Abstract
Background
Lymphoid enhancer binding factor 1 (LEF-1) has recently been reported as a potential immunohistochemical (IHC) marker for basal cell adenoma (BCA) and other salivary gland tumors, which may contribute to an increased accuracy in differentiating basaloid salivary gland neoplasms. We evaluated the utility of LEF-1 in fine needle aspiration (FNA) and resection specimens to distinguish pleomorphic adenoma (PA), BCA, basal cell adenocarcinoma (BCAC) and adenoid cystic carcinoma (ACC) as well as in non-neoplastic salivary gland (NNSG).
Methods
Cases including 66 PA (35 FNA, 31 resections), 12 BCA (5 FNA, 7 resections), 42 ACC (11 FNA, 31 resections), 1 BCAC FNA and 10 NNSG (5 FNA, 5 resections) were obtained and stained for LEF-1.
Results
On cell block (CB), 51% of PA and 60% of BCA were LEF-1 positive while 91% of ACC were LEF-1 negative. Among resections, there was a higher percentage of LEF-1 positive PA (84%) and BCA (86%), and a higher percentage of LEF-1 negative ACC (97%). LEF-1 staining had a low to moderate sensitivity for detecting benign basaloid neoplasms on FNA CB and resection specimens (92% and 97%, respectively), but a higher specificity (92% and 97% respectively), and positive predictive value (95% and 97% respectively).
Conclusion
When comparing benign (PA and BCA) and the most common malignant basaloid salivary gland tumor (ACC) lesion, positive LEF-1 favors a benign neoplasm. Additional studies with LEF-1, specifically including other rare basaloid salivary gland neoplasms are needed to further clarify the role of LEF-1 in diagnosing these lesions on FNA.
Keywords: Basaloid neoplasms, immunohistochemistry, LEF-1, salivary gland neoplasms, aspiration cytology
INTRODUCTION
Cytologically bland basaloid salivary gland tumors include benign and malignant tumors composed of cells with high nuclear/cytoplasmic ratio and absence of nuclear pleomorphism. The differential diagnosis of basaloid neoplasms usually includes pleomorphic adenoma (PA), basal cell adenoma (BCA), basal cell adenocarcinoma (BCAC), and adenoid cystic carcinoma (ACC). Due to the extent of their morphologic overlap and lack of reliable special studies, they currently represent a major challenge in the correct subclassification of salivary gland lesions on fine needle aspiration (FNA). Furthermore, all these basaloid tumors have a common differentiation toward intercalated ducts and show combined epithelial and myoepithelial differentiation contributing to the challenge of distinguishing them with immunohistochemical stains.
Basaloid cells can also be seen on FNA aspirates of non-neoplastic salivary gland diseases and non-salivary gland lesions including chronic sialadenitis, metastases, and adnexal neoplasms1. In chronic sialadenitis basaloid ductal cells appear morphologically similar to those of basaloid salivary gland neoplasms; however, basaloid cells in chronic sialadenitis are less numerous and are arranged in smaller groups in a background of chronic inflammation1. Metastases to salivary glands and primary adnexal lesions that can be misinterpreted as basaloid salivary gland neoplasms include metastatic basaloid squamous cell carcinoma, metastatic small cell neuroendocrine carcinoma, Merkel cell carcinoma, cutaneous basal cell carcinoma, and pilomatricoma. Due to this remarkable morphologic overlap, the use of immunohistochemistry (IHC) is often suggested to aid in refining the differential diagnosis of these lesions.
Lymphoid enhancer binding factor 1 (LEF-1) is a protein expressed by a gene transcriptionally regulated by the Wnt3a pathway. This pathway influences normal development of airway submucosal glands, hair follicles, teeth, and mammary glands2, 3, 4. LEF-1 has been recently reported as a potential IHC marker for BCA and other salivary gland tumors, and may contribute to an increased accuracy in differentiating basaloid salivary gland neoplasms on FNA5. In this study, we expanded on this work by considering LEF-1 expression and utility in FNA cell block (CB) and resection specimens of PA, BCA, and ACC as well as in non-neoplastic salivary gland (NNSG).
