Abstract
The cyanobacterium Synechococcus PCC7942 was transformed with various carotenogenic genes, and the resulting transformants either accumulated higher amounts of β-carotene and zeaxanthin or showed a shift in the carotenoid pattern toward the formation of zeaxanthin. These transformants were exposed to ultraviolet-B (UV-B) radiation, and the degradation of phycobilins, the inactivation of photosynthetic oxygen evolution, and the activity of photosystem II were determined. In the genetically modified cells, the influence on destruction of phycobilins was negligible. However, protection of photosynthetic reactions against UV-B damage was observed and was dependent on the carotenoid concentrations in the different transformants. Furthermore, it was shown that endogenous zeaxanthin is more effective than β-carotene. Our results suggest that carotenoids exert their protective function as antioxidants to inactivate UV-B-induced radicals in the photosynthetic membrane.
Because of the gradual depletion of ozone in the atmosphere, the UV spectral region of sunlight is increasing at the earth's surface (Caldwell et al., 1989). Radiation in the UV-B range of approximately 300 nm interferes with various metabolic reactions, primarily by generating free radicals and active oxygen species (Foyer et al., 1994). These deleterious compounds are inactivated by antioxidants. Several natural products have the potential to exhibit antioxidative properties. Among them are the carotenoids, which protect against photodynamic action in different ways: They are effective quenchers of triplet-state photosensitizers and protect against singlet oxygen and peroxy radicals (Krinsky, 1989). The photoprotective function of carotenoids is essential for photosynthetic organisms. Nonphotosynthetic organisms suffer from photooxidative stress caused by light and near-UV radiation, which requires the presence of antioxidative protection systems (Moradas-Fereira et al., 1996).
Protection against UV-B radiation by carotenoids was demonstrated in several fungi and bacteria. Some strains of Ustilago violacea accumulate Cyt c and may or may not contain carotenoids. Treatment of those strains with visible and UV radiation showed that carotenogenic strains are more resistant to death caused by light, demonstrating the effectiveness of carotenoids as protectants (Will et al., 1984). Similar studies carried out with the fungus Neurospora crassa gave comparable results (Blanc et al., 1976). Studies with transformed Escherichia coli revealed that effective protection against UV-B radiation depends either on the chemical structure of the synthesized carotenoids or on their accumulated amounts (Sandmann et al., 1998).
In photosynthetic organisms, UV-B radiation exerts direct effects on photosynthetic light reactions and carbon reduction (Teramura and Ziska, 1996). Several protection and repair mechanisms have been elucidated, but little is known about how carotenoids alleviate UV-B stress exerted on the cell and on the photosynthesis apparatus. Therefore, we modified the carotenoid content in Synechococcus PCC7942 by the introduction of different carotenogenic genes and examined the effects of UV-B radiation on these transformants. Transformants were obtained in which either the amounts of the endogenous carotenoids β-carotene and zeaxanthin were increased or the pigment pattern was shifted toward zeaxanthin. From studies of UV-B irradiation in Synechococcus PCC7942, it is well established that phycobilins decrease (Pandey et al., 1997), with a concurrent loss of energy transfer from phycobilisomes to the photosystems (Nedunchezhian et al., 1996). Impairment of photosynthetic electron transport also occurs, which in particular affects PSII activity (Campbell et al., 1998). Therefore, phycobilin contents and electron-transport rates were used as parameters to evaluate the protective effect of carotenoids in the transformants.
MATERIALS AND METHODS
Cultivation of the Cyanobacterium and Genetic Manipulations
The modified Synechococcus PCC7942 strain R2-PIM8 (van der Plas et al., 1990) with an integration platform in the metF gene for pBBR322-derived plasmids was used for insertion of the genes crtB and crtZ from Erwinia uredovora (Misawa et al., 1990) and pys from Synechocystis PCC6803 (Martínez-Férez et al., 1994). crtB and pys encode phytoene synthase and crtZ encodes β-carotene hydroxylase. All transformants were cultivated for 2 d in BG11 medium (Rippka et al., 1979) supplemented with 30 μg/mL methionin at 30°C. The cultures were illuminated with a light intensity of 40 μmol m−2 s−1 and gassed with air enriched to 1% to 2% (v/v) with CO2. R2-PIM8 was grown with 10 μg mL−1 streptomycin, and all resulting transformants were grown in the presence of 10 μg mL−1 kanamycin and 1 μg mL−1 ampicillin.
