Abstract
Background
Empirical evidences for efficacy of hot spring (HS) water in inflammatory skin disorders have not been substantiated with sufficient, immunological “hard evidence”. Mageumsan HS water, characterized by its weakly-alkaline properties and low total dissolved solids content, has been known to alleviate various immune-inflammatory skin diseases, including atopic dermatitis (AD).
Objective
The trial attempted to quantitatively analyze in vitro expression levels of chemical mediators in cutaneous inflammation from HaCaT cell line treated with Mageumsan HS, and suggest the likely mode of action through which it exerts the apparent anti-inflammatory effects in AD.
Methods
Using membrane-based human antibody array kit, customized to include 30 different, keratinocyte-derived mediator proteins, their expression levels (including interleukin [IL]-1, IL-6, IL-8, thymic stromal lymphopoietin, thymus and activation-regulated chemokine, and granulocyte macrophage colony-stimulating factor) were assessed in vitro. Selected key proteins were further quantified with enzyme-linked immunosorbent assay.
Results
There was a clear pattern of overall suppression of the mediators, especially those noted for their pro-inflammatory role in AD (monocyte chemoattractant protein [MCP]-1, regulated on activation, normal T cell expressed and secreted, cutaneous T-cell-attracting chemokine, Eotaxin, and macrophage inflammatory protein-1α, etc.). Also, reduced expression of involucrin and cytokeratin 1 was also reduced in the HS-treated group.
Conclusion
The present study has shown that Mageumsan HS water may exert its effects on inflammatory skin disorders through regulation of proinflammatory cytokines. These evidences are to be supported with further future investigations to elucidate immunological mechanism behind these beneficial effects of HS water in the chronically inflamed skin of AD.
Keywords: Atopic dermatitis, Hot spring water, Immune-regulation, Keratinocyte-derived cytokines/chemokines, Protein array analysis
INTRODUCTION
Hot spring (HS) water hydrotherapy has been regarded as a solid option in atopic dermatitis (AD) and other skin conditions of defective barrier. This less conventional mode of treatment is steadily gaining acceptance among the field professionals akin to balneology, in part due to its relative freedom from safety issues. Historical accounts, in which HS water is used as means of alleviating ailments—both cutaneous and extracutaneous—can be traced to as far back as antiquity1; one of the better documented sources include formal institutionalization of HS balneotherapy for what amounts to the present-day Hansen disease, during the reign of King Sejong. In line with such historical and cultural framework, the peninsula of Korean is endowed with vast sources of natural HS water at various regions. The waters from these geographic locations, some four hundred in in total2, differ from one another in terms of its physicochemical properties. It has been established by previous literatures that HS water in Korea can be categorized into five main subtypes on hydrochemical basis3: i.e., (1) low total dissolved solute (TDS) Ca(Na)-HCO3 type, (2) Na(Ca)-HCO3 type, (3) high TDS Na(Ca)-Cl type, (4) acidic Ca-HCO3 type, and (5) Ca(Na)-SO4 type. The distinct makeup of composite anions and cations within each subtype is believed to be ultimately responsible for the array of clinical effects they promulgate on human skin4.
In previous engagements, the authors have reported a significant clinical improvement as assessed by modified SCORing Atopic Dermatitis (SCORAD) and reduction in trans-epidermal water loss in HS water-treated AD mouse models5. In a laboratory trial, in HS-treated keratinocyte (KC) cell colonies, there was a marked decline in serum tumor necrosis factor (TNF)-α and interleukin (IL)-6 levels, two of which are the most pivotal cytokines in elicitation of immune-inflammatory process5, under stimulation of toll-like receptor (TLR) agonist 3, a potent inducer of expression of proinflammatory cytokines and chemokines6. Meanwhile, skewing of T-cell population into Treg subset was shown to have been facilitated by HS water treatment. In our animal models of experimentally-induced psoriasis, a disorder of aberrant epidermal turnover, there was a statistically meaningful decease in the total mRNA loads of IL-17 and IL-23, arguably the two most pivotal cytokines in the pathogenesis and regulation of the autoimmune inflammatory process involved7.
These previous efforts have precipitated the present in vitro study, in which the authors' aim was to put together a qualitative and quantitative analysis of expression patterns of chemical mediators released from human keratinocyte cell lines (HaCaT cells). For this investigation, we have used Magumsan HS, which has been known for its empirical effects on AD. This Na-Cl type HS possesses all of typical attributes of Korean HS. In all, this endeavor sought to render the best recapitulation of the changes in the overall immunological milieu within cutaneous KC interacting with HS water.