MATERIAL AND METHODS
After Institutional Review Board (IRB) approval was obtained, consecutive cases of basaloid salivary gland neoplasms and NNSG from 2010 through 2014 were retrieved from the cytology and surgical pathology archives of the Emory University Hospital Pathology Department. For cytology cases, only FNA cases with sufficient cellularity in the CB were selected. These cases did not include core needle biopsies. Due to the nature of the study, informed consent was not necessary.
Cases including 66 PA (35 FNA, 31 resections), 12 BCA (5 FNA, 7 resections), 42 ACC (11 FNA, 31 resections), 1 BCAC FNA and 10 NNSG (5 FNA, 5 resections) were obtained.
Among the malignant cytology cases (ACC and BCAC), 83% (10/12) had surgical follow-up. The remainder of these patients had evidence of metastatic disease and therefore an excision was not performed. All 5 BCA and 16 of 35 (45.7%) PA FNA cases were surgically excised. Among patients diagnosed with PA without a resection, there was an equal proportion of patients being lost to follow up or having other medical conditions for which an excision was not warranted. One of the cases diagnosed as PA on FNA was re-classified as carcinoma ex-PA on resection and re-review of the FNA confirmed an absence of a malignant component on the FNA smears. The diagnosis for the 10 NNSG cases (5 FNA CB and 5 resections) included chronic sialadenitis and benign parotid gland.
IHC for LEF-1 was performed on the Bond III (Leica, Buffalo Grove, IL). All slides were incubated with the primary antibody LEF-1 (LEF-1 clone EP2030Y, Abcam, Carlsbad, CA) at a dilution of 1:50 for 15 minutes, Bond epitope retrieval solution 1 (ER1™) for 20 minutes, post primary polymer for 8 minutes, blocked with 3% hydrogen peroxide for 5 minutes, 3, 3-diaminobenzidine (DAB, brown chromogen) for 10 minutes, and hematoxylin as counterstain for 5 minutes. These incubations were performed at room temperature; between incubations, sections were washed with Tris-buffered saline (Bond wash solution). Positive controls of known positive tissues (pancreatic solid pseudopapillary tumor) and negative controls with primary antibody replaced with Tris buffer, were run with the patient/study slides.
Intensity of IHC was graded as absent (0), weak (1+), moderate (2+), or strong (3+). The staining distribution was also evaluated, with <50% considered focal staining and ≥50% considered diffuse staining.
LEF-1 positivity was defined as 1+ intensity and greater than 5% of tumor cell positivity or intensity of 2–3+ regardless of percentage. LEF-1 negativity was defined as cases with absent staining and cases with 1+ intensity in less than 5% of tumor cells.
Statistical analysis including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for any given basaloid neoplasm were calculated by combining all other subtypes of basaloid neoplasms as the comparison group. Therefore, the values obtained for the above were dependent upon the distribution of types of basaloid neoplasms included in the data analysis. We assessed whether LEF-1 differentiated among common basaloid neoplasms by conducting q chi-square test using the exact multinomial distribution, as implemented in SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Table 1 and Table 2 present LEF-1 expression and statistical analysis results on basaloid salivary gland neoplasms FNA CB and resection specimens, respectively. On CB, 51% of PA and 60% of BCA were LEF-1 positive. 91% of ACC were negative. On resections, there was a higher percentage of LEF-1 positive in PA (84%) and BCA (86%), as well as higher rate of LEF-1 positivity in ACC LEF (97%). The differential staining patterns with PA and BCA on resections could be explained by a significant proportion of cases with focal and weak staining. None of the NNSG samples were immunoreactive for LEF-1 on CB or resections.
Table 1.
LEF-1 expression in basaloid salivary gland fine needle aspiration (FNA) CB (cell block)
| Type (n) | Positive (%) | Negative (%) |
|---|---|---|
| PA (35) | 18/35 (51) | 17/35 (49) |
| BCA (5) | 3/5 (60) | 2/5 (40) |
| ACC (11) | 1/11 (9) | 10/11 (91) |
| BCAC (1) | 0/1 (0) | 1/1 (100) |
| NNSG (5) | 0/5 (0) | 5/5 (100) |
PA: pleomorphic adenoma, BCA: basal cell adenoma, ACC: adenoid cystic carcinoma, BCAC: Basal cell adenocarcinoma, NNSG: non-neoplastic salivary gland
Table 2.
