and amount of enzyme was confirmed by plotting initial velocities with at least three different enzyme concentrations. Km values were calculated from double-reciprocal plots of the data. Kinetics data were subjected to linear regression analysis and the correlation of the points to the lines was >0.98.
Extraction and Purification of CS
All manipulations were performed at 4°C. At various points in the purification, protocol samples were assayed for CS activity and the protein content was determined by the method of Bradford (1976), using BSA as a standard. Frozen leaves (100 g fresh weight) were ground in a prechilled mortar with 1200 mL of 50 mm sodium phosphate buffer, pH 7.3, containing 5 mm 2-mercaptoethanol, 5 mm Na2EDTA, 5% (v/v) glycerol, 1 mg of aprotinin, 2.5% (w/v) insoluble polyvinylpolypyrrolidone, and 0.5% (w/v) sodium ascorbate. The homogenate was filtered through three layers of gauze and centrifuged at 10,000g for 15 min. The supernatant was 50% saturated with (NH4)2SO4 and centrifuged at 10,000g for 15 min; the supernatant was then adjusted to 80% saturation with (NH4)2SO4 and recentrifuged. The pellet was collected, resuspended in 10 mm sodium phosphate buffer, pH 7.2, containing 2 mm 2-mercaptoethanol, 2 mm Na2EDTA, and 20% (v/v) glycerol (buffer A), and desalted by the PD-10 column. The desalted fraction was loaded onto a hydroxyapatite column (15 mm i.d. × 160 mm) and equilibrated with buffer A. Proteins binding to the column were eluted at a flow rate of 0.3 mL min−1, with a linear gradient of 10 to 200 mm sodium phosphate buffer, pH 7.2, containing 2 mm 2-mercaptoethanol, 2 mm Na2EDTA, and 20% (v/v) glycerol.
Successive 23-mL fractions were collected, and the aliquots were assayed for CS activity. Active fractions were pooled and concentrated by precipitation in an 80% saturated solution of (NH4)2SO4. After the sample was centrifuged the pellet was dissolved in 50 mm Tris-HCl buffer, pH 8.5, containing 2 mm 2-mercaptoethanol, 2 mm Na2EDTA, and 20% (v/v) glycerol, and desalted as described above. The sample was applied to a fast-protein liquid chromatography anion-exchange column (8 mm i.d. × 15 mm; Shodex, Showa Denko) and equilibrated with 50 mm Tris-HCl buffer, pH 8.4, containing 2 mm 2-mercaptoethanol, 2 mm Na2EDTA, 20 mm KCl, and 20% (w/v) glycerol. After application of the sample, the column was eluted with the same buffer for 20 min at a flow rate of 0.45 mL min−1 before a 100-min linear gradient of 20 − 750 mm KCl in 50 mm Tris-HCl buffer, pH 8.4, containing 2 mm 2-mercaptoethanol, 2 mm Na2EDTA, and 20% (v/v) glycerol was initiated.
Successive 7.5-mL fractions were collected, and the aliquots were assayed for CS activity. The active fractions were pooled and desalted as described above, applied to a 1-mL adenosine-agarose column (8 mm i.d. × 120 mm), and equilibrated with 50 mm Tris-HCl buffer, pH 8.4, containing 2 mm 2-mercaptoethanol, 2 mm Na2EDTA, and 20% (v/v) glycerol. The column was washed with 2 mL of the same buffer and 2 mL of 50 mm Tris-HCl buffer, pH 8.5, containing 2 mm 2-mercaptoethanol, 2 mm Na2EDTA, 0.2 m NaCl, and 20% (v/v) glycerol. The bound proteins were eluted with 3 mL of 50 mm Tris-HCl buffer, pH 8.4, containing 2 mm 2-mercaptoethanol, 2 mm Na2EDTA, 0.2 m NaCl, 2 mm SAM, and 20% (w/v) glycerol. The eluent was applied to a column (16 mm i.d. × 600 mm; HiLoad Superdex 200, Pharmacia Japan) and equilibrated with 50 mm Tris-HCl buffer, pH 8.4, containing 2 mm 2-mercaptoethanol, 2 mm Na2EDTA, 150 mm KCl, and 20% (v/v) glycerol. The column was then eluted with the same buffer at a flow rate of 0.75 mL min−1. Successive 5.8-mL fractions were collected, and the aliquots were assayed for CS activity. After each purification step, aliquots of CS activity were subjected to SDS-PAGE, as described below.
