Abstract
Scope
The primary disorder underlying metabolic syndrome is insulin resistance due to excess body weight and abdominal visceral fat accumulation. In this study, it is asked if dietary intake of an ethanolic extract from Russian tarragon (Artemisia dracunculus L., termed PMI5011), shown to improve glucose utilization by enhancing insulin signaling in skeletal muscle, could prevent obesity-induced insulin resistance, skeletal muscle metabolic inflexibility, and ectopic lipid accumulation in the skeletal muscle and liver.
Methods and results
Male wild-type mice are fed a high-fat diet alone or supplemented with PMI5011 (1% w/w) over 3 months. Dietary intake of PMI5011 improved fatty acid oxidation and metabolic flexibility in the skeletal muscle, reduced insulin levels, and enhanced insulin signaling in the skeletal muscle and liver independent of robust changes in expression of factors that control fatty acid oxidation. This corresponds with significantly reduced lipid accumulation in the skeletal muscle and liver, although body weight gain is comparable to a high-fat diet alone.
Conclusion
Previous studies showed that PMI5011 enhances insulin sensitivity in the setting of established obesity-induced insulin resistance. The current study demonstrates that dietary intake of PMI5011 prevents high-fat diet-induced insulin resistance, metabolic dysfunction, and ectopic lipid accumulation in the skeletal muscle and liver without reducing body weight.
Keywords: insulin resistance, liver, metabolic syndrome, obesity, skeletal muscles
1. Introduction
One-third of adults in the United States are estimated to have metabolic syndrome (MetS) with increased prevalence in both men and women over 65 years of age.[1–3] MetS is a cluster of obesity-related risk factors for type 2 diabetes and cardiovascular disorders that includes insulin resistance as the central underlying disorder, coupled with dyslipidemia, hypertension, and visceral (intra-abdominal) fat accumulation.[4] Visceral fat is a strong predictor of obesity-induced insulin resistance,[5] but insulin resistance in obese individuals also corresponds with lipid accumulation in insulin-responsive, non-adipose tissues, primarily skeletal muscle and liver.[6] There is evidence that ectopic fat deposition in skeletal muscle and liver is more important than overall adiposity and body weight in the relationship between insulin resistance and MetS in obesity.[6,7]
The prevalence of MetS and the risk it poses for developing type 2 diabetes and cardiovascular disease has produced specific recommendations for lifestyle modifications about weight loss, diet, and exercise to treat MetS.[8] However, behavioral changes are challenging to maintain and pharmaceutical approaches to treatment are often needed. These approaches focus on multiple molecular targets that regulate insulin signaling and lipid metabolism to enhance insulin responsiveness and reduce ectopic lipid accumulation.[9] Although several of the currently available pharmacological interventions improve insulin sensitivity and lipid profiles, the safety of these approaches has been questioned.[10,11] This has increased interest in alternative approaches to treatment or prevention of obesity-induced insulin resistance and MetS. In particular, plant sources of therapeutic compounds are receiving renewed attention, given the history of botanically based compounds proved to be effective in treating chronic diseases, including type 2 diabetes.[12] Along these lines, Artemisia dracunculus L. or Russian tarragon is a perennial herb with a long history as a traditional treatment for diabetes.[13] In previous studies, we found that an ethanolic extract of A. dracunculus L. termed PMI5011 enhances insulin signaling and improves lipid metabolism in skeletal muscle in vitro.[14–16] Subsequent studies in animal models of obesity and insulin resistance demonstrated that PMI5011-mediated changes in skeletal muscle insulin signaling improve blood glucose and insulin levels.[17] Like other studies focused on treatment of MetS after onset of obesity-related insulin resistance, our studies with PMI5011 examined dietary intervention with a botanical extract to decrease insulin resistance rather than prevent obesity-related metabolic dysfunction. Fischer et al.[18] recently reported that reversal of obesity-induced glucose intolerance with weight loss does not completely reverse adipose tissue inflammation or hepatic lipid accumulation, suggesting a renewed emphasis on prevention is needed.
In the current study, we sought to determine if early dietary supplementation with PMI5011 would prevent obesity-related insulin resistance and ectopic lipid accumulation. Herein, we show that PMI5011 is effective in lowering insulin levels and improving HOMA-IR in mice fed a high-fat diet supplemented with PMI501,1 although body weight gain and percent fat mass were comparable to the high-fat diet (HFD) alone. Moreover, PMI5011 supplementation substantially reduced skeletal muscle and hepatic lipid accumulation. Thus, dietary intake of PMI5011 may be an effective approach for preventing obesity-induced metabolic dysfunction.
2. Experimental Section
2.1. Sourcing and Characterization of PMI5011 Extract
The PMI5011 botanical extract from Artemisia dracunculus L. was provided by the Botanical and Dietary Supplement Research Center at Pennington Biomedical Research Center. Detailed information about quality control, preparation, and biochemical characterization of PMI5011 was previously reported.[12,19–23] In our earlier studies, five active compounds were isolated from PMI5011 by activity guided fractionation with in vitro assays (Table 1). DMC-2 was active in more in vitro assays than the other compounds and was validated as having hypoglycemic activity in hyperglycemic mice. DMC-2 is therefore considered to be the primary marker bioactive compound. DMC-2 and sakuranetin were quantified in PMI5011as shown in Table 1. Concentrations of DMC-1 and davidigenin were approximated from the DMC-2 standard curve because they are not commercially available but have very similar chemical structures. The concentration of 6-demethoxycapillarisin was approximated from the sakuranetin standard curve because it is not commercially available and has a similar chemical structure. The fingerprints (mass spectral and UV) of these active compounds are very reproducible,[22] but the amounts of each compound and their relative abundance can vary to some degree depending on the batch of A. dracunculus crop that was used as the starting material and laboratory scale processing. Small variations in the plant material can result from seasonal/environmental conditions.