MATERIALS AND METHODS
Cell culture
This study was exempted from IRB review by Institutional Review Board at Uijeongbu St. Mary's Hospital, The Catholic University of Korea (IRB no. UC15EISE0043). HaCaT (human keratinocyte cell line), provided by the courtesy of Professor Tae-Yoon Kim (College of Medicine, The Catholic University of Korea) was cultured in Dulbeco's Modified Eagle Medium (DMEM; Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL) and 100 U/ml penicillin/streptomycin (Gibco-BRL) at 37℃ in an incubator containing 5% CO2. Subcultures were done when 80% to 90% confluence level was reached.
Preparation of HS water
The study water was sourced from Magumsan Hot Spring Baths (located in Changwon, Korea). For quality control purposes, repeated bouts of hydrochemical analysis were carried out (Table 1). To circumvent possible distortion of the true concentration, ion-paring reagent (for cation analysis) was acidified (by preventing precipitation and adsorption). Cation and dissolved silica levels were determined using inductively coupled plasma atomic emission spectroscopy, while IC was used for anion analysis. Upon arrival at our facility, HS water was immediately filtered through 0.22 µm filters to eliminate the possibility of microbial contamination, and was stored at 4℃ throughout the trial. Initial osmolarity was determined using Micro-Osmometer 210 (FISKE Associate, Norwood, MA, USA). To maintain optimal osmolarity, a powdered form of DMEM media was dissolved into the HS water to produce a mixture, then stored in a refrigerator until use.
Table 1. Hydrochemical composition of Magumsan hot spring water (pH=8.02).
| Parameter | Value (mg/L) |
|---|---|
| Alkalinity | 47.29 |
| Na+ | 221.69 |
| Ca2+ | 44.79 |
| K+ | 7.11 |
| Mg2+ | 0.88 |
| SiO2 | 35.71 |
| Li2+ | 0.20 |
| Sr2+ | 0.70 |
| Mn2+ | 0.006 |
| Cu2+ | 0.02 |
| Zn2+ | 0.006 |
| Br− | 0.12 |
| F− | 0.63 |
| Cl− | 315.24 |
| NO3− | 2.49 |
| SO42− | 102.22 |
| Total dissolved solids | 362 |
TLR stimulation
For the best possible rendering of the pro-inflammatory microenvironment in vivo, the HS water-treated DMEM media was treated with TLR agonist 3 (poly I:C). The composition of poly TLR 3 (poly I:C) was as follows: Tripamitoyl-S-glyceryl-cysteine (Pam3Cys, 1 µl/ml), heat-killed Listeria monocytogenes (HKLM, 106 cells/ml), polyriboinosinic polyribocytidylic acid (poly [I:C], 10 µl/ml; InvivoGen, San Diego, CA, USA), lipopolysaccharide (LPS, 10 µl/ml), flagellin (10 µl/ml), and Pam2CGDPKHPKSF (FSL-1, 1 µl/ml). HS was added simultaneously or pretreated 2 hours before poly (I:C) treatment. Cells were cultured for 1, 4, 10, or 24 hours in each treatment group.
Cytotoxicity assay
A part of the preliminary investigations was WST-1-based cell cytotoxicity assay (Roche, Indianapolis, IN, USA) for determination of optimal cell and poly (I:C) concentration. For 3-(4,5-dimethylthizol-2-yl) 2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO, USA) assay, cells were seeded onto 96-well microtiter plates at a density of 3×104/200 µl in fresh medium, and then treated with HS water spring water at a serially diluted concentration. To any detect time-dependent effect, the cultured media were observed sequentially at hours 1, 4, 10, and 24. At a specific time point, as indicated by the protocol, 20 µl of MTT (5 mg/ml in phosphate buffered saline) was added to each well, and the plates were again incubated for another 4 hours. The supernatant was subsequently discarded, and 200 µl dimethyl sulfoxide was added to each well. To prevent precipitation of dark blue formazan crystals, the plates were covered with aluminum foil, then gently shaken for 15 minutes, for determination of the absorption spectrum at 570 nm.