Standard methods were used for genetic manipulations according to Sambrook et al. (1989). DNA fragments were analyzed on agarose gels and restriction fragments were purified using Elu-Quick (Schleicher & Schuell). Genomic DNA was isolated according to the protocol described by Ausubel et al. (1995), and Southern hybridization was carried out with a nonradioactive digoxygenin system (Boehringer Mannheim) according to the supplier's instruction manual. Transformation of Synechococcus PCC7942-PIM8 was carried out according to the method of van den Hondel et al. (1980), with modifications as previously described (Windhövel et al., 1994).
UV-B Treatment of Cells and Determination of Phycocyanin and Carotenoids
Samples (45 mL) of 2-d-old cultures (chlorophyll adjusted to 3 μg mL−1 cell suspension) were pipetted into a Petri dish (12 cm in diameter), and the cells were exposed to UV-B radiation as indicated. The radiation chamber was equipped with four fluorescent UV-B lamps (TL40W/12, Philips, Eindhoven, The Netherlands), which exhibited their emission maximum at 312 nm and had a cutoff at 270 nm. The samples were exposed to a fluence rate of 6.8 W m−2 as determined with a UV-B sensor (model IL 1700, International Light, Newburyport, MA).
For determination of phycocyanin, including allophycocyanin, centrifuged cells from 2-mL samples were resuspended in 0.2 m Tris-HCl buffer, pH 7.5, and incubated with 1.5 mg of lysozyme overnight at 30°C under constant shaking. The spectra were recorded from the supernatant after centrifugation, and the amount of phycocyanin was calculated as phycocyanin plus allophycocyanin (in micrograms per milliliter) by their absorbance using the equation: phycocyanin + allophycocyanin content = 0.273 E620 + 0.024 E650. The carotenoid content of the Synechococcus PCC7942 transformants was determined after the 2-d growth period. Cells were heated in methanol containing 6% KOH for 15 min at 55°C. The carotenoids were partitioned into hexane:diethyl ether (10:1, v/v), and the upper phase was collected. Carotenoids were separated by HPLC on a 25-cm Nucleosil C18, 3-μm column with acetonitrile:methanol:2-propanol (85:10:5, v/v) at a flow rate of 1 mL/min (Ernst and Sandmann, 1988). Spectra were recorded on-line from the elution peaks using a photodiode array detector (model 400, Kontron Instruments, Eching, Germany).
Measurement of Photosynthesis Activities
Photosynthesis activities were determined with cells prior to or following a 6-h treatment with UV-B. Oxygen evolution was measured with a Clarke-type electrode under saturating white light of 400 μmol m−2 s−1 (Böger et al., 1981). For determination of PSII activity, benzoquinone and potassium ferricyanide (1 mm each) were added as electron acceptors. All values were related to the chlorophyll content of the cells. Chlorophyll was determined after extraction with methanol at 65°C from its absorbance and calculated as: chlorophyll a (μg mL−1) = 16.5 E665 − 8.3 E650.
Differences in photosynthesis activities and other parameters between the transformants and before and after radiation were analyzed by Student's two-tailed t test. Results have a 5% level of significance.
RESULTS
Construction and Genetic Analysis of Transformant Strains
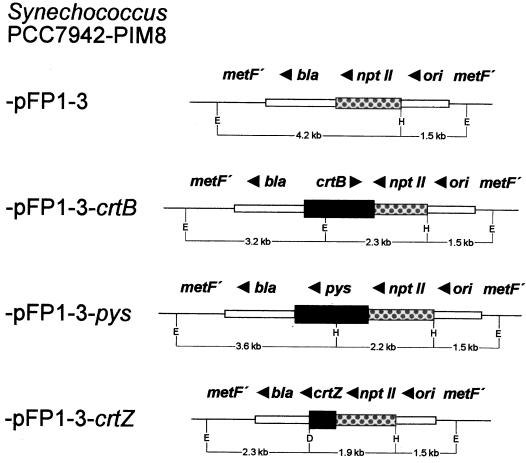
The plasmid pFP1-3 was constructed by insertion of the 1.5-kb HindIII/SalI fragment of the ntpII gene (Putnoky et al., 1983) into pUC19 (Yanisch-Perron et al., 1985). This plasmid was used to construct three integration vectors: pFP1-3-crtB with the phytoene synthase gene from Erwinia uredovora (Misawa et al., 1990), pFP1-3-crtZ with the β-carotene hydroxylase gene from the same bacterium, and pFP1-3-pys with the phytoene synthase gene from Synechocystis PCC6803 (Martínez-Férez et al., 1994). pFP1-3-crtB resulted from a 1.5-kb PvuII/HpaI fragment from pCar25 (Misawa et al., 1990), filled in with Klenow enzyme and ligated into the SmaI site of pFP1-3. The plasmid pFP1-3-crtZ was constructed using a 3.0-kb SnaBI/EcoRI fragment from pCar25 after treatment with Klenow enzyme and ligation into the SmaI site of pFP1-3. The resulting construct was then digested with DraII to delete two other genes from E. uredovora and religated. The plasmid pFP1-3-pys was obtained by starting with a 3.3-kb BamHI/KpnI fragment from plasmid pMF1041 (Martínez-Férez et al., 1994) and blunt-ending it with Klenow enzyme to ligate it into the SmaI site of pFP1-3. The resulting plasmid was then digested with ClaI to delete a 1.3-kb region downstream of the pys gene and religated.