TABLE 1.
Approximate Concentrations of Active Components of PMI5011
| Active compound isolated from PMI5011 | Approximate Concentration |
|---|---|
| *2′,4′-dihydroxy-4-methoxydihydrochalcone (DMC-2) | 1.3 μg/ml |
| 2′,4-dihydroxy-4′-methoxydihydrochalcone (DMC-1) | 0.7 μg/ml |
| Davidigenin | 0.5 μg/ml |
| 6-demethoxycapillarisin | 1.2 μg/ml |
| * Sakuranetin | 2.6 μg/ml |
DMC-2 and sakuranetin are quantified using standard curves of pure compounds, other compounds are approximated from standard curves of DMC-2 (DMC-1, davidigenin) and sakuranetin (6-demethoxycapillarisin) because compounds are not available.
2.2. Experimental Animals
Male C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). All animal experiments were approved by the Pennington Biomedical Research Center Animal Care and Use Committee (protocol #922). The animals were singly housed in a controlled and monitored environment with a 12-hr light-dark cycle at 24 °C and 70% relative humidity. At 4 weeks of age, the mice were randomly assigned (n = 14 per group) to a defined low-fat diet (LFD; 10% kcal fat, Research Diets, #D12450H, matching sucrose levels to the HFD #D12451) or the low-fat diet supplemented with 1% (w/w) PMI5011. After 4 weeks, the mice were switched to an HFD (45% kcal fat, Research Diets, #D12451) or the HFD supplemented with PMI5011, formulated with the same mass PMI5011 kcal−1 as contained in the LFD and were fed ad libitum for 3 months thereafter. The 45% fat content is similar to the fat intake (30–40% of energy intake) for adult men and women in the United States.[24] Body weight and food intake were measured weekly and body composition was measured biweekly by NMR. Activity, food intake, and indirect calorimetry were measured at 12 weeks on each diet (TSE PhenoMaster). The mice were acclimated to the TSE chambers for 2 days prior to data collection over 4 days. At the end of the study, the mice were euthanized between 7–11 AM. Human insulin (Humulin, Eli Lilly, Indianapolis, IN) was administered to a subgroup of the control and PMI5011 mice (seven per group) at a dose of 1.5 U kg−1 10 min prior to euthanasia to assay insulin signaling.
2.3. Glucose and Insulin Tolerance Tests
For the glucose (GTT) and insulin (ITT) tolerance tests, the amount of glucose or insulin administered was normalized to body weight (37.7±0.91 g body weight) at 10 weeks on the HFDs. The mice were fasted 4 h[25,26] prior to administering 2 g kg−1 body weight of glucose/mouse (GTT) or 1 U kg−1 body weight of insulin/mouse (HumulinR) (ITT) by intraperitoneal injection.
2.4. Blood Chemistry
Glucose levels were measured in whole blood using a Breeze2 glucometer (Bayer, Leverkusen, Germany). Fasting insulin levels were assayed via ELISA (Crystal Chem, Downers Grove, IL). Serum nonesterified fatty acids (Abcam, Cambridge, MA), triglycerides (Eagle Diagnostics, CedarHill, TX), and total cholesterol (Cell Biolabs, San Diego, CA) levels were assayed according to manufacturers’ instructions. The index of homeostatis model assessment of insulin resistance, e.g., HOMA-IR [insulin (μU L−1) × glucose (mm)/22.5] of each animal was calculated from fasting glucose and insulin levels.[27] Triglyceride levels in skeletal muscle and liver were assayed according to Folch et al.[28] and reported as mg dL−1. The tissue was carefully cleaned of any visible fat before assaying triglyceride levels.
2.5. Immunohistochemistry
The gastrocnemius muscle and liver was fixed in 10% formalin, embedded in paraffin, and sectioned onto slides. The sections were hematoxylin and eosin (H&E) stained and scanned (NanoZoomer Digital Pathology, Hamamatsu Corp., Bridgewater, NJ).
2.6. Fatty Acid Oxidation Assay
Mixed gastrocnemius muscle homogenates were prepared as described.[29] Palmitate oxidation was assessed in the whole muscle homogenates as described by Hulver et al.[30] with 14CO2 collected over 60 min. When present, pyruvate was added at a final concentration of 10 mm. CO2 levels were normalized to total protein and palmitate oxidation reported as nmol CO2 mg protein−1 h−1.
2.7. Analysis of Protein Expression
Tissue from four randomly selected mice was used for protein expression analysis. The skeletal muscle and liver lysates were prepared from powdered tissue by homogenizing in 25 mm HEPES, pH 7.4, 1% Igepal CA630, 137 mm NaCl, 1 mm PMSF, 10 μgmL−1 aprotinin, 1 μgmL−1 pepstatin, 5 μgmL−1 leupeptin, 10 mm Na4P2O7, 100 mm NaF, and 2 mm NaVO4. The samples were centrifuged at 14 000 × g for 10 min at 4 °C. Protein concentrations were determined using a BCA assay (Thermo Fisher Scientific, Rockford, IL). The tissue supernatants (50 μg) were resolved by SDS-PAGE and subjected to immunoblotting using chemiluminescence detection (Thermo Fisher Scientific, Rockford, IL) and quantified as described.[31] Nitrocellulose membranes were incubated with antibodies (Supporting Information Table S1) for 1–2 h at room temperature or overnight at 4 °C as indicated.
2.8. Analysis of Gene Expression
Total RNA was purified from powdered skeletal muscle tissue or liver using Direct-zol RNA miniPrep (ZYMO Research, Irvine, CA). RNA (500 ng) was reverse transcribed using Multiscribe Reverse Transcriptase (Applied Biosystems, ThermoFisher Scientific, Waltham, MA) with random primers at 37 °C for 2 h. Realtime PCR was performed with PowerUP SYBR Green Master Mix (Applied Biosystems), using the 7900 Real-Time PCR system and universal cycling conditions (50 °C for 2 min; 95 °C for 10 min; 40 cycles of 95 °C for 15 s, and 60 °C for 1min; followed by 95 °C for 15 s, 60 °C for 15 s, and 95 °C for 15 s). The assays were performed in triplicate, the results were normalized to Cyclophilin B mRNA, and analyzed using the 2−ΔΔCT method with the control diet used as the calibrator. The gene list is provided in Supporting Information Table S2.