LEF-1 expression in basaloid salivary gland resection
| Type (n) | Positive (%) | Negative (%) |
|---|---|---|
| PA (31) | 26/31 (84) | 5/31 (16) |
| BCA (7) | 6/7 (86) | 1/7 (14) |
| ACC (31) | 1/31 (3) | 30/31 (97) |
| NNSG (5) | 0/5 (0) | 5/5 (100) |
PA: pleomorphic adenoma, BCA: basal cell adenoma, ACC: adenoid cystic carcinoma, NNSG: non-neoplastic salivary gland
When examining only the 11 FNA cases with corresponding resections included in this study (same patient), there were 3 PA, 4 BCA, and 4 ACC. The 3 PA FNA cases were LEF-1 negative, but upon resection they were all LEF-1 positive with a mostly weak and diffuse staining pattern. Among the 4 BCA and 4 ACC the staining pattern and distribution of the FNA and resection cases were identical with the three LEF-1+ BCA on CB also showing immunoreactivity on the resection and the single LEF-1 negative BCA also being negative on resection. All four of the ACC with surgical follow-up were negative on FNA CB and resection.
Tables 3 and 4 compare LEF-1 expression in benign vs. malignant basaloid salivary gland neoplasms on FNA CB and resection. When compared as a group, benign basaloid salivary gland neoplasms (PA and BCA) are more frequently LEF-1 positive, both in FNA CB (52.5%) and in resections (84%), whereas malignant (ACC and BCAC) were mostly negative (92% and 97%, respectively).
Table 3.
LEF-1 expression in benign vs. malignant basaloid salivary gland FNA CB
| Type (n) | Positive (%) | Negative (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Benign (PA + BCA) (40) | 21/40 (52.5) | 19/40 (47.5) | 52.5 | 92 | 95 | 37 |
| Malignant (ACC + BCAC) (12) | 1/12 (8) | 11/12 (92) | 8 | 47 | 5 | 63 |
PA: pleomorphic adenoma, BCA: basal cell adenoma, ACC: adenoid cystic carcinoma, BCAC: Basal cell adenocarcinoma, NNSG: non-neoplastic salivary gland
Table 4.
LEF-1 expression in benign vs. malignant resected basaloid salivary gland neoplasms
| Type (n) | Positive (%) | Negative (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Benign (PA + BCA) (38) | 32/38 (84) | 6/38 (16) | 84 | 97 | 97 | 83 |
| Malignant (ACC) (31) | 1/31 (3) | 30/31 (97) | 3 | 16 | 3 | 17 |
PA: pleomorphic adenoma, BCA: basal cell adenoma, ACC: adenoid cystic carcinoma, NNSG: non-neoplastic salivary gland.
Although the sensitivity of LEF-1 staining in detecting benign basaloid neoplasms was low to modest in FNA CB and resections (52.5% and 84% respectively, tables 3 and 4), specificity was higher (92% and 97% respectively). Additionally, the positive predictive values were also high (95% and 97% respectively) indicating a higher likelihood of a benign neoplasm when LEF-1 is positive.