Figure 1 shows the genomic region of the integration platform of the four transformants. The control strain PIM8-pFP1-3 carries only the kanamycin resistance gene together with a functional bla gene for ampicillin resistance from pUC19. In PIM8-pFP1-3-pys the phytoene synthase gene pys is cotranscribed with nptII, which is under a strong promoter and lacks a transcription stop signal. The same is true with PIM8-pFP1-3-crtZ, in which the β-carotene hydroxylase gene crtZ is cotranscribed with ntpII. In PIM8-pFP1-3-crtB the phytoene synthase gene crtB from E. uredovora is also located downstream from the nptII gene. However, orientation is in the opposite direction, and it was not possible to obtain transformants in which crtB had the same orientation as nptII. Nevertheless, by northern analysis we could demonstrate that substantial amounts of the crtB transcript were formed and that in vitro phytoene synthase activity in PIM8-pFP1-3-crtB was almost twice as high as in PIM8-pFP1-3 (data not shown).
Figure 1.
Genetic map of the integration platform of Synechococcus R2-PIM8 transformants with inserted crt genes (van der Plas et al., 1990). Integrated plasmids were: pFP1-3 without a crt gene; pFP1-3-crtB, which includes the coding region of the phytoene synthase gene from E. uredovora; pFP1-3-pys with the coding region of the phytoene synthase gene from Synechocystis PCC6803; and pFP1-3-crtZ with the coding region of the β-carotene hydroxylase gene from E. uredovora. MetF′, Gene of the methionin biosynthesis pathway; bla, ampicillin resistance gene; nptII, kanamycin resistance gene; ori, origin of replication; D, DraII; E, EcoRV; H, HindIII.
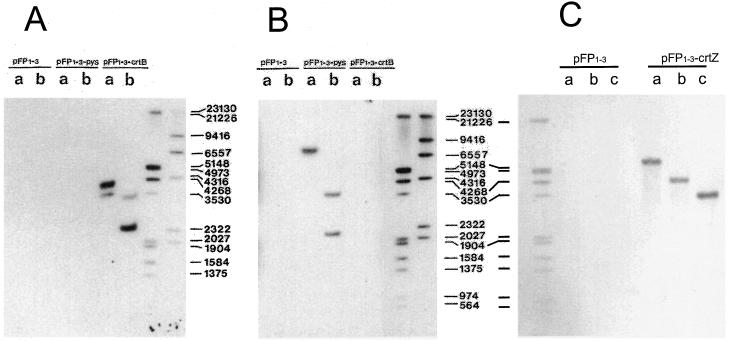
Integration of carotenogenic genes into the Synechococcus PCC7942 genome was confirmed by Southern hybridization (Fig. 2). The probes against crtB, crtZ, and pys reacted specifically with the corresponding genes in blots Figure 2, A to C, respectively. The restriction pattern obtained for the EcoRV, EcoRV/HindIII, and EcoRV/DraII digests showed the expected bands with the sizes calculated from Figure 1. The major part of the genes were used to synthesize the individual probes. Accordingly, two bands were detected in the EcoRV digest of the crtB-containing transformant because an EcoRV site is present in crtB. In the EcoRV/HindIII double-digest, the larger 3.8-kb fragment is cut to a size of 2.3 kb as calculated from Figure 1. The EcoRV/HindIII digests of the pys-containing transformant resulted in splitting of the 7.3-kb EcoRV fragment into two detectable bands. In the crtZ-containing transformant digested with EcoRV, EcoRV/HindIII, or EcoRV/DraII, the probe detected a single band of decreasing size.