2.9. Statistical Analysis
Normal distribution of the data for glucose and insulin levels, food intake, and body weight was determined using the D’Agostino–Pearson omnibus K2 normality test. Western blot data was quantitated using Un-Scan-It software (version 3, Silk Scientific). Statistical significance was determined using a two-tailed t-test or repeated measures analysis of variance. All statistical analysis was carried out using JMP Pro13 (SAS Institute) and GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). Variability is expressed as the mean ± SD.
3. Results
3.1. Effect of PMI5011 on Body Composition
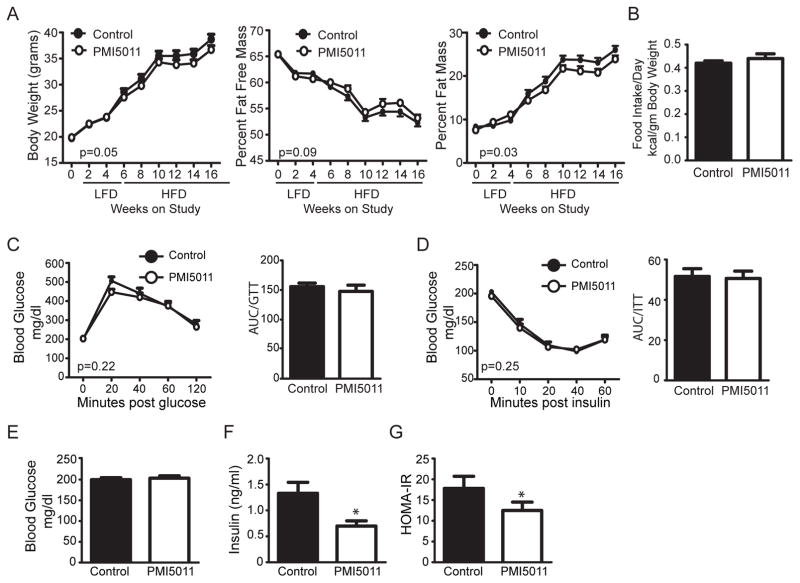
Previous studies established that PMI5011 enhances insulin signaling in the skeletal muscle and improves insulin sensitivity on a preexisting background of insulin resistance in vitro and in vivo.[14,16,17,19,32,33] To determine if obesity-related insulin resistance can be prevented by dietary supplementation with PMI5011, we carried out a feeding study in male C56BL/6J mice given a LFD alone or supplemented with PMI5011 (1% w/w) beginning at 4 weeks of age. After 1month, the diet was switched to a 45% HFD alone or supplemented with PMI5011and these diets were maintained for 3months. As shown in Figure 1A, PMI5011 supplementation led to a small decrease in body weight at 10 weeks on the HFD that was associated with a reduction in the percent fat mass while food intake is unaffected (Figure 1B).
Figure 1.
Dietary supplementation with an extract from Artemisia dracunculus L. (PMI5011) improves insulin levels in obese mice. A) Body weight, percent fat mass, and percent fat free mass were measured at the indicated time points in male mice fed a defined low-fat (LFD) or high-fat (HFD) diet alone (Control) or supplemented with PMI5011 (PMI5011). B) Food intake was monitored weekly and measured while in the TSE metabolic chambers. C) Glucose and D) insulin tolerance tests were performed at 10 weeks on the HFD alone (Control) or supplemented with PMI5011 (PMI5011). E) Glucose and F) insulin were assayed at the end of study and G) HOMA-IR was determined using the end of study insulin and glucose values. Statistical significance is reported as the mean ± SEM; compared to control, *p < 0.05.
We next assayed the effect of PMI5011 on glucose homeostasis and insulin sensitivity. Glucose tolerance testing shows PMI5011 does not significantly change the response to a glucose load (Figure 1C) and baseline glucose levels are unchanged by PMI5011 (Figure 1C and E). Although insulin tolerance testing indicates insulin sensitivity is not significantly enhanced by PMI5011 (Figure 1D), fasting insulin levels are reduced by dietary intake of PMI5011 (Figure 1F), consistent with decreased resistance to insulin’s action as indicated by the decreased HOMA-IR (Figure 1G).
3.2. Indirect Calorimetry in Mice Fed HFD Supplemented with PMI5011
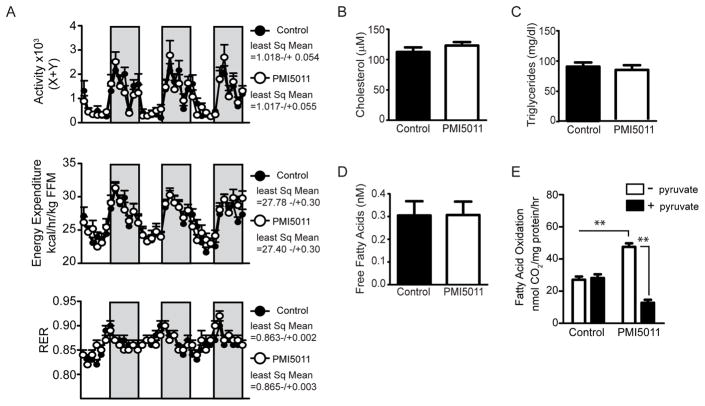
The small changes in body weight and fat mass when the HFD was supplemented with PMI5011 suggested PMI5011 affects energy balance, although energy intake was unchanged (Figure 1B). To assess energy expenditure, we carried out indirect calorimetry after 12 weeks of dietary supplementation. As shown in Figure 2A, activity and energy expenditure were not significantly altered by PMI5011 when averaged over 3 days, although energy expenditure trends upward by the third active period (dark bars, night). This is also the case for the respiratory exchange ratio (RER), a measure of metabolic flexibility, the ability to switch between carbohydrates or lipids as an energy source, depending on nutrient availability. Moreover, the reduced insulin levels in PMI5011-fed mice did not correlate with changes in circulating fatty acids, triglycerides, or cholesterol (Figure 2B–D).