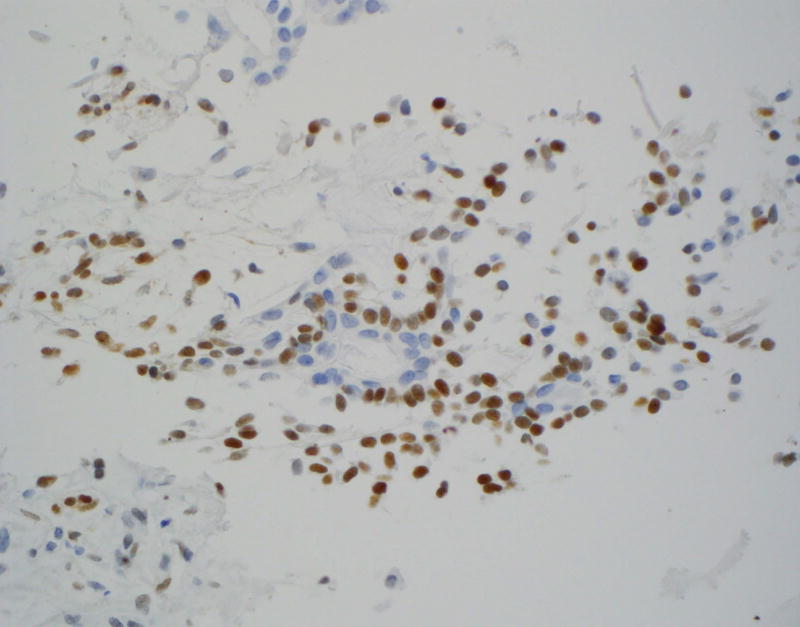
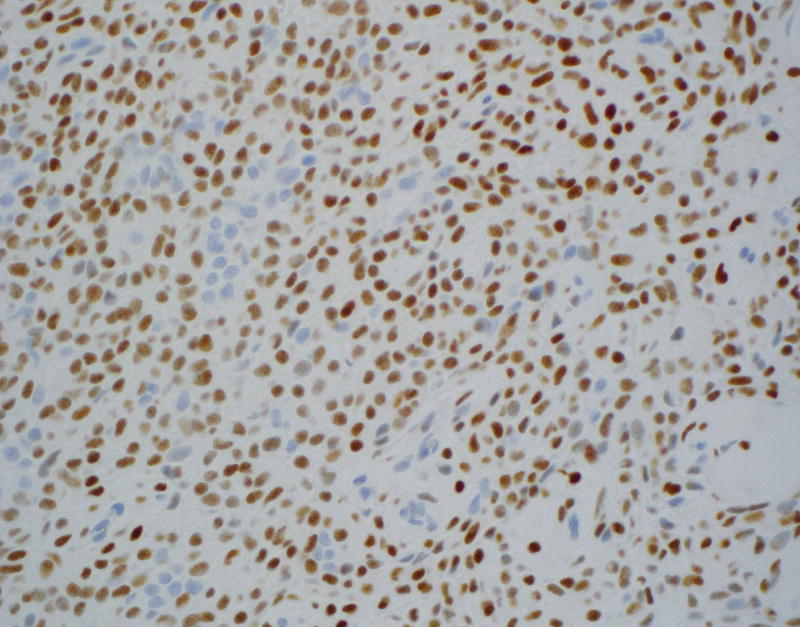
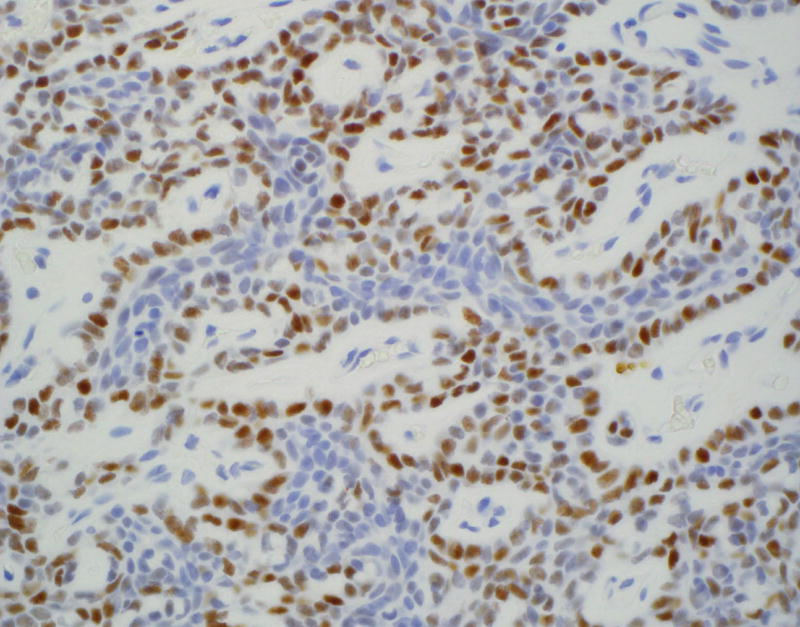
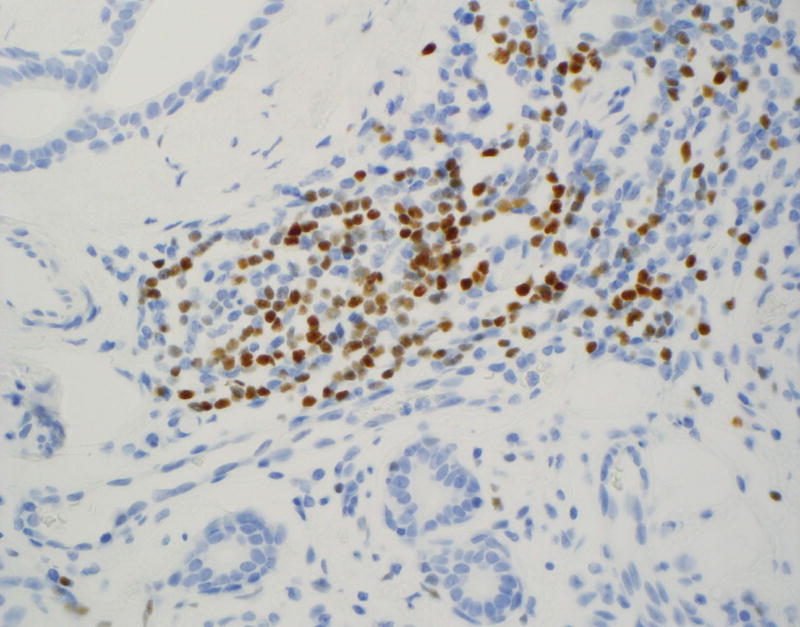
LEF-1 significantly differentiated among the types of basaloid neoplasms in both FNA (p = 0.001; Table 1) and resection specimens (p < 0.001; Table 2). Moreover, LEF-1 significantly differentiated between malignant and benign lesions in both FNA (p = 0.008; Table 3) and resection specimens (p < 0.001; Table 4). Table 5 shows the LEF-1 expression patterns in FNA CB and resections according to staining intensity and distribution. Faint and focal staining with LEF-1 was noted on the rare (1 CB and 1 resection) LEF-1 positive ACC. In contrast, LEF-1 staining of PA and BCA was mostly moderate to strong (Figure 1A), with a predominance of focal staining on PA CB, diffuse staining on resected PA (Figure 1B), and focal staining on both BCA CB and resection. LEF-1 staining