Abstract
Purpose
Cranial irradiation with 56Fe, a form of space radiation, causes hippocampus-dependent cognitive changes. 56Fe irradiation also increases reactive oxygen species (ROS) levels, which may contribute to these changes. Therefore, we investigated the effects of the antioxidant alpha lipoic acid (ALA) on cognition following sham-irradiation and irradiation.
Material and Methods
Male mice were irradiated (brain only) with 56Fe (3Gy) or sham-irradiated at 6–9 months of age. Half of the mice remained fed a regular chow and the other half of the mice were fed a caloric-matched diet containing ALA starting two-weeks prior to irradiation and throughout cognitive testing. Following cognitive testing, levels of 3-nitrotyrosine (3NT), a marker of oxidative protein stress, and levels of microtubule-associated protein (MAP-2), a dendritic protein important for cognition, were assessed using immunohistochemistry and confocal microscopy.
Results
ALA prevented radiation-induced impairments in spatial memory retention in the hippocampal and cortical dependent water maze probe trials following reversal learning. However, in sham-irradiated mice, ALA treatment impaired cortical-dependent novel object recognition and amygdala-dependent cued fear conditioning.
Conclusion
ALA might have differential effects on the brain under normal physiological conditions and those involving environmental challenges such as cranial irradiation.
Keywords: 56Fe irradiation, antioxidants, cognition, wild-type mice, sex
Introduction
During space missions, astronauts are exposed to high energy charged 56Fe particles. This is associated with long-term cognitive changes related to the dose delivered to the medial temporal lobe, the site of the hippocampus, a critical brain structure for learning and memory [1]. These changes might involve alterations in the level of reactive oxygen species (ROS), which are a major component of oxidative stress and increased by irradiation [2]. Although ROS are involved in learning and memory, a prolonged increase in ROS can lead to cell damage and cell death through oxidation of cellular components such as lipids, proteins and DNA [3]. Because of its high oxygen consumption and relatively low levels of antioxidants, the brain is particularly vulnerable to ROS damage. When the production of ROS exceeds the ability of the cell to repair itself and is greater than what the antioxidants can scavenge, oxidative stress occurs. Restoration of the balance between ROS and antioxidants may prevent oxidative damage. Therefore, supplementation of antioxidants might also ameliorate the effects of irradiation on cognitive function.
The antioxidant, α-lipoic acid (ALA) can directly scavenge ROS and it appears to restore age-related reductions in the antioxidant glutathione. ALA also participates in the recycling of vitamin C and vitamin E and is able to chelate redox active transition metals thereby inhibiting production of other ROS such as hydrogen peroxide and the hydroxyl radical. In addition to its antioxidant properties, ALA in its reduced form, dihydrolipoic acid (DHLA), increases acetylcholine production by activation of choline acetyltransferase and appears to increase glucose uptake in insulin-resistant neurons. Because ROS are also required for learning and memory, the potential effects of ALA on the cognitive function of sham-irradiated and irradiated mice are not clear.
The protective properties of ALA on the brain have been observed under a variety of challenges including irradiation [2]. ALA (200mg/kg, i.p.) administered to mice prior to whole body 56Fe irradiation (1.5 Gy), prevented radiation-induced deficits in water maze reference memory and attenuates measures of oxidative stress in the cerebellum. The purpose of this study was to assess whether ALA can also prevent radiation-induced impairments in other cognitive domains. To assess the effects of ALA and irradiation treatments on oxidative stress, levels of 3-nitrotyrosine were assessed in brain regions important for the assessed behaviors using immunohistochemical methods.
Microtubule associated protein 2 (MAP2), a protein important for the assembly of microtubules, particularly in the dendritic arbor, is associated with changes in learning and memory [4]. Therefore, the effects of ALA on MAP-2 immunoreactivity in the hippocampus and cortex of behaviorally tested sham-irradiated and irradiated mice were assessed as well.