Photoaffinity Labeling with [methyl-14C]SAM
The following procedure, based on the method of Wanek and Richter (1995), was used. Aliquots containing 2.6 μg of protein obtained by adenosine-agarose chromatography, as described above, were mixed with 64 μm [methyl-14C]SAM (3.7 kBq) in 0.1 m Tris-HCl, pH 8.4, containing 0.8 mm Na2EDTA, 0.8 mm 2-mercaptoethanol, and 8% (v/v) glycerol in a final volume of 25 μL. Samples comprising 8-μL droplets were placed on a 4°C cooling plate and irradiated for 40 min with UV light (254 nm, 710 μW cm−2; model HP-6C, Atto Corp., Tokyo) at a distance of 0.8 cm with or without 200 μm S-adenosyl-l-homocysteine; inhibition of 98% resulted. The reaction was stopped by the addition of an SDS sample buffer; after the sample was boiled, the protein was subjected to SDS-PAGE.
SDS-PAGE
SDS-PAGE was performed in minigels (12% polyacrylamide) according to the method of Laemmli (1970), after which the proteins were visualized with Coomassie brilliant blue (Quick CBB, Wako Pure Chemical Industries, Osaka) and silver staining (Daiichi Pure Chemicals, Tokyo), using the modified methods of Oakley et al. (1980). In both cases, the gels were incubated with staining solutions for approximately 30 min. After the gels were dry, autoradiography was conducted using an Image-Analyzer system (FLA-2000, Fuji Chemical Measurement, Mitaka, Japan). Exposure time for the imaging plates was approximately 18 h.
Terminal Sequencing
The purified CS on a gel of SDS-PAGE was transferred to a PVDF membrane, and the N-terminal sequence was obtained for the first 20 amino acids by the Edman degradation method, using a pulse-liquid sequencer with an online phenylthiohydantoin analyzer (Perkin Elmer).
RESULTS
Purification of CS
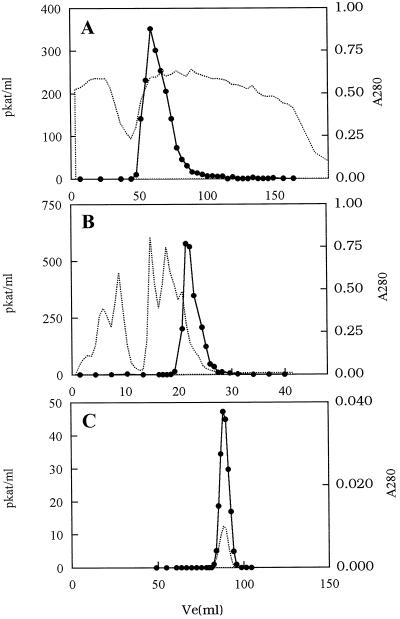
The results of a typical purification are summarized in Table I. CS activity in the frozen leaves at −80°C was stable for more than 3 months, but after extraction, it rapidly lost activity. After 24 h at 4°C, activity in the crude extract was reduced to 20% of its original value. However, when 20% glycerol was added to the cell-free preparations and the samples were stored at 4°C for 24 h, activity was reduced to 75% of its original value. This facilitated the purification of CS activity as summarized in Table I. The specific activity of CS in the initial crude extract (step 1) was 10.9 pkat mg−1; after ammonium sulfate precipitation (step 2), it increased 2-fold with about 50% recovery. Hydroxyapatite chromatography (step 3), during which CS eluted as a single peak in approximately 50 mm phosphate (Fig. 2A), increased the specific activity to 91 pkat mg−1 with an overall recovery of 38.6%. A further increase of approximately 2.5-fold in the specific activity of CS and about 50% recovery was achieved with anion-exchange chromatography (step 4; Fig. 2B). The key step, however, was the use of affinity chromatography with adenosine-agarose support. After the affinity column was washed with 0.2 m NaCl, CS activity was eluted with a 2 mm SAM buffer. This procedure yielded an overall recovery of 3.2% and 232-fold purification (step 5). Further purification was achieved with gel-filtration chromatography (step 6; HiLoad Superdex 200, Pharmacia Japan; Fig. 2C). The elution volume of the CS peak corresponded to 61 kD. After gel-filtration chromatography (step 6), recovery of the original CS activity was 3.6% , accompanied by a 523-fold increase in specific activity at 5700 pkat mg−1 protein. The recovery of CS activity from this final step was slightly higher than that estimated after affinity chromatography (step 5), presumably because the presence of SAM in the affinity-chromatography eluting buffer lowered the specific activity of the [14C]SAM in the incubation medium, resulting in underestimation of CS activity in step 5.