of BCA and some PA was marked by an accentuated LEF-1 staining of the myoepithelial cells at the periphery of the tumor nests (Figure 1C).
Table 5.
LEF-1 staining intensity and distribution in FNA CB and resected basaloid salivary gland neoplasms
| N= | Focal + (<50%) | Diffuse + (≥50%) | Weak + (1+) | Moderate to Strong + (2–3+) |
||||
|---|---|---|---|---|---|---|---|---|
| CB | R | CB | R | CB | R | CB | R | |
| PA (CB=35, Resections =31) | 14 (40%) | 8 (25.8%) | 4 (11.4%) | 18 (58.1%) | 7 (20%) | 7 (22.6%) | 11 (31.4%) | 19 (61.3%) |
| BCA (CB=5, Resections =7) | 3 (60%) | 5 (71.4%) | 0 | 1 (14.3%) | 1 (20%) | 3 (42.9%) | 2 (40%) | 3 (42.9%) |
| ACC (CB=11, Resections =31) | 1 (9.1%) | 1 (3.2%) | 0 | 0 | 1 (9.1%) | 1 (3.2%) | 0 | 0 |
PA: pleomorphic adenoma, BCA: basal cell adenoma, ACC: adenoid cystic carcinoma
Figure 1.
Immunohistochemical staining on pleomorphic adenoma (PA) and basal cell adenoma (BCA) for LEF-1.