Figure 2.
Southern genome analysis of Synechococcus PIM8 transformed with pFP1-3, pFP1-3-crtB, pFP1-3-pys, and pFP1-3-crtZ. Hybridization was with a digoxygenin-labeled crtB probe (A), a pys probe (B), or a crtZ probe (C). DNA was digested with EcoRV (a), EcoRV and HindIII (b), or EcoRV and DraII (c). Sizes are indicated in bp.
Carotenoid Content of Synechococcus PCC7942 Transformants
HPLC separation of extracted pigments after saponification showed that Synechococcus PCC7942 contains β-carotene and its 3,3′-dihydroxy derivative zeaxanthin as major carotenoids. Depending on growth conditions, trace amounts of β-cryptoxanthin (3-hydroxy-β-carotene) could be detected as an intermediate. Analysis of the carotenoids from the control strain PIM8-pFP1-3 and from PIM8-pFP1-3-crtZ with the inserted crtZ gene demonstrated a much higher zeaxanthin peak in the latter at the expense of β-carotene. Growth at a light intensity of 50 μmol m−2 s−1 of PIM8-pFP1-3 resulted in a total carotenoid content of 3.17 mg g−1 dry weight (Table I) and a β-carotene to zeaxanthin ratio of about 2:1. Integration of one of the phytoene synthase genes, crtB or pys, resulted in an increase of the carotenoid content of the transformants. In crtB, a value of 3.83 mg g−1 dry weight represents a 20% increase over the control, whereas PIM8-pFP1-3-pys with a 5.13 mg g−1 dry weight contained about 60% more carotenoids than the control transformant PIM8-pFP1-3. Nevertheless, the β-carotene to zeaxanthin ratio did not change much in the transformants with an additional phytoene synthase gene. In transformant PIM8-pFP1-3-crtZ with the β-carotene hydroxylase gene crtZ from E. uredovora, total carotenoids were in the same range as in the control. However, a stronger conversion of β-carotene to zeaxanthin was observed. The share of zeaxanthin increased from 32% of total carotenoids in the control to 55% in PIM8-pFP1-3-crtZ.
Table I.
Carotenoid composition of Synechococcus PCC7942 transformants
| Transformants of Strain PIM8 | Colored Carotenoidsa | Distribution
|
|||
|---|---|---|---|---|---|
| β-Carotene | β-Cryptoxanthin | Zeaxanthin | Hydroxy-zeaxanthin | ||
| mg g−1 dry wt | % | ||||
| -pFP1-3 | 3.17 ± 0.28 | 56.6 ± 5.2 | 2.9 ± 0.3 | 32.0 ± 3.2 | 8.5 ± 0.8 |
| -pFP1-3-crtB | 3.83 ± 0.30 | 61.1 ± 5.1 | 2.6 ± 0.3 | 28.7 ± 3.0 | 7.5 ± 0.9 |
| -pFP1-3-pys | 5.13 ± 0.38 | 58.2 ± 4.4 | 3.1 ± 0.4 | 32.1 ± 3.4 | 6.6 ± 0.8 |
| -pFP1-3-crtZ | 3.40 ± 0.24 | 30.9 ± 3.2 | 3.9 ± 0.4 | 55.1 ± 4.7 | 10.1 ± 0.9 |
Carotenoid concentrations are the means ± sd of at least five determinations.
UV-B Treatment and Response
Treatment of the control transformant PIM8-pFP1-3 with UV-B radiation of an intensity of 6.8 W m−2 during a 6-h period decreased the phycocyanin content and photosynthesis activity (Table II). The latter was measured under saturating light conditions to compare the entire photosynthesis capacity of the transformants before and after UV-B treatment. In all transformants the remaining phycocyanin concentration was decreased by about one-half after exposure. Photosynthesis activity of intact cells, measured as oxygen evolution involving PSII and PSI, was very similar in the control and PIM8-pFP1-3-crtB. Untreated transformants PIM8-pFP1-3-pys and PIM8-pFP1-3-crtZ exhibited lower and increased photosynthesis activities, respectively. The higher rate of oxygen evolution for PIM8-pFP1-3-crtZ was significant at a 5% level. The PSII activities of all transformants did not differ significantly. Protection of overall photosynthesis activity and PSII activity after UV-B treatment was best with PIM8-pFP1-3-crtZ. Here, photosynthesis was about 2-fold higher and PSII activity was almost 3-fold higher than in the control (Table II). Residual activities were also higher in the irradiated PIM8-pFP1-3-pys transformant. For UV-B-treated PIM8-pFP1-3-crtB a significant difference was found only in PSII activity, which was 2-fold higher than in the control.