Materials and Methods
Mice
Male C57Bl/6J mice were bred and housed in our colony. Food and water were provided ad libitum, and there was a constant 12-on/12-off light cycle (on at 6:00 am; off at 6:00 pm). At approximately 6–9 months of age, mice were maintained on a regular chow diet (PicoLab Rodent Diet 20, #5053; PMI Nutrition International, St. Louis, MO) or placed on a matched diet to which ALA was added (Rodent Pico Chow 20 + 0.165 % LA, Animal Specialties Inc ,Woodburn, OR (part of PMI)) (see supplementary data for spec sheets). The concentration of ALA used in the current study was based on previous studies showing neuroprotective effects when this concentration was administered through the diet for up to 6 months [5]. Three weeks later the mice were sham-irradiated or irradiated, and three months after irradiation the mice were behaviorally tested, as described below.
56Fe Irradiation
Two weeks after placing mice on the selected diets, they were shipped from our facility to Brookhaven National Laboratories (BNL) in Upton NY and allowed to acclimate for 1 week before sham-irradiation or irradiation. Following i.p. anesthesia (ketamine (Sigma), 80 mg/kg and xylazine (Sigma), 20 mg/kg), mice were sham-irradiated (n = 7–8 per diet) or head only irradiated (n = 6–7 per diet) with 56Fe at a dose of 3 Gy (600 MeV/amu). Sham-irradiated mice underwent the same procedure except that they were not irradiated. Four days later, the mice were shipped back to our facility. Mice remained on their selected diets throughout behavioral testing. All procedures were according to the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committees of our facility and BNL.
Behavioral Testing
Behavioral testing began 3 months following sham-irradiation or irradiation. Cognitive function was assessed using the novel location and novel object recognition tests, contextual and cued fear conditioning test, and water maze test, as described in the supplement.
Immunohistochemistry
Following behavioral testing, the mice were processed for 3NT and MAP-2 immunohistochemistry, as summarized in the supplement.
Results
Condition of mice during experimental procedures
ALA fed sham-irradiated and irradiated mice did not show any signs of distress or sick-like behaviors. Furthermore, there were no group differences in locomotor activity in any of the behavioral tests (see also Table 1, supplement) to suggest any potential physical adverse effects of either irradiation or ALA treatment.
Measures of exploratory and anxiety-like behaviors in the open field and elevated zero maze tests
There were no effects of irradiation, ALA or interaction between the two treatments for percent time spent in the center region of the chamber, entries made into the center area or distance moved in the open field test (Table 1, supplement), indicating that neither treatment affected exploratory or anxiety-like behaviors. There was also no effect of irradiation, ALA, or interaction between the treatments for time spent in the open areas of the maze, entries into the open areas or distance moved in the elevated zero maze (Table 1, supplement). Thus, the results of both the open field test and the elevated zero maze test indicate that neither ALA nor irradiation affected exploratory or anxiety-like behaviors.
Novel location and novel object recognition tasks
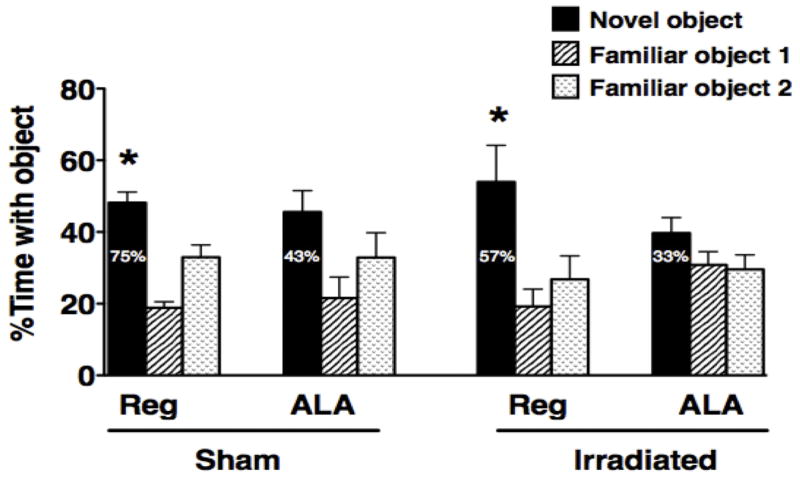
None of the groups of mice exhibited novel location recognition, i.e. exploration of a familiar object in a novel location (data not shown) but some groups did show novel object recognition. To detect novel location recognition may require a more sensitive paradigm. There might be a greater motivation to explore a displaced object than motivation to explore a novel object. In the cortical-dependent novel object recognition task, sham-irradiated and irradiated mice receiving the regular diet showed novel object recognition and spent more time exploring the novel object than either familiar object but ALA-fed sham-irradiated and irradiated mice did not (sham-irradiated control diet, F2,23 = 27.83; P < 0.001; irradiated control diet, F2,20 = 5.93; P < 0.01, Dunnett’s post-hoc, Fig. 1). As there are three objects in this test, change levels would be 33%. Similarly, the majority of mice on regular diet explored the novel object more than the familiar objects whereas the majority of ALA-fed mice did show this preference (Fig. 1).