Figure 2.
PMI5011 increases fatty acid oxidation and improves skeletal muscle metabolic flexibility, but does not affect the respiratory exchange ratio (RER) or the plasma lipid profile. A) Activity, energy expenditure, and RER were measured by indirect calorimetry at 12 weeks on the HFD alone or supplemented with PMI5011. Least squares means were determined using the data collected over 3 consecutive days. Energy expenditure is normalized to fat-free mass (FFM). Dark bars indicate 7PM–7AM. B) Cholesterol, C) triglycerides, and D) free fatty acids levels were determined at the end of the study. E) Fatty acid oxidation assays were carried out in mixed gastrocnemius muscle homogenates exposed to [1–14C] palmitate ± 10 mm pyruvate. Statistical significance is reported as the mean ± SD, **p < 0.01.
3.3. Dietary Intake of PMI5011 Increases Metabolic Flexibility in Skeletal Muscle Independent of Transcriptional Regulation of Fatty Acid Oxidation
The skeletal muscle is the primary site of insulin-stimulated glucose disposal and increased dietary lipid intake is closely linked with resistance to insulin action in the skeletal muscle. In the metabolic inflexibility associated with insulin resistance, the ability of skeletal muscle to switch from fatty acid uptake and oxidation during fasting to insulin-stimulated glucose uptake and oxidation with feeding is compromised.[34,35] Recent evidence further suggests that metabolic flexibility is not strictly a result of alternations in insulin signaling as rats fed an HFD exhibit impaired substrate switching at the level of the mitochondria.[36] To determine if the metabolic inflexibility suggested by the RER was reflected in a reduced ability to oxidize fatty acids in skeletal muscle, we assayed fatty acid oxidation in gastrocnemius muscle homogenate. The ability to switch between fat and carbohydrate oxidation was determined by assaying suppression of fatty acid oxidation in the presence of pyruvate (Figure 2E) as a surrogate for carbohydrate oxidation. Fatty acid oxidation in the control mice fed the HFD was not suppressed by pyruvate, likely suggesting the HFD induced metabolic inflexibility at the level of the mitochondria. When the HFD was supplemented with PMI5011, fatty acid oxidation increased and was significantly suppressed in the presence of pyruvate (Figure 2E). Importantly, PMI5011 supplementation increased the ability of skeletal muscle to oxidize fatty acids while consuming an HFD and prevented the development of mitochondrial metabolic inflexibility in the skeletal muscle.
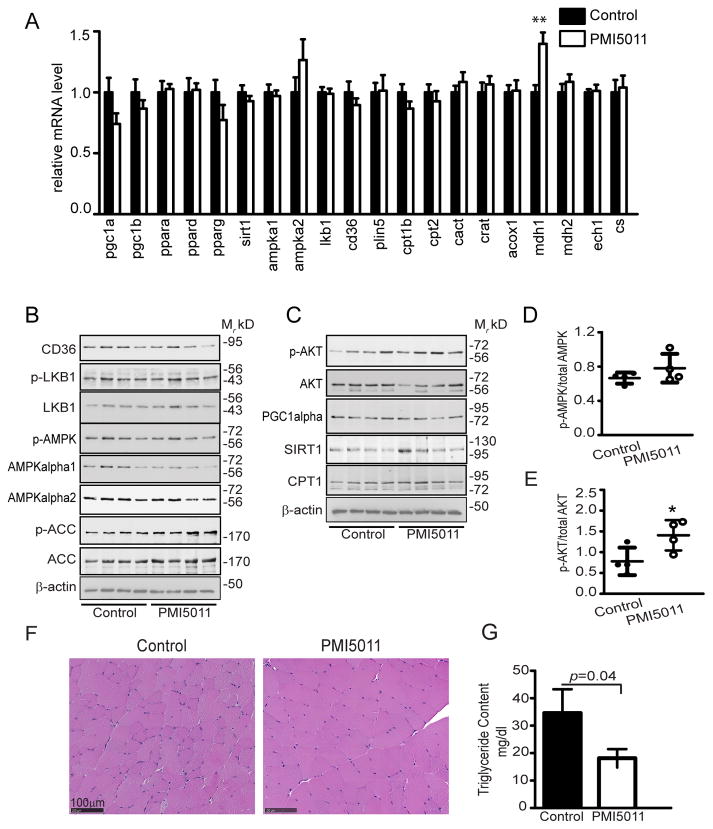
To determine if PMI5011-mediated changes in fatty acid oxidation are transcriptionally regulated, we assayed the expression of a broad panel of genes involved in lipid metabolism (Figure 3A, Supporting Information Table S2). Small, but statistically insignificant changes occur for transcriptional regulators of fatty acid oxidation (pgc-1alpha, pgc-1b, pparalpha, ppardelta, ppargamma, and sirt1) (Figure 3A). This pattern holds for genes encoding proteins involved in lipid uptake and storage (amp-kalpha1, ampkalpha2, lkbalpha, cd36, and plin5), fatty acid oxidation (cpt1beta, cpt2, cact, crat, acox1, mdh2, and ech1), or mitochondria biogenesis (citrate synthase, cs). However, expression of the gene encoding the cytoplasmic malate dehydrogenase (mdh1) that participates in the malate/aspartate shuttle was increased with PMI5011 supplementation.
Figure 3.