Membrane-based human antibody array
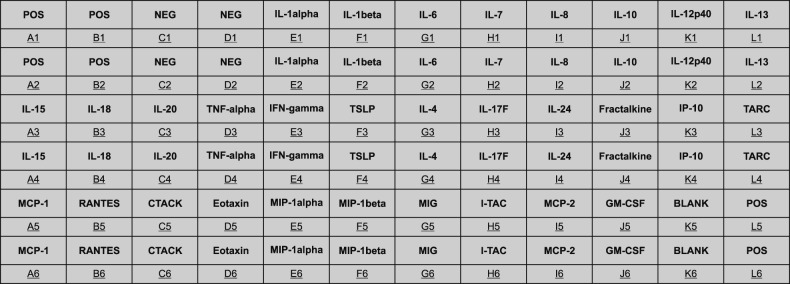
The primary assessment tool was custom-made Raybio® C-Series Custom Cytokine Antibody Array (Catalog No: AAX-CUST; RayBiotech, Inc., Norcross, GA, USA), designed to quantify relative protein expression profile across the four different treatment groups: the control (DMEM media only), only HS-treated, only TLR agonist poly (I:C) treated, and both HS and the TLR agonist poly (I:C)-treated groups. The microarray membrane is tagged with 30 specific antibodies (provided in the public domain by http://www.raybiotech.com/custom-antibody-array-sandwich-based.html) against mediator proteins in the supernatant. Upon thorough review of literature, KC-derived cytokines and chemokines that are most pertinent to AD-related skin inflammation were included. The microarray was designed to provide investigators with a panoramic view of the overall effects of HS water upon human KCs (Fig. 1).
Fig. 1. Arrangement of custom-made cytokine/chemokine-specific antibody microarray of 30 chemical mediators of keratinocyte origin. POS: positive control, NEG: negative control, IL: interleukin, TNF: tumor necrosis factor, IFN: interferon, TSLP: thymic stromal lymphopoietin, IP: interferon-γ induced protein, TARC: thymus and activation-regulated chemokine, MCP: monocyte chemoattractant protein, RANTES: regulated on activation, normal T cell expressed and secreted, CTACK: cutaneous T-cell-attracting chemokine, MIP: macrophage inflammatory protein, MIG: monokine induced by γ-interferon, I-TAC: interferon-inducible T-cell α chemoattractant, MCP: monocyte chemoattractant protein, GM-CSF: granulocyte macrophage colony-stimulating factor.
This assay was carried out in accordance with manufacturer's instructions: first, concentrations of the 12 cell supernatants in the control, the HS group, the TLR agonist poly (I:C) treatment group, HS and the TLR agonist poly (I:C) treatment group were measured using bicinchoninic acid assay.
The array membranes were blocked with 2 ml of blocking buffer at room temperature (RT) for 30 minutes. The membranes were washed three times with 2 ml of wash buffers I and II at RT for five minutes with through shaking and then incubated with 1ml Biotinylated Antibody Cocktail at RT for 2 hours or overnight at 4℃. After the membranes were cleansed in the same manner, 2 ml of 1-fold horseradish peroxidase–conjugated streptavidin at RT for 2 hours. After the membranes were cleansed likewise, 4 ml of mixed chemiluminescent detection buffer were added to the membranes the manners in which the entire surface would be covered. The entire system was then allowed to stabilize at RT for two minutes. Finally, several films were acquired after exposing the membranes to radiographic film (Kodak Industrex Processor Model; Carestream Health, Inc., Rochester, NY, USA). The two films deemed most suitable for comparing spot signal intensity were selected.
Comparison of the four groups in spot signal intensity was meticulously done with naked eye. For any inter-group difference that was detected, density measurement was done using densitometry software Image J (NIH website along with an array plug-in) after the two films were converted to computer files. The images were adjusted for positive control normalization and background subtraction using RayBiotech® Microsoft Excel-based Analysis Software Tools.
Protein enzyme-linked immunosorbent assay quantification
Statistically significant microarray results (differences among the control, HS-treated, TLR agonist poly (I:C) treated, and both HS/poly (I:C) treated groups) were tested for validity with ELISA protein quantification. In addition, the expression level of thymus and activation-regulated chemokine (TARC), a chemokine known to be suppressed after treatment with HS water, was also measured. Concentrations of interferon-γ induced protein (IP)-10, TARC, regulated on activation, normal T cell expressed and secreted (RANTES), and Eotaxin were measured in all 12 samples using commercially available ELISA kits (Quantikine Elisa kits, catalog number DIP100, DDN00, DRN00B, and DTX00, respectively; R&D Systems, Minneapolis, MN, USA). Concentration of each sample was measured twice, and then the two values were averaged. All ELISAs in HaCaT supernatant treated with poly (I:C) in presence or absence of HS water were quantified as indicated by the manufacturer's protocols.