Table I.
Purification of CS from young developing tea leaves
| Step | Fraction | Volume | Total Activity | Total Protein | Specific Activity | Purification | Yield |
|---|---|---|---|---|---|---|---|
| mL | pkat | mg | pkat mg−1 | fold | % | ||
| 1 | Crude extract | 930 | 6330 | 581 | 10.9 | 1.0 | 100 |
| 2 | Ammonium sulfate | 33.8 | 3410 | 155 | 22.0 | 2.0 | 53.9 |
| 3 | Hydroxyapatite | 23.0 | 2630 | 28.9 | 91.0 | 8.0 | 41.5 |
| 4 | Shodex IEC QA-824 | 7.5 | 1070 | 4.82 | 221 | 20.3 | 16.9 |
| 5 | Adenosine-agarose | 2.0 | 202 | 0.08 | 2530 | 232 | 3.2 |
| 6 | Superdex 200 | 5.8 | 228 | 0.04 | 5700 | 523 | 3.6 |
Figure 2.
Purification of CS from young tea leaves. A, Hydroxyapatite chromatography of proteins precipitated with 50% to 80% saturated (NH4)2SO4. B, Shodex IEC QA-824 anion-exchange chromatography of the active fraction from hydroxyapatite chromatography. C, HiLoad Superdex 200 gel filtration of the elution from adenosine-agarose chromatography. The dotted lines indicate A280 and solid circles represent CS activity.
SDS-PAGE Analysis
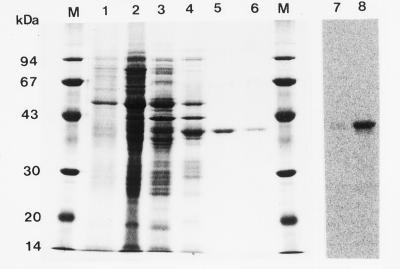
Aliquots of CS activity at various points in the purification sequence were analyzed by SDS-PAGE; the gel was then stained (Fig. 3). Initially, the samples contained a heterogeneous mixture of proteins (lanes 1–4), but the efficiency of the affinity-chromatography step with adenosine-agarose is evident in a comparison of lanes 4 and 5. The molecular mass of the single polypeptide band after affinity chromatography was estimated with SDS-PAGE to be 41 kD. The single band was observed and confirmed by both Coomassie Blue (lane 5) and silver staining (not shown).
Figure 3.
SDS-PAGE analysis of proteins at various stages of purification and photoaffinity labeling of CS from the adenosine-agarose fraction. Fractions from each purification step were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. Lane 1, Crude extract (6 μg); lane 2, 50% to 80% saturated (NH4)2SO4 precipitate (23 μg); lane 3, hydroxyapatite (12 μg); lane 4, Shodex anion-exchange chromatography (7.4 μg); lane 5, adenosine-agarose (2.0 μg); and lane 6, Hi-Load adenosine-agarose chromatography (0.7 μg). Lanes 7 and 8, SDS-PAGE after photoaffinity labeling of CS with [methyl-14C]SAM (1.96 GBq mmol−1). Radioactivity was visualized by an image-analyzer system. Lane 7, With SAH; lane 8, without SAH; lane M, molecular mass marker proteins.