Figure 1.
Novel Object Recognition of sham-irradiated and irradiated mice receiving a regular diet or a diet containing ALA. *P < 0.05 versus the other two objects.
Hippocampus- and amygdala-dependent conditioned fear
There were no pre-existing differences between the four different treatment groups on freezing behavior or distance moved prior to fear conditioning training as assessed by analysis of the first two minutes of the training trial before mice received the tone or shock (data not shown).
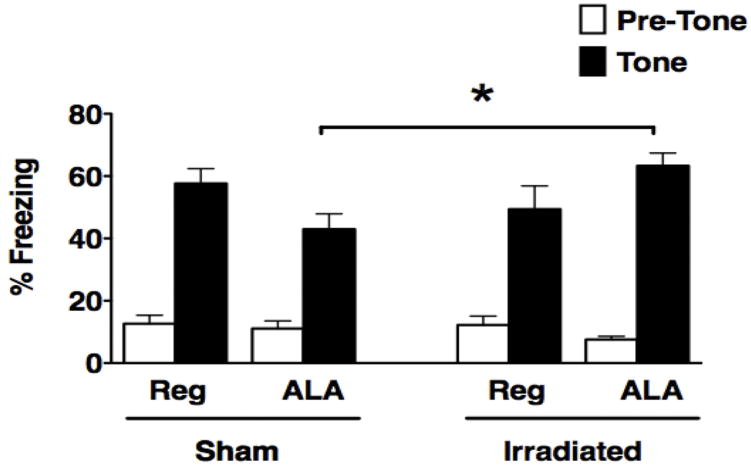
There was no overall effect of irradiation, diet or significant group interactions observed during contextual fear conditioning on day two when the mice were reintroduced to the fear conditioning chamber (data not shown). However, in the cued fear conditioning test, there was a diet by irradiation interaction (F1,26 = 5.2; P < 0.05, Fig. 2). While ALA reduced fear conditioning is sham-irradiated mice it enhanced cued fear conditioning in irradiated mice. As a consequence, within ALA-fed mice, sham-irradiated mice froze less in response to the tone compared to irradiated mice (P < 0.05, Fisher’s PLSD). This effect of irradiation was not observed in regular diet-fed mice.
Figure 2.
Cued fear conditioning of sham-irradiated and irradiated mice receiving a regular diet or a diet containing ALA. Freezing measures of sham-irradiated and irradiated female and male mice with or without ALA supplementation during the pretone (white bars) and tone (black bars) in the cued fear conditioning task are shown. *P < 0.05.
Spatial learning and memory in the water maze
There were no group differences in swim speeds during the visible platform training sessions in the water maze. Therefore, latency to the platform was used as a measure of performance. All groups of mice learned the task and improved their performance during the visible platform training (F2.3,55.1 = 77.99; P < 0.001, Greenhouse-Geisser, repeated measures ANOVA), and there were no significant effects of the treatments or interactions between them (data not shown).
During the hidden platform training sessions, all groups of mice learned the first platform location (effect of session: F3,72 = 23.95; P < 0.001, repeated measures ANOVA) and the second platform location (effect of session: F2.05,49.29= 11.90; P < 0.001, Greenhouse-Geiser, repeated measures ANOVA). There were no interactions between irradiation and diet for test performance during the sessions. Analysis of the percent time spent swimming in the outer zone (thigmotaxic behavior) showed no difference in thigmotaxis during the visible platform sessions (data not shown). However, during the first hidden platform training sessions, there was a significant effect of diet on thigmotaxis in session 5 (session x diet interaction, F3,72 = 3.38; P < 0.05). Mice receiving the ALA-containing diet spent more time in the peripheral zone of the pool than mice receiving the control diet (18.1 ± 2.3% versus 11.6 ± 2.1%, respectively). However, a group difference in thigmotaxic behavior was only observed in this first hidden platform session. There were no group differences in thigmotaxic behavior during the training sessions for the second hidden platform location.