Statistical analysis
Data are expressed as means±standard error of the mean. One-way ANOVA tests were applied to analyze the results in the four group (at p<0.05), using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA). An independent t-test was used to determine statistical significance.
RESULTS
Hydrochemical analysis
Hydrochemical composition of the study water is summarily given in Table 1. The temperature was measured to be 38.1℃, which places the water into the loosely defined category of moderate-high thermal HS water. The pH (8.02) was typical of weakly alkaline Korean HS water. Electrical conductivity was 1,291 µS/cm, and dissolved oxygen was 3.15 mg/L. TDS was slightly above the median for Korean HS water (239 mg/L) at 362 mg/L. The predominant cation and anion were Na+ and Cl−, respectively (hence, an NaCl type HS water). The amount of trace element was negligible.
Cytokine/chemokine array profiles
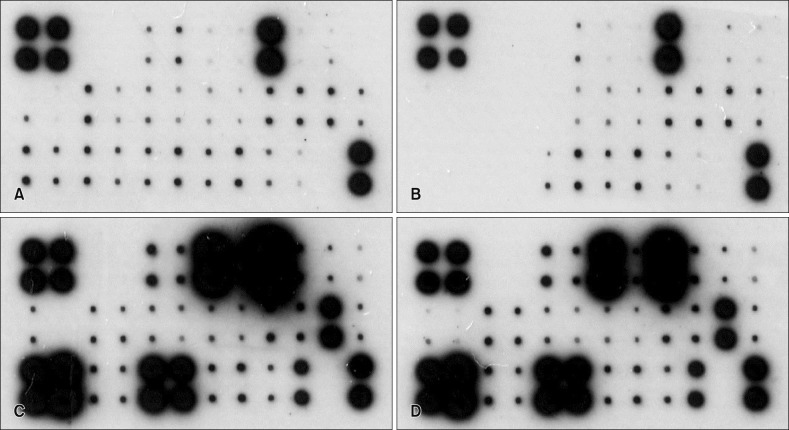
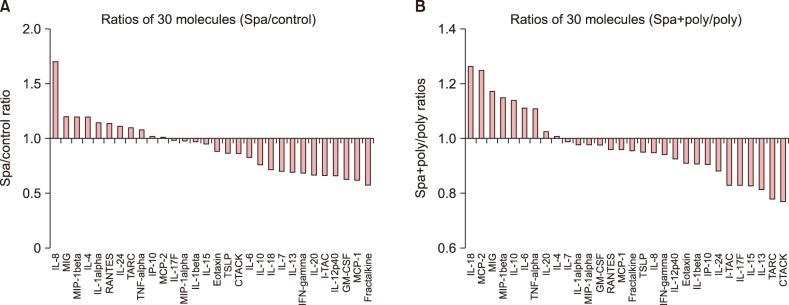
The result for cytokine/chemokine microarray is summarized in Fig. 2. Overall, the expression of proinflammatory mediators in the HS-treated group (Fig. 2B) was suppressed (Fig. 2A). The level of expression is reflected in the relative size and color density of the respective black dots for each mediator. When treated with TLR 3 poly (I:C), a powerful stimulator of proinflammatory chemical mediators, a robust augmentation of protein expression is seen (Fig. 2C). In contrast, this augmentative effect is conspicuously offset upon treatment with the HS water (Fig. 2D). The intensity difference of expression for each mediator with respect to the positive control in the array was converted to the numerical integral density (IntDensity, measured in AU, shown in Fig. 3), so that the influence that are exclusively attributable to the study water may be better grasped. In Fig. 3A, the overall trend of downregulation was seen throughout virtually all the mediators, some of which were IL-1β, IL-15, IL-18, IL-20, IFN-γ, fractalkine, IP-10, monocyte chemoattractant protein (MCP)-1, cutaneous T-cell-attracting chemokine (CTACK), Eotaxin, and macrophage inflammatory protein (MIP)-1α. In Fig. 3B, in which the effects due to poly (I:C) were left out, the expression level of 11 mediators, including IL-8, IL-15, IL-17F, IL-24, fractalkine, IP-10, TARC, MCP-1, RANTES, CTACK, interferon-inducible T-cell α chemoattractant, and granulocyte macrophage colony-stimulating factor (GM-CSF) were reduced, while in seven cytokines/chemokines (IL-6, IL-10, IL-20, TNF-α, MIP-1β and MCP-2) the expression intensity was increased.