Photoaffinity Labeling with [methyl-14C]SAM
To investigate whether the single SDS-PAGE band was a SAM-binding protein, photoaffinity labeling (irradiation with UV light in the presence of [methyl-14C]SAM; Fig. 3, lane 8) was carried out according to the method of James et al. (1995). This procedure confirmed the labeling of the 41-kD protein. The labeling was completely prevented by SAH, a potent inhibitor of methyltransferase (Fig. 3, lane 7).
Kinetic Properties of CS
The substrate specificity of the final enzyme preparation was tested, and its individual activities are summarized in the first line of Table II. When dimethylxanthines were used as substrates, paraxanthine was the best methyl acceptor, followed by theobromine, which was 12% as active as paraxanthine. Trace quantities of theophylline were converted to caffeine by 7-N-methylation. 7-Methylxanthine was the most effective substrate of the three monomethylxanthines; the product was theobromine. 3-Methylxanthine and 1-methylxanthine were not effective substrates, yielding low levels of theophylline. This suggests that CS catalyzed only 3-N- and 1-N-methyltransferase activity, with the former being generally more active than the latter. The purified CS did not catalyze the conversion of xanthosine to 7-methylxanthosine or of XMP to 7-methyl-XMP.
Table II.
Comparison of the substrate specificity of SAM-dependent N-methyltransferases from various plant sources
| Plant | Substrate/Methylation Positiona
|
References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7-mX/3N | 3-mX/1N | 1-mX/3N | Tb/1N | Tp/7N | Px/3N | X/3N | XR/7N | XMP/7N | ||
| % | ||||||||||
| Tea | ||||||||||
| Leaves (purified) | 100 | 17.6 | 4.2 | 26.8 | tr | 210 | tr | nd | nd | This study |
| Leaves (partially purified) | 100 | 14.0 | 20.0 | 21.4 | nd | 206 | – | 10.3 | – | Kato et al. (1996) |
| Leaves (crude) | 100 | tr | 4.5 | 25 | 2.5 | 250 | nd | nd | nd | Suzuki and Takahashi (1975) |
| Leaves (crude) | 100 | – | – | 20 | – | – | 16 | 56 | nd | Negishi et al. (1985) |
| Cocoa tea | ||||||||||
| Leaves (crude) | 100 | – | – | nd | – | nd | – | – | – | Ashihara et al. (1998) |
| Coffee | ||||||||||
| Endosperm (partially purified) | 100 | – | – | 185 | – | – | – | – | – | Mazzafera et al. (1994) |
| Fruits (crude) | 100 | – | 5.7 | 127 | 4.6 | 175 | nd | nd | – | Roberts and Waller (1979) |
| Leaves (crude)b | 100 | – | – | – | – | – | – | – | 104 | Schulthess et al. (1996) |
The relative activity is indicated as the percentage of the activity with 7-mX. CS activity with 7-mX (100%) in this work was 2.7 nkat mg−1 protein. nd, Not detected; tr, trace; –, not determined.
mX, Methylxanthine; Tb, theobromine; Tp, theophylline; Px, paraxanthine; X, xanthine; XR, xanthosine.
Km value of coffee leaf enzyme for 7-mX (0.4 mm) is lower than that for Tb (0.5 mm) (Mosli Waldhauser, 1997a).
To compare enzyme-substrate affinity, kinetics parameters were determined. Lineweaver-Burk plots gave a Km value of 21 μm for SAM in the presence of 200 μm paraxanthine. The Km values for paraxanthine, 7-methylxanthine, and theobromine in saturating 50 μm SAM were 24, 186, and 344 μm, respectively.
pH Dependence of CS Activity
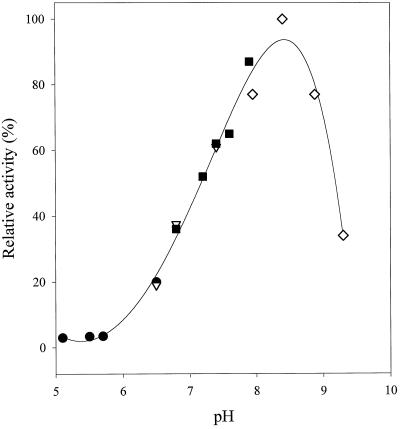
The pH-dependent activity curve for the methylation of paraxanthine showed a distinct maximum at pH 8.5 and was apparently unaffected by any of the buffers that we used (Fig. 4).