Table II.
Effect of UV-B radiation on phycocyanin content, photosynthetic oxygen production by cells, and PSII activity
| Pigment and Activity | 0 h UV-B | 6 h UV-B |
|---|---|---|
| Phycocyanin | μg g−1 dry wt | |
| PIM8-pFP1-3 (control strain) | 41.9 ± 2.7 | 22.0 ± 2.1 |
| PIM8-pFP1-3-crtB | 43.1 ± 2.8 | 24.8 ± 1.6 |
| PIM8-pFP1-3-pys | 45.4 ± 4.2 | 27.3 ± 2.7 |
| PIM8-pFP1-3-crtZ | 41.5 ± 3.7 | 21.7 ± 1.8 |
| Oxygen production | μmol O2 mg−1 Chl h−1 | |
| PIM8-pFP1-3 | 132 ± 11 | 42 ± 3 |
| PIM8-pFP1-3-crtB | 140 ± 16 | 52 ± 4 |
| PIM8-pFP1-3-pys | 114 ± 12 | 66 ± 7 |
| PIM8-pFP1-3-crtZ | 152 ± 15 | 91 ± 9 |
| PSII activity | μmol O2 mg−1 Chl h−1 | |
| PIM8-pFP1-3 | 585 ± 64 | 56 ± 6 |
| PIM8-pFP1-3-crtB | 602 ± 76 | 109 ± 8 |
| PIM8-pFP1-3-pys | 577 ± 66 | 118 ± 11 |
| PIM8-pFP1-3-crtZ | 569 ± 79 | 160 ± 9 |
All values are means ± sd of at least five determinations. The intensity of the UV-B radiation was 6.8 W m−2. Oxygen consumption rates in the dark were between 10% and 20% of the photosynthesis rates and did not differ among the transformants.
DISCUSSION
It was demonstrated previously that in higher plants the phytoene-synthesis step is rate limiting for the entire carotenoid-biosynthesis pathway (Fray et al., 1995; Kumagai et al., 1995). Our results showing elevated carotenoid contents in Synechococcus PCC7942 transformants expressing the foreign phytoene synthase genes crtB or pys (Table I) indicate that the same bottleneck exists in cyanobacterial carotenoid biosynthesis. With the bacterial gene crtZ it was possible to shift the carotenogenic pathway toward the synthesis of zeaxanthin. Thus, Synechococcus PCC7942 transformants with a higher and altered carotenoid content allowed us to investigate which carotenoids are promising candidates in the protection of photosynthesis against damage by UV-B radiation (Middleton and Teramuara, 1993).
The damage to photosynthesis by UV-B radiation is caused by the generation of free radicals rather than singlet oxygen (Hideg and Vass, 1996). These radicals accumulate in the thylakoids and are responsible for peroxidation reactions that destroy various components of the photosynthesis apparatus (Malanga et al., 1997), among which are the D1 and D2 proteins of PSII, which are highly susceptible to peroxidative conditions (Greenberg et al., 1989; Jansen et al., 1996). Protection against UV-B can occur at different levels (for review, see Teramura and Ziska, 1996). Plants can accumulate UV-B-screening compounds such as flavonoids (Middleton and Teramura, 1993), the antioxidant system can inactivate oxygen radicals, or the effective replacement of damaged constituents can resist UV-B stress, as was shown for the D1 protein (Campbell et al., 1998). In this context, carotenoids may have both a screening and an antioxidant function. The latter has been established for many carotenoid structures (Woodall et al., 1997). In cyanobacteria, soluble carotenoid proteins (Diverse-Pierlussi and Krogmann, 1988) or carotenoids in the cell wall (Jürgens and Weckesser, 1985) could act as a filter for UV-B radiation.