Fig. 2. Cytokine protein microarray in the human keratinocyte cell cultured after 24 hours. (A) Dulbeco's Modified Eagle Medium (DMEM; Gibco-BRL, USA) media (without fetal bovine serum, osmorality 336 mOSM/kg). (B) DMEM media with Magumsan hot spring (HS) water (osmorality 350 mOSM/kg). (C) DMEM media treated with toll-like receptor 3 agonist poly (I:C) (10 µg/ml). (D) DMEM media with Magumsan HS water treated with poly (I:C) (10 µg/ml).
Fig. 3. The ratios of the 30 mediator molecules expressed at different levels. (A) is the ratio of hot spring (HS)-treated group to the control. The ratio ranged from 0.58 (Fractalkine) to 1.70 (IL-8). (B) is the ratio of HS and poly (I:C) group to poly (I:C) group. The ratio ranged from 0.77 (CTACK) to 1.26 (IL-18). IL: interleukin, MIG: monokine induced by γ-interferon, MIP: macrophage inflammatory protein, RANTES: regulated on activation, normal T cell expressed and secreted, TARC: thymus and activation-regulated chemokine, TNF: tumor necrosis factor, IP: interferon-γ induced protein, MCP: monocyte chemoattractant protein, TSLP: thymic stromal lymphopoietin, CTACK: cutaneous T-cell-attracting chemokine, IFN: interferon, GM-CSF: granulocyte macrophage colony-stimulating factor.
Enzyme-linked immunosorbent assay
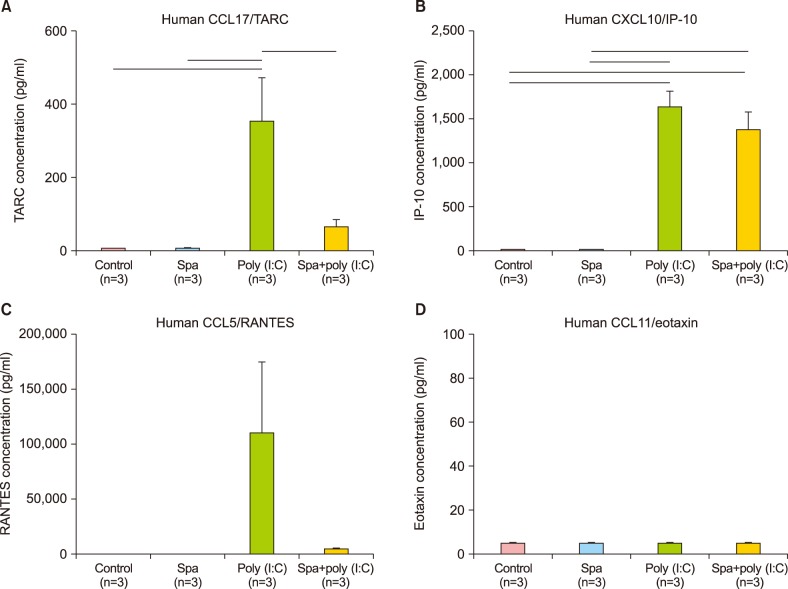
ELISA technique was used to quantify the expression levels of four key mediators, which are renowned for their inflammation-instigating roles in AD, namely, CXCL10/IP-10, CCL17/TARC, CCL5/RANTES, and CCL11/Eotaxin.
The density of TARC expression was significantly decreased in HS and poly (I:C)-treated group versus poly (I:C)-treated group (p<0.001) (Fig. 4A). Also, the density of IP-10 was lower in HS and poly (I:C)-treated group versus poly (I:C) group (p<0.001) (Fig. 4B). Thus, the ratio of decline (0.84) was much smaller than that observed for TARC (0.18) (Fig. 3B). RANTES levels followed downward trend as well. The absolute concentration of Eotaxin was much lower than TARC, IP-10, or RANTES (Table 2).
Fig. 4. (A~D) The concentrations of four key molecules as measured by enzyme-linked immunosorbent assay in human keratinocyte cells treated with hot spring (HS), poly (I:C), HS with poly (I:C), and controls (n=3). The density of TARC expression was significantly decreased in HS with poly (I:C)-treated group versus poly (I:C) only group (p<0.001). The density of IP-10 was also measured to be lower in HS with poly (I:C)-treated group versus poly (I:C) only group (p<0.001), thus the ratio (0.84) was much smaller than that observed for TARC (0.18). RANTES followed the downward trend as well. The absolute concentration of Eotaxin was much lower than those of TARC, IP-10, or RANTES. TARC: thymus and activation-regulated chemokine, IP-10: interferon-γ induced protein-10, RANTES: regulated on activation, normal T cell expressed and secreted.