Figure 4.
Effect of pH on the activity of tea-leaf CS. CS was assayed in 50 mm Tris-HCl (⋄), 50 mm sodium phosphate (▪), 50 mm Pipes (▿), and 50 mm Mes (•) buffers.
N-Terminal Sequence
The N-terminal sequence of CS is shown in Figure 5. In a database search (SwissProt protein database; FASTA program; Pearson and Lipman, 1988), we found no homology with any other protein sequence.
Figure 5.
The 20-amino acid N-terminal sequence obtained for CS.
DISCUSSION
A single N-methyltransferase that has broad substrate specificity was purified to electrophoretic homogeneity. The key step was affinity chromatography on adenosine-agarose. This method was previously used with success to purify an S-methyltransferase of plant origin (James et al., 1995). The tea CS enzyme used 7-methylxanthine and theobromine as substrates; it could therefore convert 7-methylxanthine to caffeine. The final specific activity of CS with paraxanthine, 7-methylxanthine, and theobromine as the substrates was 5.7, 2.7, and 0.72 nkat mg−1 protein, respectively. These values were 6- to 1,000,000-fold higher than those reported for partially purified preparations from coffee (Mazzafera, 1994; Gillies et al., 1995; Mosli Waldhauser et al., 1997a) and 50-fold higher than those we had obtained in an earlier study with a partially purified preparation from young tea leaves (Kato et al., 1996). The apparent molecular mass of tea-leaf CS was 61 kD, as estimated by gel-filtration chromatography, and 41 kD, as estimated by SDS-PAGE. The value obtained from gel filtration is broadly comparable with the estimated molecular masses of partially purified enzymes from coffee endosperm (54 kD; Mazzafera et al., 1994) and coffee leaves (67 kD; Mosli Waldhauser et al., 1997a).
The methyl-acceptor specificity of the N-methyltransferases in crude, partially purified, and highly purified preparations from tea, cocoa tea, and coffee are summarized in Table II. The broad substrate specificity of the purified CS obtained in the present investigation is very similar to that of crude tea-leaf extracts, as reported by Suzuki and Takahashi (1975). CS therefore seems to be a major tea N-methyltransferase. Although paraxanthine is the best methyl acceptor for both tea and coffee N-methyltransferases, the substrate specificity of tea CS is different from that of coffee fruits and cocoa-tea leaves. Theobromine is a better methyl acceptor than 7-methylxanthine in preparations from coffee endosperm (Mazzafera et al., 1994) and coffee fruits (Roberts and Waller, 1979) but not in preparations from the tea-leaf enzyme (Table II). Mosli Waldhauser et al. (1997b) reported recently that the Km for 7-methylxanthine (approximately 0.4 mm) was lower than that for theobromine (approximately 0.5 mm) in purified preparations from coffee leaves. Therefore, the substrate specificities observed in enzyme preparations from leaves of tea and coffee appear to be similar.
The properties of the N-methyltransferase of cocoa tea, a theobromine-accumulating plant, are different from those of coffee and tea; the cocoa tea enzyme can use 7-methylxanthine as a methyl acceptor, but it cannot use theobromine or paraxanthine (Ashihara et al., 1998). The data summarized in Table II indicate distinct variations in the properties of the N-methyltransferases; these variations may cause the differing spectra of purine alkaloids that accumulate in the three species.
Two routes for the synthesis of 7-methylxanthine have been proposed. Negishi et al. (1985) demonstrated the presence of N-methyltransferase activity in tea-leaf extracts, in which xanthosine, but not XMP, was an active methyl acceptor. In contrast, Schulthess et al. (1996) reported that the N-methyltransferase from coffee leaves catalyzed the methylation of XMP as well as xanthosine and proposed that XMP, not xanthosine, is the in situ acceptor for the first methyl group acceptor in caffeine biosynthesis. The present study shows that purified tea CS does not methylate either xanthosine or XMP and, therefore, does not catalyze the first methylation step in the caffeine biosynthesis pathway.