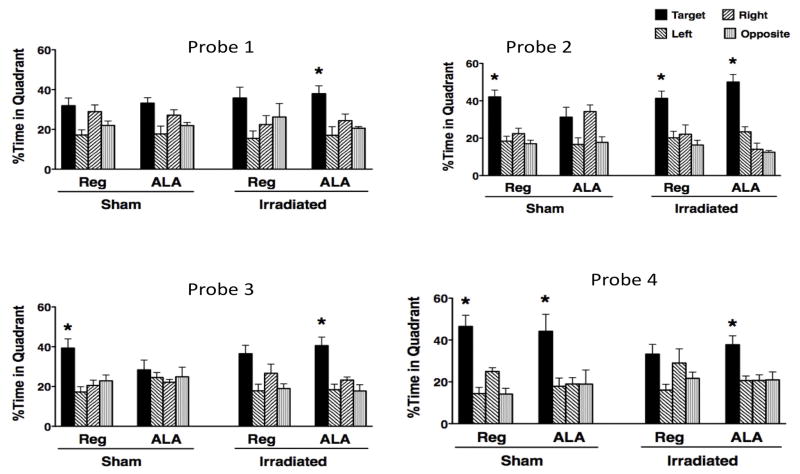
In the first probe trial, following one day of training for the first platform location, only irradiated mice on the ALA diet showed a significant preference for the target quadrant (P < 0.05, Probe 1, Fig. 3). Upon further training, all groups of mice except sham-irradiated ALA fed mice showed a target preference during the second probe trial (Probe 2, Fig. 3).
Figure 3.
Spatial memory retention of sham-irradiated and irradiated mice receiving a regular diet or a diet containing ALA in for the first hidden platform location (Probes 1 and 2) and second hidden platform location (Probes 3 and 4) in the water maze probe trials is shown. Spatial memory retention was measured as the percent time spent in the target quadrant where the platform was previously located compared to the other three non-target quadrants. *P < 0.05 versus the three non-target quadrants.
In the first probe trial following reversal training (Probe 3, Fig. 3), sham-irradiated mice on regular diet and irradiated ALA-fed mice were the only groups of mice that showed a target quadrant preference during the third probe trial (P < 0.05). Upon further training, all groups of mice except irradiated mice receiving the regular diet showed a target preference in the fourth probe trial (Probe 4, Fig. 3). Similar effects of irradiation and ALA diet on the water maze probe trials were observed when the data were analyzed using cumulative distance to the platform as the performance measure (data not shown).
3NT Immunoreactivity
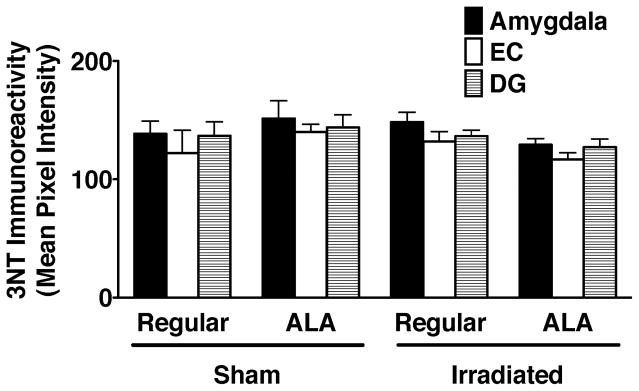
Next, we determined potential effects of irradiation and diet on the mean pixel intensity of 3NT immunoreactivity in the dentate gyrus, enthorhinal cortex, and amygdala (Fig. 4). While there were no significant effects of irradiation or diet, there was a trend towards lower 3NT levels in irradiated mice receiving a diet containing ALA than irradiated mice receiving a regular diet. In sham-irradiated mice, the opposite pattern is seen: higher 3NT levels in sham-irradiated mice receiving ALA than sham-irradiated mice receiving a regular diet.
Figure 4.
3NT immunoreactivity in the amygdala, enthorhinal cortex, and dentate gyrus of sham-irradiated and irradiated mice receiving a regular diet or a diet containing ALA. Mean pixel intensities are shown.