Increasing amounts of zeaxanthin and β-carotene in the transformants (Table I) resulted in protection of photosynthesis against UV-B damage (Table II). This effect was most pronounced on PSII activity. A positive relationship between carotenoid content and relief from UV-B inactivation was evident. The protection in the transformant in which carotenoid biosynthesis is shifted toward formation of zeaxanthin indicates that in Synechococcus PCC7942 zeaxanthin provides the highest protective potential. It was shown recently that zeaxanthin is the most effective protectant against UV-B radiation in E. coli transformants (Sandmann et al., 1998) and that it prevents radical peroxidation processes in liposomes much better than β-carotene (Woodall et al., 1997). Furthermore, only destruction of membrane-located processes such as photosynthetic electron transport by UV-B could be prevented, but protection of (soluble) phycocyanins against UV-B was not observed, since they are located outside of the photosynthetic membrane and are not in contact with carotenoids (Table II).
The genetic modification of Synechococcus PCC7942 made it possible to elucidate the primary role of carotenoids in protecting photosynthesis as quenchers of radicals generated in the thylakoid membrane upon UV-B radiation. Light-saturation curves are available for some of the Synechococcus PCC7942 transformants (Windhövel et al., 1995); however, for a better understanding of carotenoid function in the primary photosynthesis reaction, a more detailed study including measurements of additional photosynthesis parameters is needed. Because cyanobacterial species other than Synechococcus PC7942 synthesize carotenoid structures that are different from those shown in Table I, it may be possible to find other carotenoids with even better protective properties than zeaxanthin.
Footnotes
This work was supported by a grant from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, Germany (grant no. 07UVB07).
LITERATURE CITED
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JD, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1995. [Google Scholar]
- Blanc PL, Tuveson RW, Sargent ML. Inactivation of carotenoid-producing and albino strains of Neurospora crassa by visible light, black light and ultraviolet radiation. J Bacteriol. 1976;125:616–625. doi: 10.1128/jb.125.2.616-625.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böger P, Sandmann G, Miller R. Herbicide resistance in a mutant of the microalga Bummilleriopsis filiformis. Photosynth Res. 1981;2:61–74. doi: 10.1007/BF00036166. [DOI] [PubMed] [Google Scholar]
- Caldwell MM, Teramura AH, Tevini M. The changing solar ultraviolet climate and the ecological consequence for higher plants. Trends Ecol Evol. 1989;4:363–367. doi: 10.1016/0169-5347(89)90100-6. [DOI] [PubMed] [Google Scholar]
- Campbell D, Eriksson M, Öquist G, Gustafsson P, Clarke AK. The cyanobacterium Synechococcus resists UV-B by exchanging photosystem II reaction-center D1 proteins. Proc Natl Acad Sci USA. 1998;95:364–369. doi: 10.1073/pnas.95.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diverse-Pierlussi M, Krogmann DW. Biochim Biophys Acta. 1988;933:372–377. [Google Scholar]
- Ernst S, Sandmann G. Poly-cis carotene pathway in the Scenedesmus mutant C-6D. Arch Microbiol. 1988;150:590–594. [Google Scholar]
- Foyer CH, Lelendais M, Kunert K-J. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PM, Grierson D. Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J. 1995;8:693–701. [Google Scholar]
- Greenberg B, Gaba V, Canaani O, Malkin S, Mattoo AK, Edelman M. Separate photosensitizers mediate degradation of the 32-kDa photosystem II reaction center protein in the visible and UV spectral regions. Proc Natl Acad Sci USA. 1989;86:6617–6620. doi: 10.1073/pnas.86.17.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideg E, Vass I. UV-B induced free radical production in plant leaves and isolated thylakoid membranes. Plant Sci. 1996;115:251–260. [Google Scholar]
- Jansen MAK, Greenberg BM, Edelman M, Mattoo AK, Gaba V. Accelerated degradation of the D2 protein of photosystem II under ultraviolet radiation. Photochem Photobiol. 1996;63:814–817. [Google Scholar]