Table 2. Effect of Magumsan HS on in vitro secretion of TARC, IP-10, RANTES and Eotaxin of human keratinocyte cells as measured by enzyme-linked immunosorbent assay.
| Variable | Tx Group | Concentration (pg/ml) | Ratio | p-value | |
|---|---|---|---|---|---|
| HS/Control | HS+poly/poly | ||||
| TARC | Control | 3.09±0.98 | 0.99 | <0.001 | |
| HS | 3.05±1.46 | ||||
| Poly (I:C) | 353.0±117.2 | 0.18 | |||
| HS+poly (I:C) | 64.04±19.85 | ||||
| IP-10 | Control | 3.63±1.27 | 0.65 | <0.001 | |
| HS | 2.33±1.32 | ||||
| Poly (I:C) | 1,628±177.4 | 0.84 | |||
| HS+poly (I:C) | 1,374±200.6 | ||||
| RANTES | Control | 41.67±17.02 | 0.05 | 0.064 | |
| HS | 1.90±0.96 | ||||
| Poly (I:C) | 109,896±63,129 | 0.04 | |||
| HS+poly (I:C) | 4,009±1,093 | ||||
| Eotaxin | Control | 4.26±0.93 | 1.08 | 0.99 | |
| HS | 4.61±0.80 | ||||
| Poly (I:C) | 4.87±0.83 | 0.98 | |||
| HS+poly (I:C) | 4.75±0.84 | ||||
Values are presented as mean±standard deviation. HS: hot spring, TARC: thymus and activation-regulated chemokine, IP-10: interferon-γ induced protein-10, RANTES: normal T cell expressed and secreted, Tx: treatment, poly: TLR 3 agonist poly (I:C).
DISCUSSION
AD shows a wide heterogeneity of etiological factors8,9, which underscore its chronic and often indocile course. As the multi-faceted nature of its pathophysiology become further unraveled, a gradual, shift in disease paradigm has been set in motion; the incumbent paradigm is the model of perturbed equilibrium in the adaptive arm of the immune system10,11, in which a largely Th2-process, coupled with dysregulated Treg subpopulation12 triggers a cascade of closely-intertwined immunological interactions. The breached-barrier model has given its way to one of cellular/adaptive immunity and the constituent molecular mediators (with a distinct emphasis on cytokines and chemokines), which are presently considered “bolts and screws” of the machinery. These mediators may also have a direct bearing on characteristic symptoms; studies have revealed that in AD-associated intractable pruritus-where the receptors involved are often not entirely histaminic13 -proinflammatory cytokines are prime target suspects that instigate it14,15. Naturally brought along with this paradigmatic shift was revamping in management scheme; systemic immunomodulators and biologics have taken a center-stage as an important arsenal against the immunological leg of AD patho-mechanism16. The authors, having experienced an apparent, skin barrier-reparative effect with an HS water after 4 weeks of bathing in a tub17, were prompted with the question of whether HS water brings upon beneficial effects on cellular and molecular level with certain immune-mediating action. Our results point to the evidence that Magumsan HS water was shown to have on overall suppressed expression of the key KC-derived immune mediators, which. The expression level of IL-1α, IL-6, IL-8, TNF-α, and GM-CSF are attenuated in the presence of an NaCl/CaCl HS water in vitro. In the poly (I:C)-stimulated, HS-treated group, reduction in relative level of expression was seen with IL-8, IL-15, IL-17F, IL-24, fractalkine, IP-10, TARC, MCP-1, RANTES, CTACK, and I-TAC. IL-8 is a KC-derived cytokine that acts as a chemoattractant for neutrophils during the Th2-dominated acute inflammation phase of AD18. IL-15 is renowned for its role in psoriasis, where the proinflammatory mediator is released from KC to influence survival and turnover of CD8+ cells, which in turn feeds back to the KC to enhance inflammation19. IL-24 is secreted from KC and facilitates expression of secretion of pro-inflammatory mediators IL-8, PGE2, and MMP-1, in a positive feedback regulation of epidermal inflammation in response to external and internal sources20. On the other hand, there was an up-regulation of IL-10, an anti-inflammatory cytokine, and six other proinflammatory cytokines and chemokines (IL-6, IL-20, TNF-α, MIP-1β, MCP-2, and GM-CSF). Taken together, the expression profile results reaffirm our previous findings that the HS water exerts significant curbing effects on proinflammatory mediators from KC and simultaneously contribute to holding of inflammation in check through facilitating secretion of Treg-related mediators. Even in the face of this shift of weight from barrier to immunity, one factor that remains a crucial common denominator is the role played by KCs. KCs make up the brick and mortar of the outermost layer of barricade against constant physical, chemical, and bacterial barrage from the external environment. On another level, these cells are an important command post for the immunological interplay between major elements of innate and cellular immunity21,22. A vast reservoir for innate immune mediator is one of the first responders at the scene to neutralize the threats of possible outside intrusion or noxious stimulus from within. In the case of the latter, these chemical alarm signals often take the shape of pattern recognition receptors, typified by TLR and nucleotide-binding oligomerization domain-like receptors. Our preliminary investigations led to the conviction that TLR 3 agonist is the most capable of inducing secretion of various mediator molecules. Hence, it was used categorically for recreating the in vivo environment circa KC during the Th2-driven inflammation.