A: Moderately to strong LEF-1 staining on PA cell block (CB) (×400)
B: Diffusely positive LEF-1 staining on resected PA (×400)
C: LEF-1 staining of resected BCA with accentuated LEF-1 staining of the myoepithelial cells at the periphery of the tumor nests (×400)
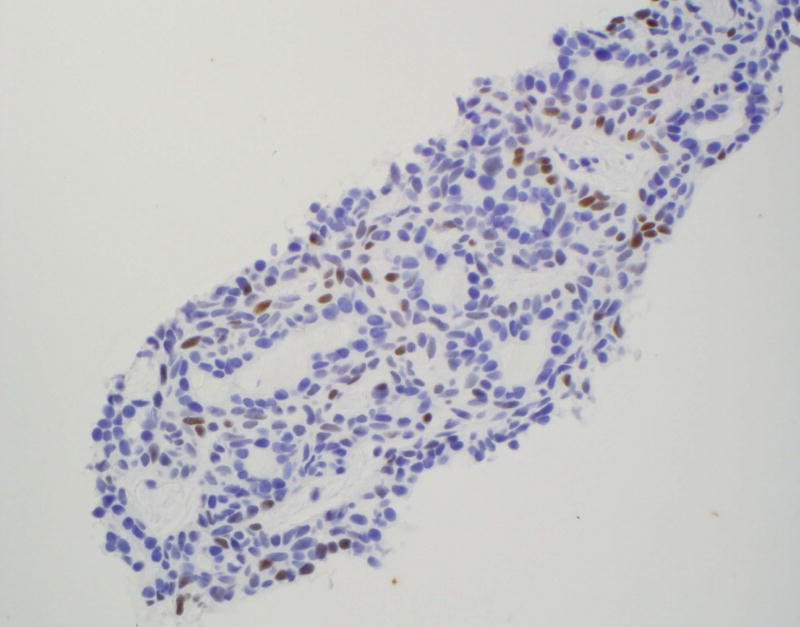
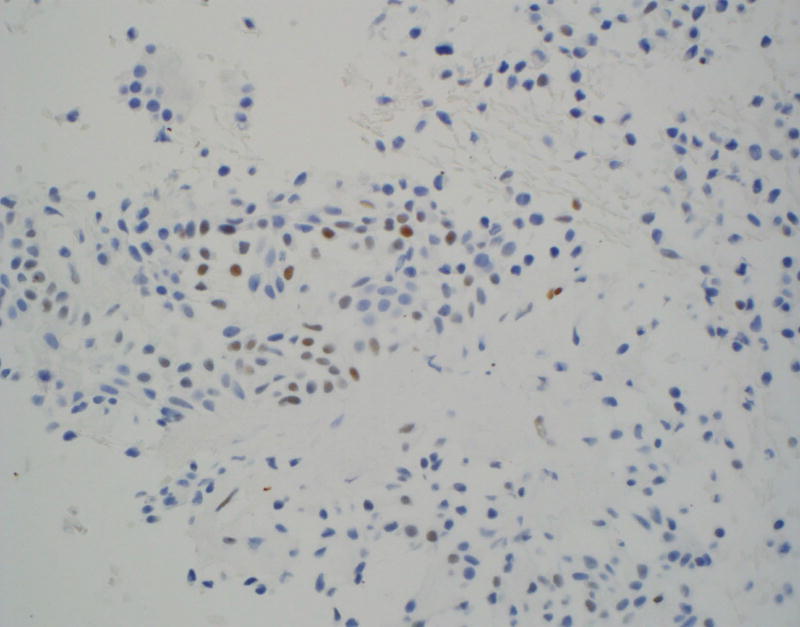
The focal staining pattern of BCA (Figure 2A) and occasional focal and weak staining of PA (Figure 2B) can represent significant pitfalls when trying to distinguish benign basaloid neoplasm from ACC, especially on CB. An additional pitfall to be aware of is the presence of LEF-1 staining of lymphocytes (Figure 2C).
Figure 2.
Pitfalls of LEF-1 staining
A and 2B: Focal LEF-1 staining of BCA and PA CB respectively (×400).
C: LEF-1 staining of lymphocytes and absence of staining in benign salivary gland resection (×400)
DISCUSSION
LEF-1 is known for its role in identifying adamantinomatous craniopharyngioma and a variety of odontogenic tumors5, 6. It is also usually expressed in selected skin adnexal tumors7, 8, 9. A newly identified staining characteristic of LEF-1 which may contribute to a more precise classification of basaloid salivary gland neoplasms is the positive staining of most BCA and BCAC5. This is especially helpful because ACC, the most common malignant neoplasm mimicking PA and BCA on cytology, has been reported to be LEF-1 negative on resections5.