MAP-2 Immunoreactivity
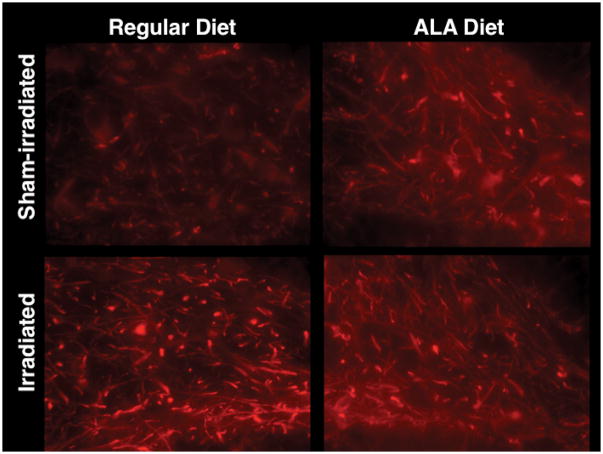
For MAP-2 immunoreactivity, there was an interaction between brain region, irradiation and diet (F4,44 = 3.52; P < 0.05). Representative images are shown in Fig. 5. In the hippocampal dentate gyrus of mice on regular diet, irradiated mice had increased MAP-2 immunoreactivity compared to sham-irradiated mice (F1,7 = 9.23; P < 0.05, Fig. 6). In sham-irradiated mice, ALA appeared to also increase MAP-2 immunoreactivity but this effect did not reach significance (P = 0.17). None of the other brain regions assessed showed similar increases in MAP-2 immunoreactivity following irradiation (data not shown).
Figure 5.
Representative images of MAP-2 immunoreactivity in the dentate gyrus of sham-irradiated and irradiated mice receiving a regular diet or a diet containing ALA.
Figure 6.
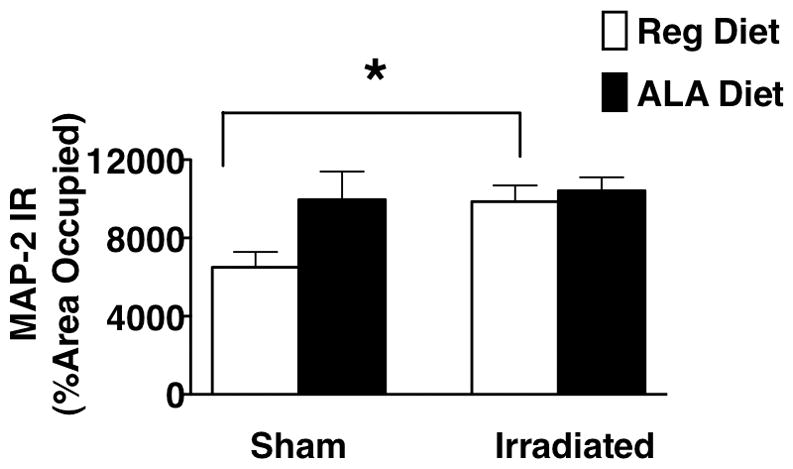
MAP-2 immunohistochemistry (IR) in the dentate gyrus of sham-irradiated and irradiated male with or without LA supplementation. In non-LA supplemented male mice, irradiation increased MAP-2 IR in the dentate gyrus of the hippocampus (*P < 0.05 versus sham-irradiated non-LA supplemented mice, Fisher’s PLSD following ANOVA).
Discussion
In this study we show that ALA prevents radiation-induced impairments in spatial memory retention in the water maze probe trials following reversal learning. However, in sham-irradiated mice ALA treatment impairs novel object recognition and cued fear conditioning. Thus, ALA might have differential effects under normal physiological conditions and those involving environmental challenges such as cranial irradiation.
In the water maze task, 56Fe irradiation to the brain only disrupted water maze probe trial performance when the task was made more challenging with reversal learning. This effect was prevented by ALA. The finding that the mice showed intact spatial memory retention for the first platform location indicates that, under the tested experimental conditions, cognitive flexibility might be particular sensitive to the effects of irradiation.