PMI5011 reduces lipid accumulation in skeletal muscle absent robust changes in expression of skeletal muscle factors involved in lipid metabolism. A) Expression of a panel of genes involved in lipid metabolism was assayed in mixed gastrocnemius muscle using qRT-PCR. B,C) Total and phospho-protein levels were determined mixed gastrocnemiusmuscle using a panel of proteins that regulate signaling events and transcriptional activity in fatty acid oxidation or insulin signaling in mice fed the HFD alone (Control) or supplemented with PMI5011 (PMI5011). D)Phosphorylation of AMPK relative to total AMPK (AMPKα1 and AMPKα2) and E) phosphorylation of AKT relative to total AKT was determined. F) H&E staining of gastrocnemius muscle mice fed a HFD alone (Control) or supplemented with PMI5011 (PMI5011). G) Triglyceride content was assayed in gastrocnemius and reported as mg dL−1. Statistical significance is reported as the mean ± SD, compared to control; *p < 0.05, **p < 0.01.
Consistent with gene expression, CD36, LKB1, AMPKα2, PGC1α, SIRT1, and CPT1 protein levels in skeletal muscle are not altered by PMI5011(Figure 3B and C, Supporting Information Figure S1). Notably, AMPK phosphorylation relative to total AMPKα1 andAMPKα2 trends upward with PMI5011 supplementation (Figure 3B and D) due to downregulation of AMPKα1 protein, while dietary intake of PMI5011 significantly increases AKT phosphorylation (Figure 3C and E) relative to total AKT protein.
3.4. PMI5011 Mediated Changes in Lipid Content of the Skeletal Muscle
To determine if increased fatty acid oxidation in the skeletal muscle corresponds to reduced lipid content, independent of transcriptional regulation of factors controlling uptake and oxidation of fatty acids, we evaluated the lipid content of the gastrocnemius muscle. Although changes in lipid accumulation are not apparent with H&E staining (Figure 3F), biochemical analysis shows triglyceride levels are significantly reduced when the HFD is supplemented with PMI5011 (Figure 3G).
3.5. PMI5011 Mediated Changes in Hepatic Gene and Protein Expression and Lipid Accumulation
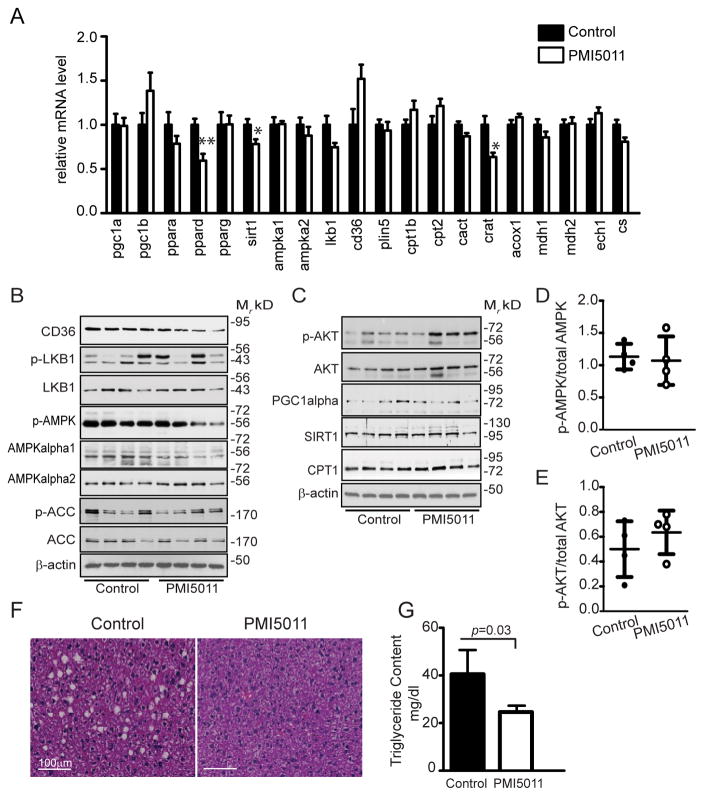
Given the role of ectopic lipid accumulation in the skeletal muscle and liver in the insulin resistance and dyslipidemia found in MetS, we also examined the effect of PMI5011 supplementation on the liver. As we observed in the skeletal muscle, many of the genes encoding proteins that regulate lipid metabolism are unaffected. However, there was a small, but significant decrease in ppardelta, sirt, and crat gene expression with PMI5011 supplementation (Figure 4A). Even so, a survey of the expression of the encoded proteins found that the regulation of gene expression by PMI5011 did not correspond to changes in protein expression. For example, SIRT1 was unchanged and hepatic levels of CD36 decreased with PMI5011 (Figure 4B, Supporting Information Figure S2). As noted in skeletal muscle, AMPK-α1 protein levels and AMPK phosphorylation are decreased with PMI5011. However, phosphorylation of AMPK relative to total AMPK is unaffected by PMI5011 (Figure 4D) owing to the parallel decrease in AMPK-α1 protein levels. Other regulators of lipid metabolism (LKB1, ACC, PCG-1α, and CPT1) were also unaffected by PMI5011 (Figure 4B, Supporting Information Figure S2). In contrast to the skeletal muscle, phosphorylation of AKT in the liver is not significantly altered with PMI5011 supplementation (Figure 4C and E).
Figure 4.