The tea xanthosine N-methyltransferase appears to be very labile or, alternatively, the amounts may vary significantly in young tea leaves. The enzyme sometimes disappears even in crude extracts during purification, although we did find some enzyme in semipurified tea-leaf extracts that were prepared previously (Kato et al., 1996; Table II). Although improbable, the possibility remains that in in situ tea CS has an active site for 7-N-methylation that is lost in in vitro preparations. It is more likely, however, that xanthosine N-methyltransferase protein differs in CS and that two distinct enzymes participate in the three methylation steps of caffeine biosynthesis.
The Km value for paraxanthine is the lowest, and the Vmax for this substrate is the highest, of the substrates tested; hence, paraxanthine is the best substrate for CS. However, as discussed in our previous paper (Kato et al., 1996), there is limited synthesis of paraxanthine from 7-methylxanthine; it is not an important methyl acceptor in vivo. The Km value for theobromine is high (>0.3 mm), which may explain the transient accumulation of theobromine in young tea leaves (Ashihara and Kubota, 1986).
The effects of the concentration of SAM and several methyl acceptors on the activity of CS show typical Michaelis-Menten-type kinetics, and feedback inhibition by caffeine could not be detected (data not shown). Therefore, it is unlikely that allosteric control of CS activity operates in tea leaves. The Km of tea CS for SAM (21 μm) in the presence of paraxanthine was similar to the values for 7-methylxanthine and theobromine (25 μm) obtained with crude tea enzyme preparations (Suzuki and Takahashi, 1975).
One of the major factors affecting the activity of CS in vitro seems to be inhibition by SAH. As shown in the photoaffinity labeling with SAM, CS was completely inhibited by SAH. SAH binds to most methyltransferases with higher affinity than SAM (Poulton, 1981). Therefore, control of the intracellular SAM-to-SAH ratio is one possible mechanism for regulating the activity of many methyltransferases, including CS. Nothing is known about such ratios in tea or coffee, but in the leaves of 6-d-old pea seedlings, the SAM and SAH content is 14.6 and 0.7 nmol g−1 fresh weight, respectively (Edwards, 1995).
Maximum CS activity was obtained at pH 8.5, the same value that was obtained in an earlier study with N-methyltransferase activity in crude tea-leaf extracts (Suzuki and Takahashi, 1975). Similar alkaline pH optima have been reported for chloroplast stroma enzymes (Foyer, 1984). CS is probably a stroma enzyme; a previous study of tea has showed that paraxanthine N-methyltransferase activity is located in chloroplasts (Kato et al., 1998). It is noteworthy that there is a marked decline in CS activity between pH 8.0 and 7.0. Stromal pH increases from approximately 7.0 to approximately 8.0 upon illumination (Edwards and Walker, 1983); thus, it is feasible that CS activity is stimulated by light. Several stromal enzymes, including ribulose-1,5-bisphosphate carboxylase, Fru-1,6-bisphosphatase, and sedoheptulose-1,7-bisphosphatase, have alkaline pH optima, and the regulation of their activities by light is one of the important mechanisms in the control of the Calvin-Benson cycle (Foyer, 1984).
The 20-amino acid N-terminal sequence obtained for CS does not show similarities with the N-methyltransferase sequence from coffee reported by Mazzafera et al. (1994). Many N-terminal sequences have been reported for plant O-methyltransferases, but there were only a few relating to plant N-methyltransferases (Joshi and Chiang, 1998). Two genes encoding plant N-methyltransferases, putrescine N-methyltransferase (Hibi et al., 1994; Walton et al., 1994; Hashimoto et al., 1998) and Rubisco large subunit N-methyltransferase (Klein and Houtz, 1995; Ying et al., 1996), have been cloned, but the amino acid sequences of these enzymes show little homology with the N-terminal sequence of tea CS.