Our study indicates that using LEF-1 on FNA CB of basaloid salivary gland neoplasms may contribute to a more precise subclassification of these lesions. Since positive staining was mostly noted on PA and BCA and only rarely on ACC, positive LEF-1 staining favors a benign basaloid salivary gland neoplasm, both on CB and resections. Similar to other IHC markers evaluated in the setting of basaloid salivary gland neoplasms, the staining intensity and distribution of LEF-1 should also be considered. Rare ACC positive cases (2 of 42, 5%, when combined CB and resected cases) were only faint and focally LEF-1 positive. Our results also indicate that negative LEF-1 staining cannot be used to favor malignancy, since several benign salivary gland aspirates and all NNSG aspirates were LEF-1 negative (32% and 100%, respectively). The latter could be a significant pitfall when interpreting aspirates of chronic sialadenitis mimicking basaloid salivary gland neoplasms.
Noteworthy is that the only 2 LEF-1 positive ACC cases (1 CB and 1 resection from different patients) were classified as poorly differentiated adenocarcinoma, suggestive of solid variant/high grade ACC and high grade ACC, respectively. The ACC CB case was clearly identifiable as a poorly differentiated carcinoma on cytology; therefore it would not have posed a diagnostic challenge to differentiate from benign basaloid salivary gland lesions.
The results of LEF-1 in ruling out ACC and detecting BCA correlate with those of Bilodeau et al who also observed negative LEF-1 staining on ACC and usually positive LEF-1 staining on BCA5. On the other hand, our results differ in regard to LEF-1 staining of PA. Bilodeau et al described negative staining of all 12 PA cases, whereas in our study most PA were positive. This difference regarding PA staining could be due to a different cut off value for positive LEF-1 staining, since in the above study only 2+ staining of greater than 50% of the tumor cells was considered LEF-1 positive and/or the different antibody clone used (sc-8591, 1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA)5.
There is likely no role for LEF-1 in differentiating primary salivary gland basaloid lesions from metastatic lesions to the parotid gland or primary skin lesions. Although non-salivary gland basaloid lesions were not tested in our study, previous studies have demonstrated reliable nuclear LEF-1 staining of squamous cell carcinoma (SCC), cutaneous basal cell carcinoma (BCC), and pilomatricoma9. Up to 24% of oral SCC are LEF-1 immunoreactive, with an increased expression in moderately to poorly differentiated tumors, an association with poorer prognosis, and an expression pattern ranging from diffuse to a predominance of expression at the periphery of the tumor nests10. Kriegl et al described LEF-1 staining in all 74 skin BCCs tested, with strong nuclear staining of the tumor cells at the periphery of tumor nests and absent nuclear staining of cells in the center of tumor nests9. When a diffuse staining pattern was present, there was stronger staining of the cells at the periphery, and weak to strong staining of tumor cells in the center of tumor nests9. Strong nuclear labeling of peripheral basaloid cells with absence of staining of transitional cells has also been described in benign and malignant pilomatricomas7, 8. Interestingly, a very similar pattern of staining with strong nuclear labeling of peripheral basaloid cells was noted in salivary gland BCA tumor cells and also some PA cases in our study (Figures 1A–1C).
There is scant data regarding the role of LEF-1 immunohistochemical staining in other groups of salivary gland neoplasms, especially oncocytoid salivary gland neoplasms. Mucoepidermoid carcinomas are negative (0 of 8 cases tested); however, to our knowledge other oncocytoid lesions have not been investigated5.
Known pitfalls of LEF-1 staining when trying to differentiate benign from malignant basaloid neoplasms include positive LEF-1 staining of most BCAC and occasional staining of epithelial-myoepithelial carcinoma (EMCA), since 11 of 16 BCAC and 4 of 19 EMCA cases tested by Bilodeau et al were LEF-1 positive. This current study did not examine EMCA and included only a single case of BCAC and therefore further investigation of LEF-1 use including these rare basaloid salivary gland neoplasms should be pursued5.