PMI5011-mediated decrease in hepatic lipid accumulation is not associated with robust changes in gene or protein expression of factors regulating lipid metabolism. A) Expression of a panel of genes involved in lipid metabolism was assayed in mixed gastrocnemius muscle using qRTPCR. B,C) Total and phospho-protein levels were determined in the liver using a panel of proteins that regulate signaling events and transcriptional activity in fatty acid oxidation or insulin signaling in mice fed the HFD alone (Control) or supplemented with PMI5011 (PMI5011). D) Phosphorylation of AMPK relative to total AMPK (AMPKα1 and AMPKα2) and E) phosphorylation of AKT relative to total AKT was determined. F) H&E staining of the liver from mice fed a HFD alone (Control) or supplemented with PMI5011 (PMI5011). G) Triglyceride content was assayed in the liver and reported as mg/dl. Statistical significance is reported as the mean ± SD, compared to control; *p < 0.05, **p < 0.01
Although reduced or unchanged mRNA and protein expression of a wide range of factors that regulate hepatic lipid metabolism (Figure 4A and B) suggested PMI5011 supplementation would not affect hepatic triglyceride levels, H&E staining and the hepatic triglyceride content show that hepatic lipid accumulation is significantly reduced in the high fat fedmice supplemented with PMI5011 (Figure 4F and G).
4. Discussion
Our earlier studies of botanically based treatment of insulin resistance indicate an extract from A. dracunculus L. (Russian tarragon) termed PMI5011 improves glucose metabolism via enhanced insulin action in skeletal muscle.[14,16,17,32,37] In the current study, we sought to determine if dietary supplementation with PMI5011 prior to the onset of HFD-induced obesity prevents development of obesity-related insulin resistance and MetS. PMI5011 suppresses ectopic lipid accumulation in the skeletal muscle and liver and improves insulin sensitivity in obese male mice fed an HFD without reducing body weight gain or affecting plasma levels of triglycerides and free fatty acids over a 20-week period.
Accumulation of visceral fat in obesity associated with insulin resistance is generally attributed to increased release of fatty acids from adipose tissue that has exceeded its capacity to store lipids. However, in agreement with our findings, other studies demonstrate that the level of circulating fatty acids is unrelated to insulin sensitivity in obesity.[38,39] Moreover, PMI5011-mediated improved insulin sensitivity and reduced ectopic lipid accumulation without substantial changes in body weight and adiposity is consistent with studies showing ectopic fat deposition in skeletal muscle and liver is more important than overall adiposity and body weight in the relationship between insulin resistance and MetS in obesity.[6,7]
Skeletal muscle is the largest contributor to whole-body glucose disposal and resistance to insulin’s action in skeletalmuscle is a primary feature of metabolic syndrome and type 2 diabetes.[40] In humans, obesity-related triglyceride accumulation in skeletal muscle is associated with insulin resistance and a reduced capacity of skeletal muscle to oxidize lipids as an energy source.[41] This is the case for the obese male mice when assayed by changes in the RER in response to nutritional cues. Although the RER indicated metabolic inflexibility at a whole body level, ex vivo assays of fatty acid oxidation in skeletal muscle homogenates show PMI5011 supplementation increased the capacity of the skeletal muscle to oxidize fatty acids. Furthermore, the improvement in skeletal muscle metabolic flexibility was reflected in the significantly reduced skeletal muscle lipid content of the PMI5011-fed male mice. Thus, the PMI5011-mediated change in skeletal muscle metabolic flexibility correlates with physiological outcomes associated with reduced risk of obesity-related metabolic syndrome.
As it stands, the mechanism by which PMI5011 increases fatty acid oxidation, improves skeletal muscle metabolic flexibility, and reduces skeletal muscle and hepatic lipid accumulation remains unclear. Fatty acid oxidation in the mitochondria requires a series of steps regulating uptake of fatty acids, transport into the cytoplasm, and entry into the mitochondria. The AMP-activated kinase (AMPK) is a key regulator of fatty acid oxidation via its interaction with the fatty acid translocase CD36,[42] followed by regulation of acetyl-CoA carboxylase (ACC) and activation of carnitine palmitoyltranferase 1 (CPT1) as well as transcriptional regulators of fatty acid oxidation such as PCG-1α.[43] The modest increase in AMPK activation in the skeletal muscle is not associated with increased ACC phosphorylation, a well-described target of AMPK activity. Changes in mRNA levels of factors affecting hepatic lipid metabolism are more robust that we observed in the skeletal muscle, but the changes are consistently less than twofold, indicating transcriptional regulation does not account for the effect of PMI5011 on skeletal muscle or hepatic lipid accumulation. Moreover, the generally downward trend in expression of a broad panel of genes that regulate fatty acid oxidation suggests these changes are compensatory in response.
Upregulation of the cytoplasmic malate dehydrogenase-1 (mdh1) mRNA in skeletal muscle points to possible transcriptional regulation of mitochondrial function by PMI5011. MDH-1 catalyzes the reduction of oxaloacetate to malate in the cytoplasm to transport reducing equivalents across the mitochondrial membrane for ATP production.[44] Petersen et al.[45] found that insulin resistance and intramuscular lipid accumulation can be attributed to dysregulation of fatty oxidation due to impaired ATP production rather than changes in lipid uptake. This raises the possibility that PMI5011-mediated increased fatty acid oxidation in the skeletal muscle and insulin responsiveness is related to changes in MDH1 activity to improve mitochondrial function. A limitation of our study is that mitochondria were not isolated to assess mitochondrial function or posttranslational modifications of mitochondrial proteins that regulate fatty acid oxidation such as SIRT-1 dependent acetylation.[46] Along these lines, there is evidence that acetylation of MDH1 enhances its catalytic activity in 3T3-L1 adipocytes.[47]
However, dietary intake of PMI5011 is associated with improved insulin sensitivity indicated by decreased insulin levels, the corresponding lower HOMA-IR, and increased AKT phosphorylation in the skeletal muscle. This is consistent with our earlier findings that PMI5011 enhances insulin signaling and improves glucose metabolism in obesity-related insulin resistance.[14,16,17] Moreover, enhanced insulin signaling and reduced lipid accumulation in skeletal muscle indicates the PMI5011-mediated stimulation of fatty acid oxidation does not lead to production of the reactive lipid intermediates that are reported to inactivate insulin signaling.[48] PMI5011-mediated enhanced insulin signaling in skeletal muscle may also indirectly account for the substantial improvement in hepatic lipid accumulation by reducing the amount of glucose available to drive hepatic lipogenesis[9,49] in the presence of obesity. A more direct effect of PMI5011 on hepatic lipid accumulation is suggested by the decrease in CD36 protein levels. Wilson et al.[50] report that reduced hepatic CD36 levels are associated with less hepatic lipid accumulation and improved insulin sensitivity in high fat fed mice. This raises the interesting possibility that PMI5011 supplementation reduces fatty acid uptake in the liver to affect lipid accumulation. Together, our data underscores the potential for dietary supplementation with PMI5011 in maintaining insulin sensitivity and preventing development of obesity related risk factors for MetS, such as ectopic skeletal muscle and hepatic lipid accumulation in the presence of increased body weight.