As has been shown with many secondary metabolism pathways (Poulton, 1981), the biosynthesis of caffeine is closely related to the stage of development, and CS activity can be detected only in young, expanding tea leaves. Detailed studies of the control of caffeine biosynthesis will be possible when CS antibodies and the cDNA encoding CS protein become available.
ACKNOWLEDGMENT
We thank Dr. Y. Takeda (National Research Institute of Vegetables, Ornamental Plants, and Tea, Makurazaki, Kagoshima, Japan) for the generous supply of plant material.
Abbreviations:
- CS
caffeine synthase
- SAH
S-adenosyl-l-homocysteine
- SAM
S-adenosyl-l-methionine
- XMP
xanthosine 5′-monophosphate
Footnotes
This work was supported in part by a Grant-in-Aid for Scientific Research (no. 10640627) from the Ministry of Education, Science, Sports and Culture of Japan (to H.A.) and by a grant from the Mishima Kaiun Memorial Foundation (to M.K.). A.C. received funding for travel between the United Kingdom and Japan from The Royal Society.
LITERATURE CITED
- Ashihara H, Crozier A. Biosynthesis and metabolism of caffeine and related purine alkaloids in plants. In: Callow JA, editor. Advances in Botanical Research, Vol 30. London: Academic Press; 1999. pp. 117–205. [Google Scholar]
- Ashihara H, Gillies FM, Crozier A. Metabolism of caffeine and related purine alkaloids in leaves of tea (Camellia sinensis L.) Plant Cell Physiol. 1997;38:413–419. [Google Scholar]
- Ashihara H, Kato M, Ye C. J Plant Res. 1998;111:599–604. [Google Scholar]
- Ashihara H, Kubota H. Patterns of adenine metabolism and caffeine biosynthesis in different parts of tea seedlings. Physiol Plant. 1986;68:275–281. [Google Scholar]
- Ashihara H, Monteiro AM, Gillies FM, Crozier A. Biosynthesis of caffeine in leaves of coffee. Plant Physiol. 1996;111:747–753. doi: 10.1104/pp.111.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann TW, Koetz R, Morath P. N-methyltransferase activities in suspension cultures of Coffea arabica L. Plant Cell Rep. 1983;2:33–35. doi: 10.1007/BF00269231. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;7:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Edwards G, Walker DA. C3, C4: Mechanisms and Cellular and Environmental Regulation of Photosynthesis. Oxford, UK: Blackwell; 1983. [DOI] [PubMed] [Google Scholar]
- Edwards R. Determination of S-adenosyl-l-methionine and S-adenosyl-l-homocystein in plants. Phytochem Anal. 1995;6:25–30. [Google Scholar]
- Foyer CH (1984) Photosynthesis. John Wiley, New York
- Fujimori N, Suzuki T, Ashihara H. Seasonal variations in biosynthetic capacity for the synthesis of caffeine in tea leaves. Phytochemistry. 1991;30:2245–2248. [Google Scholar]
- Gillies FM, Jenkins GI, Ashihara H, Crozier A (1995) In vitro biosynthesis of caffeine: stability of N-methyltransferase activity in cell-free preparations from liquid endosperm of Coffea arabica.In Proceedings of the 16th International Symposium on Coffee Science, Kyoto. Association Scientifique Internationale du Café, Paris, pp 599–605
- Hashimoto T, Shoji T, Mihara T, Oguri H, Tamaki K, Suzuki K, Yamada Y. Intraspecific variability of the tandem repeats in Nicotiana putrescine N-methyltransferases. Plant Mol Biol. 1998;37:25–37. doi: 10.1023/a:1005961122814. [DOI] [PubMed] [Google Scholar]
- Hibi N, Hashiguchi S, Hashimoto T, Yamada Y. Gene expression in tobacco low-nicotine mutants. Plant Cell. 1994;6:723–735. doi: 10.1105/tpc.6.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James F, Nolte KD, Hanson AD. Purification and properties of S-adenosyl-l-methionine:l-methionine S-methyltransferase from Wollastonia biflora leaves. J Biol Chem. 1995;270:22344–22350. doi: 10.1074/jbc.270.38.22344. [DOI] [PubMed] [Google Scholar]