Akiyama et al.23 suggested that trace metal ions (e.g., Mn++ and I−) are crucial for the bactericidal property of HS water and its ability for keeping hydrogen concentration low. On par with the notion, the most predominant cation and anion in the present investigation were Na++ and Cl−, respectively (i.e., an NaCl type HS water), which were responsible for the improvement of cutaneousd barrier homeostasis in Magumsan HS. The concentration of CaCl2 in Magumsan HS was 44 mg/L, and authors had attempted to produce a solution of CaCl2 of the same concentration (for the purposes of comparison); however, the actual osmolarity was significantly deviant from the expected value, which forced us to abandon its use. Upon a consultation with a membrane physiologist, the authors have reckoned that the experimental value is of far more relevance, which led us to use only HS water for osmolarity, which would pose a serious flaw in accurate assessment of its effects on direct cell contact. Early on in our experiment, we added 4 M NaCl determination. Osmolarity of the water was determined to be 74 mOsm/kg initially along with 1 M sucrose as to bring the DMEM media equimolar to normal cell osmolarity. Afterwards, the resulting mixture of the Magumsan water and DMEM powder was determined to be 350 mOsm/kg, which was deemed suitable.
The authors believe that our endeavor in the present investigation has left us with an alternative methodology for assessing potential immune-modulating actions of Korean HS water with varying spectrum of constituent ionic substances; this compendium represents a possible standard protocol for further sophisticated studies involving HS water. Our microarray results provided a window through which the immunological impact of an NaCl type HS water on skin overall can be grasped, and we are convinced that our work would serve as a primer for other prospective investigative trials that would hopefully fill in the missing puzzles between the apparent beneficial effects of HS water and its elaborate working mechanism. Future studies on detailed molecular machinery of immune-inflammatory regulation of HS water and varying degrees of effects based on differing mineral composition and concentrations would enable us to venture further into the territory that are left largely unchartered.
ACKNOWLEDGMENT
This work was supported by the Korean Academy of Hot Spring, Seoul, Republic of Korea.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132–140. doi: 10.1046/j.1529-8019.2003.01622.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim JW. Therapeutic effectiveness and its underlying immunologic mechanisms of Korean hot spring water on atopic dermatitis. J Korean Acad Hot Spring. 2012;1:48–54. [Google Scholar]
- 3.Kim KH, Yun ST, Park SS, Choi BY, Chang ES, Ko YG. Physicochemical characteristics of natural hot spring waters in Korea and geochemical modeling of water quality change in heating-cooling. J Korean Acad Hot Spring. 2012;1:22–33. [Google Scholar]
- 4.Kim JW, Hahn HJ, Woo SY, Yun ST, Lee JT, Kim HJ. Immunoinflammatory regulation effects of Korean hot spring water. J Jpn Soc Balneol Climatol Phys Med. 2015;78:253–270. [Google Scholar]
- 5.Lee HP, Choi YJ, Cho KA, Woo SY, Yun ST, Lee JT, et al. Effect of spa spring water on cytokine expression in human keratinocyte HaCaT cells and on differentiation of CD4(+) T cells. Ann Dermatol. 2012;24:324–336. doi: 10.5021/ad.2012.24.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi YJ, Lee HJ, Lee DH, Woo SY, Lee KH, Yun ST, et al. Therapeutic effects and immunomodulation of suanbo mineral water therapy in a murine model of atopic dermatitis. Ann Dermatol. 2013;25:462–470. doi: 10.5021/ad.2013.25.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KH, Cho KA, Kim JY, Kim JY, Baek JH, Woo SY, et al. Filaggrin knockdown and Toll-like receptor 3 (TLR3) stimulation enhanced the production of thymic stromal lymphopoietin (TSLP) from epidermal layers. Exp Dermatol. 