An adverse effect of ALA was observed in sham-irradiated mice in the first three but not fourth water probe trials. This suggests that sham-irradiated mice can overcome ALA-induced deficits when given enough training. In addition, poor memory for the first platform location (Probes 1 and 2) might have facilitated learning and memory of the new platform location, resulting in a preference for the target quadrant in Probe 4. However, poor memory retention for the new platform location during early training in the third probe trial suggests that poor memory retention for the first platform location is not sufficient to acquire memory for the second platform location. Similar to the water maze data, ALA consumption by sham-irradiated mice adversely affected performance on the amygdala-dependent cued fear conditioning task. Irradiation also reversed ALA-induced impairments in this task. Thus, emotional learning and memory in the amygdala might also be sensitive to ALA.
Compounds with antioxidant activity other than ALA are also being considered to mitigate radiation effects, for example inhibitors of angiotensin-converting enzyme (ACE) [6]. In the brain, the renin-angiotensin system (RAS) is found in neurons and glia and release renin and angiotensinogen. They interact to generate biologically-inactive angiotensin I that is cleaved by ACE to form biologically-active angiotensin II (AII). AII binds to AII type-1 and type-2 receptors (AT1R and AT2R) that are highly expressed in the hippocampus and can enhance apoptosis, inflammation, and oxidative stress. Following focal irradiation at a dose of 30 Gy, ACE inhibitors were shown to mitigate the development of white matter necrosis in the optic tract [7]. ACE inhibitors show therapeutic promise as mitigators of chronic radiation injuries. Following total body irradiation, they were shown to mitigate chronic renal failure in rats [8, 9]. Consistent with these animal studies, in patients undergoing total body irradiation and hematopoietic stem cell transplantation, ACE inhibitors were shown to be safe and efficacious in mitigating chronic renal failure [10]. However, in mice ACE inhibitors worsened the functional and histopathologic damage in kidneys after injection of an in vivo generator of alpha- and beta-particle emitting elements [11]. A related type of compound, an AT1 receptor antagonist (L-158,809), offered moderate mitigation of radiation injury to mouse kidneys [11]. When used prior to, during, and after fractionated brain irradiation, the same AT1 receptor antagonist mitigated radiation-induced impairments in novel object recognition in rats [12]. However, while not significant in the overall analysis, there was a clear trend for the AT1 receptor antagonist to reduce novel object recognition in sham-irradiated rats [12], consistent with the detrimental effects of ALA on novel object recognition in sham-irradiated mice observed in the current study.
Statins, which block the rate-limiting step of cholesterol biosynthesis and are widely used to treat hypercholesterolemia and atherosclerosis, are also being considered as mitigators for radiation injury based on their ability to reduce inflammation [13] and oxidative stress [14], including oxidative stress caused by AII. For example, statins have been shown to mitigate radiation-induced pneumonitis [15] and to delay intestinal radiation injury in rats [16]. The effects of statins on cognition under baseline conditions and following radiation are less clear. A combined diet containing the ACE inhibitor Ramipril (via drinking water) and Atorvastatin (via diet) did show mitigation of the radiation-induced reduction in doublecortin-positive immature neurons in the dentate gyrus following a dose of 10 Gy 137Cs [17]. However, the combined Ramipril/Atorvastatin diet had no mitigating effects on radiation-induced changes in contextual fear conditioning (Raber et al, unpublished observations). In addition, when Ramipril or Atorvastatin were started 24 hours following cranial irradiation with 15 Gy 137Cs, they did not mitigate contextual fear conditioning and impaired cued fear conditioning (Raber et al, unpublished observations). These divergent data, rat [13, 17] (and personal communication Dr. Jenrow) vs. mouse [12] (and current study) could be due to the time point of initiation of treatment, type (ACE inhibitor versus AT1 receptor antagonist) and dose of the drug used, rodents species used (rats versus mouse), dose and type (single dose or fractionated dose) of irradiation, and cognitive test used.
Our data indicate that MAP-2 is sensitive to irradiation and ALA. However, the discrepancy in the pattern seen with respect to treatments and behavioral outcomes suggests that changes in this marker of dendritic morphology can not fully explain the effects of irradiation or ALA on cognitive function.
In conclusion, our study supports the idea that ALA might only be beneficial following environmental challenges but be detrimental under baseline conditions. This paradoxical effect might not be limited to ALA as we have seen similar effects with Ramipril or combined treatment with Ramipril and Atorvastatin (Raber et al, unpublished observations). Future studies are warranted to determine the mechanisms underlying the effects of irradiation and ALA and other anti-oxidants on cognition. In addition, other behavioral assessments with tests sensitive to cortical function, such as working memory water maze and fear extinction are needed to further elucidate the effects of irradiation in the presence or absence of anti-oxidants.