- Joshi CP, Chiang VL. Conserved sequence motifs in plant S-adenosyl-l-methionine-dependent methyltransferases. Plant Mol Biol. 1998;37:663–674. doi: 10.1023/a:1006035210889. [DOI] [PubMed] [Google Scholar]
- Kato A, Crozier A, Ashihara H. Subcellular localization of the N-methyltransferase involved in caffeine biosynthesis in tea. Phytochemistry. 1998;48:777–779. [Google Scholar]
- Kato M, Kanehara T, Shimizu H, Suzuki T, Gillies FM, Crozier A, Ashihara H. Caffeine biosynthesis in young leaves of Camellia sinensis: in vitro studies on N-methyltransferase activity involved in the conversion of xanthosine to caffeine. Physiol Plant. 1996;98:629–636. [Google Scholar]
- Klein RR, Houtz RL. Cloning and development expression of pea ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit N-methyltransferase. Plant Mol Biol. 1995;27:249–261. doi: 10.1007/BF00020181. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacterophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Looser E, Baumann TW, Wanner H. The biosynthesis of caffeine in the coffee plant. Phytochemistry. 1974;13:2515–2518. [Google Scholar]
- Mazzafera P, Wingsle G, Olsson O, Sandberg G. S-adenosyl-l-methionine:theobromine 1-N-methyltransferase, an enzyme catalyzing the synthesis of caffeine in coffee. Phytochemistry. 1994;37:1577–1584. [Google Scholar]
- Mosli Waldhauser S, Gillies FM, Crozier A, Baumann TW. Separation of the N-7 methyltransferase, the key enzyme in caffeine biosynthesis. Phytochemistry. 1997a;45:1407–1414. doi: 10.1016/s0031-9422(97)00187-8. [DOI] [PubMed] [Google Scholar]
- Mosli Waldhauser S, Kretschmar JA, Baumann TW. Phytochemistry. 1997b;44:853–859. [Google Scholar]
- Negishi O, Ozawa T, Imagawa H. Methylation of xanthosine by tea leaf extracts and caffeine biosynthesis. Agric Biol Chem. 1985;49:887–890. [Google Scholar]
- Oakley BR, Kirsch DR, Morris NR. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980;105:361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton JE (1981) Transamination and demethylation reactions in the metabolism of secondary plant products. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants, Vol 7. Academic Press, New York, pp 667–723
- Roberts MF, Waller GR. N-methyltransferases and 7-methyl-N9-nucleoside hydrolase activity in Coffea arabica and the biosynthesis of caffeine. Phytochemistry. 1979;18:451–455. [Google Scholar]
- Schulthess BH, Morath P, Baumann TW. Caffeine biosynthesis starts with the metabolically channelled formation of 7-methyl-XMP: a new hypothesis. Phytochemistry. 1996;41:169–175. [Google Scholar]
- Suzuki T, Ashihara H, Waller GR. Purine and purine alkaloid metabolism in Camellia and Coffea plants. Phytochemistry. 1992;31:2575–2584. [Google Scholar]
- Suzuki T, Takahashi E. Biosynthesis of caffeine by tea-leaf extracts enzymic formation of theobromine from 7-methylxanthine and of caffeine from theobromine. Biochem J. 1975;146:87–96. doi: 10.1042/bj1460087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NJ, Peerless ACJ, Robins RJ, Rhodes MJC, Boswell HD, Robins DJ. Purification and properties of putrescine N-methyltransferase from transformed roots of Datura stramonium L. Planta. 1994;193:9–15. [Google Scholar]
- Wanek W, Richter A. Purification and characterization of myo-inositol 6-O-methyltransferase from Vigna umbellata Ohwi et Ohashi. Planta. 1995;197:427–434. [Google Scholar]
- Ying Z, Janney N, Houtz RL. Organization and characerization of the ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit ɛN-methyltransferase gene in tobacco. Plant Mol Biol. 1996;32:663–671. doi: 10.1007/BF00020207. [DOI] [PubMed] [Google Scholar]