2011;20:149–151. doi: 10.1111/j.1600-0625.2010.01203.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee YB, Lee JY, Lee HJ, Yun ST, Lee JT, Kim HJ, et al. Immunomodulatory effects of balneotherapy with hae-un-dae thermal water on imiquimod-induced psoriasis-like murine model. Ann Dermatol. 2014;26:221–230. doi: 10.5021/ad.2014.26.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irvine AD, Eichenfield LF, Friedlander SF, Simpson EL. Review of critical issues in the pathogenesis of atopic dermatitis. Semin Cutan Med Surg. 2016;35(5 Suppl):S89–S91. doi: 10.12788/j.sder.2016.042. [DOI] [PubMed] [Google Scholar]
- 10.Simpson EL, Irvine AD, Eichenfield LF, Friedlander SF. Update on epidemiology, diagnosis, and disease course of atopic dermatitis. Semin Cutan Med Surg. 2016;35(5 Suppl):S84–S88. doi: 10.12788/j.sder.2016.041. [DOI] [PubMed] [Google Scholar]
- 11.Sinke JD, Rutten VP, Willemse T. Immune dysregulation in atopic dermatitis. Vet Immunol Immunopathol. 2002;87:351–356. doi: 10.1016/s0165-2427(02)00066-1. [DOI] [PubMed] [Google Scholar]
- 12.Ong PY, Leung DY. Immune dysregulation in atopic dermatitis. Curr Allergy Asthma Rep. 2006;6:384–389. doi: 10.1007/s11882-996-0008-5. [DOI] [PubMed] [Google Scholar]
- 13.Ou LS, Goleva E, Hall C, Leung DY. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol. 2004;113:756–763. doi: 10.1016/j.jaci.2004.01.772. [DOI] [PubMed] [Google Scholar]
- 14.Werfel T, Biedermann T. Current novel approaches in systemic therapy of atopic dermatitis: specific inhibition of cutaneous Th2 polarized inflammation and itch. Curr Opin Allergy Clin Immunol. 2015;15:446–452. doi: 10.1097/ACI.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 15.Seltmann J, Werfel T, Wittmann M. Evidence for a regulatory loop between IFN-γ and IL-33 in skin inflammation. Exp Dermatol. 2013;22:102–107. doi: 10.1111/exd.12076. [DOI] [PubMed] [Google Scholar]
- 16.Knol EF, Hijnen D. Atopic dermatitis: a tale of two distinct pathomechanisms that make you itch. Eur J Immunol. 2016;46:2512–2515. doi: 10.1002/eji.201646708. [DOI] [PubMed] [Google Scholar]
- 17.Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-tohigh-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388:31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 18.Glowacka E, Lewkowicz P, Rotsztejn H, Zalewska A. IL-8, IL-12 and IL-10 cytokines generation by neutrophils, fibroblasts and neutrophils- fibroblasts interaction in psoriasis. Adv Med Sci. 2010;55:254–260. doi: 10.2478/v10039-010-0037-0. [DOI] [PubMed] [Google Scholar]
- 19.Bobbala D, Mayhue M, Menendez A, Ilangumaran S, Ramanathan S. Trans-presentation of interleukin-15 by interleukin-15 receptor alpha is dispensable for the pathogenesis of autoimmune type 1 diabetes. Cell Mol Immunol. 2017;14:590–596. doi: 10.1038/cmi.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin SH, Choi D, Chun YJ, Noh M. Keratinocyte-derived IL-24 plays a role in the positive feedback regulation of epidermal inflammation in response to environmental and endogenous toxic stressors. Toxicol Appl Pharmacol. 2014;280:199–206. doi: 10.1016/j.taap.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Strbo N, Yin N, Stojadinovic O. Innate and adaptive immune responses in wound epithelialization. Adv Wound Care (New Rochelle) 2014;3:492–501. doi: 10.1089/wound.2012.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665–675. doi: 10.1016/j.rdc.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama H, Yamasaki O, Tada J, Kubota K, Arata J. Antimicrobial effects of acidic hot-spring water on Staphylococcus aureus strains isolated from atopic dermatitis patients. J Dermatol Sci. 2000;24:112–118. doi: 10.1016/s0923-1811(00)00091-8. [DOI] [PubMed] [Google Scholar]