Supplementary Material
Acknowledgments
Conceived and designed the experiments: W.T.C., Z.E.F.; performed the experiments: Y.Y., T.M.M., A.P., Z.E.F.; Analyzed the data: Y.Y., T.M.M., R.L.M., R.C.N., Z.E.F.; Contributed reagents/materials/analysis tools: R.C.N., A.P., D.M.R., I.R.; Wrote original draft of manuscript or review and edited the manuscript: Y.Y., R.C.N., I.R., D.M.R., Z.E.F. This research is supported by the National Center for Complementary and Integrative Health and the Office of Dietary Supplements of the National Institutes of Health under Award Number P50AT002776 that funds the Botanical and Dietary Supplement Research Center of Pennington Biomedical Research Center and the Department of Plant Biology and Pathology in the School of Environmental and Biological Sciences (SEBS) of Rutgers University. The work also used Cell Biology Imaging core facilities that are supported in part by the Pennington Biomedical Research Center COBRE (NIH8 1P30GM118430-01) Center Grant from the National Institute of Health and was partially supported by the Pennington Biomedical Research Center NORC Center Grant P30 DK072476.
Abbreviations
- AKT
protein kinase B
- AMPK
AMP-activated protein kinase
- HFD
high fat diet
- LFD
low fat diet
- MetS
metabolic syndrome
- PMI5011
experimental designation of the extract
- RER
respiratory exchange ratio
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Yongmei Yu, Pennington Biomedical Research Center, Baton Rouge, LA.
Tamra M. Mendoza, Pennington Biomedical Research Center, Baton Rouge, LA
Dr. David M. Ribnicky, Department of Plant Biology, Rutgers University, New Brunswick, NJ
Dr. Alexander Poulev, Department of Plant Biology, Rutgers University, New Brunswick, NJ
Dr. Robert C. Noland, Pennington Biomedical Research Center, Baton Rouge, LA
Dr. Randall L. Mynatt, Pennington Biomedical Research Center, Baton Rouge, LA
Dr. Ilya Raskin, Department of Plant Biology, Rutgers University, New Brunswick, NJ
Dr. William T. Cefalu, Pennington Biomedical Research Center, Baton Rouge, LA.
Dr. Z. Elizabeth Floyd, Pennington Biomedical Research Center, Baton Rouge, LA
References
- 1.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. JAMA. 2015;313:1973. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 2.Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S. J Am Coll Cardiol. 2013;62:697. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuk JL, Ardern CI. Diabetes Care. 2010;33:2457. doi: 10.2337/dc10-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neill S, O’Driscoll L. Obes Rev. 2015;16:1. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 5.Cuthbertson DJ, Steele T, Wilding JP, Halford JC, Harrold JA, Hamer M, Karpe F. Int J Obes (Lond) 2017;41:853. doi: 10.1038/ijo.2017.4. [DOI] [PubMed] [Google Scholar]
- 6.Yki-Jarvinen H. J R Soc Med. 2002;95:39. [PMC free article] [PubMed] [Google Scholar]
- 7.Grundy SM. Eur J Clin Invest. 2015;45:1209. doi: 10.1111/eci.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalle Grave R, Calugi S, Centis E, Marzocchi R, El Ghoch M, Marchesini G. Diabetes Metab Syndr Obes. 2010;3:373. doi: 10.2147/DMSOTT.S13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuel VT, Shulman GI. Cell Metab. 2018;27:22. doi: 10.1016/j.cmet.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicoletti P, Aithal GP, Bjornsson ES, Andrade RJ, Sawle A, Arrese M, Barnhart HX, Bondon-Guitton E, Hayashi PH, Bessone F, Carvajal A, Cascorbi I, Cirulli ET, Chalasani N, Conforti A, Coulthard SA, Daly MJ, Day CP, Dillon JF, Fontana RJ, Grove J, Hallberg P, Hernández N, Ibáñez L, Kullak-Ublick GA, Laitinen T, Larrey D, Lucena MI, Maitland-van der Zee AH, Martin JH, Molokhia M, Pirmohamed M, Powell EE, Qin S, Serrano J, Stephens C, Stolz A, Wadelius M, Watkins PB, Floratos A, Shen Y, Nelson MR, Urban TJ, Daly AK International Drug-Induced Liver Injury Consortium, Drug-Induced Liver Injury Network Investigators International Serious Adverse Events Consortium. Gastroenterology. 2016;152:1078. [Google Scholar]
- 11.Stafylas PC, Sarafidis PA, Lasaridis AN. Int J Cardiol. 2009;131:298. doi: 10.1016/j.ijcard.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt B, Ribnicky DM, Poulev A, Logendra S, Cefalu WT, Raskin I. Metabolism. 2008;57:S3. doi: 10.1016/j.metabol.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanston-Flatt SK, Flatt PR, Day C, Bailey CJ. Proc Nutr Soc. 1991;50:641. doi: 10.1079/pns19910077. [DOI] [PubMed] [Google Scholar]
- 14.Kheterpal I, Coleman L, Ku G, Wang ZQ, Ribnicky D, Cefalu WT. Phytother Res. 2010;24:1278. doi: 10.1002/ptr.3093. [DOI] [PubMed] [Google Scholar]