Other challenges noted in our study were the frequent presence of focal and faint staining of PA and BCA on FNA CB and positive staining of lymphocytes. LEF-1 is normally expressed in normal T- and pro-B-lymphocytes, predominantly in paracortical regions, shows overexpression in chronic lymphocytic leukemia/small lymphocytic lymphoma, and in a smaller subset of lymphoid cells of other lymphomas11.
In conclusion, this study clearly identifies a strong role for LEF-1 as a diagnostic marker aiding in the subclassification of certain basaloid tumors of the salivary gland on FNA CB and resections.
When comparing common benign (PA and BCA) and the most common malignant (ACC) basaloid tumor, positive LEF-1 favors a benign neoplasm, with positive predictive values of 95% and 97% on CB and resections respectively; however, additional studies with LEF-1, especially as a panel with other IHC markers and including other rare basaloid salivary gland neoplasms are needed to further clarify LEF-1’s role in diagnosing basaloid salivary gland neoplasms on FNA.
Acknowledgments
Funding sources: Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000454.
Footnotes
Conflict of interest: The authors have no conflicts of interest.
References
- 1.Krane JF, Faquin WC. Salivary Gland. In: Cibas ES, Ducatman BS, editors. Cytology Diagnostic Principles and Clinical Correlates. Elsevier Saunders; 2014. pp. 303–304. [Google Scholar]
- 2.Liu X, Driskell RR, Luo M, et al. Characterization of Lef-1 Promoter Segments that Facilitate Inductive Developmental Expression in Skin. J of Investigative Dermatol. 2004;123(2):264–274. doi: 10.1111/j.0022-202X.2004.23201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan D, Yue Y, Zhou W, et al. Submucosal gland development in the airway is controlled by lymphoid enhancer binding factor 1 (LEF1) Development. 1999;126:4441–53. doi: 10.1242/dev.126.20.4441. [DOI] [PubMed] [Google Scholar]
- 4.Driskell RR, Goodheart M, Neff T, et al. Wnt3a regulates LEF-1 expression during airway submucosal gland morphogenesis. Dev Biol. 2007;305:90–102. doi: 10.1016/j.ydbio.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilodeau EA, Acquafondata M, Barnes EL, et al. A comparative analysis of LEF-1 in odontogenic and salivary tumors. Human Pathol. 2015;46:255–259. doi: 10.1016/j.humpath.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Sekine S, Takata T, Shibata T, et al. Expression of enamel proteins and LEF-1 in adamantinomatous craniopharyngioma: evidence for its odontogenic epithelial differentiation. Histopathology. 2004;45(6):573–9. doi: 10.1111/j.1365-2559.2004.02029.x. [DOI] [PubMed] [Google Scholar]
- 7.Cribrier B, Worret WI, Braun-Falco M, et al. Expression patterns of hair and epithelial keratins and transcriptin factors HOXC13, LEF1, and β- catenin in a malignant pilomatricoma: a histological and immunohistochemical study. J of Cutaneous Pathol. 2006;33:1–9. doi: 10.1111/j.0303-6987.2006.00308.x. [DOI] [PubMed] [Google Scholar]
- 8.Cribrier B, Peltre B, Grosshans E, et al. On the regulation of hair keratin expression: lessons from studies in Pilomatricomas. J Invest Dermatol. 2004;122:1078–1083. doi: 10.1111/j.0022-202X.2004.22513.x. [DOI] [PubMed] [Google Scholar]
- 9.Kriegl L, Horst D, Kirchner T, et al. LEF-1 expression in basal cell carcinomas. British J of Dermatol. 2009;160:1335–1365. doi: 10.1111/j.1365-2133.2009.09144.x. [DOI] [PubMed] [Google Scholar]
- 10.Su MC, Chen CT, Huang FI, et al. Expression of LEF1 is an independent prognostic factor for patients with oral squamous cell carcinoma. J of the Formaosan Medical Association. 2014;113:934–939. doi: 10.1016/j.jfma.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Tandon B, Peterson L, Gao J, et al. Nuclear overexpression of lymphoid- enhancer-binding factor 1 identified chronic lymphocytic leukemia/small lymphocytic lymphoma in small B-cell lymphomas. Modern Pathol. 2011;24:1433–1443. doi: 10.1038/modpathol.2011.103. [DOI] [PubMed] [Google Scholar]