Supplementary Material
Acknowledgments
This work was supported by NASA Grant NNJ06HE63G and Alzheimer’s Association Grant IIRG-05-14021.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Abayomi OK. Pathogenesis of cognitive decline following therapeutic irradiation for head and neck tumors. Acta Oncol. 2002;41:346–51. doi: 10.1080/028418602760169389. [DOI] [PubMed] [Google Scholar]
- 2.Manda K, Ueno M, Anzai K. Memory impairment, oxidative damage and apoptosis induced by space radiation: ameliorative potential of alpha-lipoc acid. Behav Brain Res. 2008;187:387–395. doi: 10.1016/j.bbr.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term postentiation: contributory or inhibitory? J Neurosci Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- 4.Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharman EH, Bondy SC. Effects of age and dietary antioxidants on cerebral electron transport chain activity. Neurobiol Aging. 2001;22:629–34. doi: 10.1016/s0197-4580(01)00226-3. [DOI] [PubMed] [Google Scholar]
- 6.Cohen E, Irving B, Drobyski W, Klein J, Passweg J, Talano J-A, Jucket M, Moulder J. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2008;70:1546–1551. doi: 10.1016/j.ijrobp.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, Brown S, Jenrow K, RYU S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 2008;87:279–286. doi: 10.1007/s11060-008-9520-x. [DOI] [PubMed] [Google Scholar]
- 8.Ryu S, Koloszvary A, Jenrow K, Brown S, KIM J. Mitigation of radiation-induced optic neuropathy in rats by ACE inhibitor ramipril: importance of ramipril dose and treatment time. J Neurooncol. 2007;82:119–124. doi: 10.1007/s11060-006-9256-4. [DOI] [PubMed] [Google Scholar]
- 9.Moulder J, Fish B, Cohen E. ACE inhibitors and AII receptor antagonists in the treatment and prevention of bone marrow transplantation. Curr Pharm Des. 2003;9:737–749. doi: 10.2174/1381612033455422. [DOI] [PubMed] [Google Scholar]
- 10.Moulder J, Fish B, Cohen E. Noncontinuous use of angiotensin converting enzyme inhibitors in the treatment of experimental bone marrow transplant nephropathy. Bone Marrow Transplant. 1997;19:729–735. doi: 10.1038/sj.bmt.1700732. [DOI] [PubMed] [Google Scholar]
- 11.Jaggi J, Seshan S, Mcdevitt M, Sgouros G, Hyjek E, Scheinberg D. Mitigation of radiation nephropathy after internal alpha-particle irradiation of kidneys. Int J Radiat Oncol Biol Phys. 2006;64:1503–1512. doi: 10.1016/j.ijrobp.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 12.Robbins M, Payne V, Tomasi E, ALE The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2009;73:499–505. doi: 10.1016/j.ijrobp.2008.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diomede L, Albani M, Sottocorno M. In vivo anti-inflammatory effect of statins is mediated by non-sterol mevalonate products. Arterioscler Thromb Vasc Biol. 2001;21:1327–1332. doi: 10.1161/hq0801.094222. [DOI] [PubMed] [Google Scholar]
- 14.Haendeler J, Hoffman J, Zeiher A, DIMMELER S. Antioxidant effects of statins via S-nitrosylation and activation of thioredoxin in endothelial cells. Circulation. 2004;110:856–861. doi: 10.1161/01.CIR.0000138743.09012.93. [DOI] [PubMed] [Google Scholar]
- 15.William J, Hernady E, Johnston C. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 2004;161:560–567. doi: 10.1667/rr3168. [DOI] [PubMed] [Google Scholar]
- 16.Haydont V, Gilliot O, Rivera S. Successful mitigation of delayed intestinal radiation injury using pravastatin is not associated with acute injury improvement or tumor protection. Int J Radiat Oncol Biol Phys. 2007;68:1483–1490. doi: 10.1016/j.ijrobp.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Jenrow K, Brown S, Liu J, Kolozsvary A, Lpannowski K, KIM J. Ramipril mitigates radiation-induced impairments of neurogenesis in the rat dentate gyrus. Radiat Oncol. 2010;5:1–10. doi: 10.1186/1748-717X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.