- 15.Obanda DN, Cefalu WT. J Nutr Biochem. 2013;24:1529. doi: 10.1016/j.jnutbio.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang ZQ, Ribnicky D, Zhang XH, Raskin I, Yu Y, Cefalu WT. Metabolism. 2008;57:S58. doi: 10.1016/j.metabol.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang ZQ, Ribnicky D, Zhang XH, Zuberi A, Raskin I, Yu Y, Cefalu WT. J Nutr Biochem. 2011;22:71. doi: 10.1016/j.jnutbio.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer IP, Irmler M, Meyer CW, Sachs SJ, Neff F, de Angelis MH, Beckers J, Tschop MH, Hofmann SM, Ussar S. Int J Obes (Lond) 2017 doi: 10.1038/ijo.2017.224. https://doi.org/10.1038/ijo.2017.224. [DOI] [PMC free article] [PubMed]
- 19.Ribnicky DM, Poulev A, Watford M, Cefalu WT, Raskin I. Phytomedicine. 2006;13:550. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Ribnicky DM, Kuhn P, Poulev A, Logendra S, Zuberi A, Cefalu WT, Raskin I. Int J Pharm. 2009;370:87. doi: 10.1016/j.ijpharm.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuberi AR. Metabolism. 2008;57:S10. doi: 10.1016/j.metabol.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logendra S, Ribnicky DM, Yang H, Poulev A, Ma J, Kennelly EJ, Raskin I. Phytochemistry. 2006;67:1539. doi: 10.1016/j.phytochem.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Govorko D, Logendra S, Wang Y, Esposito D, Komarnytsky S, Ribnicky D, Poulev A, Wang Z, Cefalu WT, Raskin I. Am J Physiol Endocrinol Metab. 2007;293:E1503. doi: 10.1152/ajpendo.00420.2007. [DOI] [PubMed] [Google Scholar]
- 24.U. S. D. A. Service. Nutrient Intakes from Food: Mean Amounts of Consumed per Individual, by Gender and Age, What We Eat in America, NHANES 2009–2010. U. S. D. A. Service; Sutton, TX: 2012. [Google Scholar]
- 25.McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. Am J Physiol Endocrinol Metab. 2009;297:E849. doi: 10.1152/ajpendo.90996.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Am J Physiol Endocrinol Metab. 2008;295:E1323. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Diabetologia. 1985;28:412. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Folch J, Lees M, Sloane Stanley GH. J Biol Chem. 1957;226:497. [PubMed] [Google Scholar]
- 29.Noland RC, Woodlief TL, Whitfield BR, Manning SM, Evans JR, Dudek RW, Lust RM, Cortright RN. Am J Physiol Endocrinol Metab. 2007;293:E986. doi: 10.1152/ajpendo.00399.2006. [DOI] [PubMed] [Google Scholar]
- 30.Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, MacDougald KG, Cline GW, Shulman GI, Dohm GL, Hormard JA. Am J Physiol Endocrinol Metab. 2003;284:E741. doi: 10.1152/ajpendo.00514.2002. [DOI] [PubMed] [Google Scholar]
- 31.Wang ZQ, Floyd ZE, Qin J, Liu X, Yu Y, Zhang XH, Wagner JD, Cefalu WT. Diabetes. 2009;58:1488. doi: 10.2337/db08-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kheterpal I, Scherp P, Kelley L, Wang Z, Johnson W, Ribnicky D, Cefalu WT. Nutrition. 2014;30:S43. doi: 10.1016/j.nut.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandanmagsar B, Haynie KR, Wicks SE, Bermudez EM, Mendoza TM, Ribnicky D, Cefalu WT, Mynatt RL. Diabetes Obes Metab. 2014;16:728. doi: 10.1111/dom.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley DE, Mandarino LJ. Diabetes. 2000;49:677. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 35.Galgani JE, Moro C, Ravussin E. Am J Physiol Endocrinol Metab. 2008;295:E1009. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. J Biol Chem. 2009;284:22840. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obanda DN, Ribnicky DM, Raskin I, Cefalu WT. Nutrition. 2014;30:S59. doi: 10.1016/j.nut.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez TL, Sutherland JP, Wolfe P, Allian-Sauer M, Capell WH, Talley ND, Wyatt HR, Foster GD, Hill JO, Eckel RH. Am J Clin Nutr. 2010;91:578. doi: 10.3945/ajcn.2009.27909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karpe F, Dickmann JR, Frayn KN. Diabetes. 2011;60:2441. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeFronzo RA, Tripathy D. Diabetes Care. 2009;32:S157. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Am J Physiol. 1999;277:E1130. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 42.Samovski D, Sun J, Pietka T, Gross RW, Eckel RH, Su X, Stahl PD, Abumrad NA. Diabetes. 2015;64:353. doi: 10.2337/db14-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomson DM, Winder WW. Acta Physiol (Oxf) 2009;196:147. doi: 10.1111/j.1748-1716.2009.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minarik P, Tomaskova N, Kollarova M, Antalik M. Gen Physiol Biophys. 2002;21:257. [PubMed] [Google Scholar]
- 45.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. N Engl J Med. 2004;350:664. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. FEBS Lett. 2008;582:46. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim EY, Kim WK, Kang HJ, Kim JH, Chung SJ, Seo YS, Park SG, Lee SC, Bae KH. J Lipid Res. 2012;53:1864. doi: 10.1194/jlr.M026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Cell Metab. 2008;7:45. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI. Proc Natl Acad Sci USA. 2007;104:12587. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson CG, Tran JL, Erion DM, Vera NB, Febbraio M, Weiss EJ. Endocrinology. 2016;157:570. doi: 10.1210/en.2015-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.