- Jürgens UJ, Weckesser J. Carotenoid-containing outer membrane of Synechocystis sp. strain PCC6714. J Bacteriol. 1985;164:384–389. doi: 10.1128/jb.164.1.384-389.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky NI. Antioxidant function of carotenoids. Free Radical Biol Med. 1989;7:617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- Kumagai MH, Donson J, Della-Cioppa G, Harvey D, Hanley K, Grill LK. Cytoplasmic inhibition of carotenoid biosynthesis with virus-derived RNA. Proc Natl Acad Sci USA. 1995;92:1679–1683. doi: 10.1073/pnas.92.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanga G, Calmanovici G, Puntarulo S. Oxidative damage to chloroplasts from Chlorella vulgaris exposed to ultraviolet-B radiation. Physiol Plant. 1997;101:455–462. [Google Scholar]
- Martínez-Férez I, Fernandez-Gonzalez B, Sandmann G, Vioque A (1994) Cloning and expression in E. coli of the gene coding for phytoene synthase from the cyanobacterium Synechocystis sp. PCC6803. Biochim Biophys Acta 1218: 145–152 [DOI] [PubMed]
- Middleton EM, Teramura AH. The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol. 1993;103:741–752. doi: 10.1104/pp.103.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol. 1990;172:6704–6712. doi: 10.1128/jb.172.12.6704-6712.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradas-Fereira PV, Costa P, Piper, Mager W. The molecular defenses against reactive oxygen species in yeast. Mol Microbiol. 1996;19:651–658. doi: 10.1046/j.1365-2958.1996.403940.x. [DOI] [PubMed] [Google Scholar]
- Nedunchezhian N, Ravindran KC, Abadia A, Abadia J, Kulandaivelu G. Damages of photosynthetic apparatus in Anacystis nidulans by ultraviolet-B radiation. Biol Plant. 1996;38:53–59. [Google Scholar]
- Pandey R, Chauhan S, Singhal GS. UVB-induced photodamage to phycobilisomes of Synechococcus sp. PCC 7942. J Photochem Photobiol B. 1997;40:228–232. doi: 10.1016/s1011-1344(97)00060-2. [DOI] [PubMed] [Google Scholar]
- Putnoky E, Kiss GB, Ott I, Kondorosi A. Tn5 carries a streptomycin resistance determination downstream from the kanamycin resistance gene. Mol Gen Genet. 1983;191:288–294. doi: 10.1007/BF00334828. [DOI] [PubMed] [Google Scholar]
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignment, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sandmann G, Kuhn S, Böger P. Evaluation of structurally different carotenoids in Escherichia coli transformants as protectants against UV-B radiation. Appl Environ Microbiol. 1998;64:1972–1974. doi: 10.1128/aem.64.5.1972-1974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramura AH, Ziska LH. Ultraviolet-B radiation and photosynthesis. In: Baker NR, editor. Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 435–450. [Google Scholar]
- van den Hondel CAMJJ, Verbeek S, van den Ende A, Weisbeek P, Borrias WE, van Arkel GA. Introduction of transposon Tn901 into a plasmid of Anacystis nidulans: preparation for cloning in cyanobacteria. Proc Natl Acad Sci USA. 1980;77:1570–1574. doi: 10.1073/pnas.77.3.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas J, Hegemann H, de Vrieze G, Tuyl M, Borrias M, Weisbeek P. Genomic integration system based on pBR322 sequences for the cyanobacterium Synechococcus sp. PCC7942: transfer of genes encoding plastocyanin and ferredoxin. Gene. 1990;95:39–48. doi: 10.1016/0378-1119(90)90411-j. [DOI] [PubMed] [Google Scholar]
- Will OH, Newl NA, Reppe CR. The photosensitivity of pigmented and non-pigmented strains of Ustilago violacea. Curr Microbiol. 1984;10:295–302. [Google Scholar]
- Windhövel U, Gatzek S, Böger P. A foreign phytoene synthase gene modifies the photosynthesis rate of Synechococcus PCC7942. In: Mathis P, editor. Photosynthesis: From Light to Biosphere. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 457–460. [Google Scholar]
- Windhövel U, Geiges B, Sandmann G, Böger P. Expression of Erwinia uredovora phytoene desaturase in Synechococcus PCC7942 leading to resistance against a bleaching herbicide. Plant Physiol. 1994;104:119–125. doi: 10.1104/pp.104.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall AA, Britton G, Jackson MJ. Carotenoids and protection of phospholipids in solution or liposomes against oxidation by peroxyl radicals: relationship between carotenoid structure and protective ability. Biochim Biophys Acta. 1997;1336:575–586. doi: 10.1016/s0304-4165(97)00007-x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C, Vieira J, Messing J. nucleotide sequence of M13 mp18 and pUC19 vectors Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]