Abstract
Recognition of non-SCID and secondary causes of T cell lymphopenia detected by TREC newborn screening is important in directing subsequent care and identifying those who would not benefit from more invasive interventions. Here, we report two infants with low TRECs and severe, but self-resolving, T cell lymphopenia identified by SCID NBS that were caused by in utero exposure to purine antimetabolites.
Keywords: Purine antimetabolites, TREC newborn screening, T cell lymphopenia, azathioprine, 6-mercaptopurine, maternal immunosuppression, neonatal lymphopenia
To the Editor
Implementation of the T-cell receptor excision circle (TREC) assay as part of the Recommended Uniform Screening Panel of Core Conditions in the United States (U.S.) has allowed for the prompt detection and management of T cell lymphopenia in newborns.1 For those with severe combined immune deficiency (SCID), hematopoietic stem cell transplant (HSCT) at an early age (<3.5 months) and/or prior to onset of infection results in the highest 5-year survival rates, emphasizing the important role that screening for T cell lymphopenia plays in the newborn screening (NBS) panel.2 In the U.S., TREC analysis is performed in 39 states, Washington D.C., and Puerto Rico, and identifies infants with a broad range of genetic conditions that affect T cell generation or maturation including typical SCID, leaky SCID, syndromes that result in low T cell numbers, as well as secondary causes of T cell lymphopenia.
Common non-SCID and secondary causes of T cell lymphopenia include extreme prematurity, DiGeorge syndrome, trisomy 21, CHARGE syndrome, ataxia telangiectasia, post-surgical extravasation of fluid and lymphocytes, and gastrointestinal malformations resulting in lymphocyte loss. Recognition of these underlying disorders directs subsequent care and identifies those who would not benefit from HSCT. Here, we report two infants with low TRECs and severe T cell lymphopenia identified by SCID NBS that were caused by in utero exposure to purine antimetabolites.
Infant A presented at four weeks of age for evaluation of depressed TRECs (9 TRECs/uL with normal β-actin copy number of 16,100) and T cell lymphopenia by flow cytometry. Initial CD4 T cell count at 25 days of life was 198 cells/μL with normal B and NK lymphocyte numbers (Figure 1B). He was born in California, of Hispanic descent, at 36 weeks by emergent caesarian section for maternal pre-eclampsia and fetal bradycardia. The prenatal and post-delivery periods were otherwise unremarkable. The maternal history was notable for systemic lupus erythematosus (SLE) complicated by lupus nephritis treated with mycophenolate mofetil, then switched to azathioprine (AZA; 100mg/day) at week 2 of gestation. At the time of initial evaluation, the infant was in excellent state of health and thriving. He was started on prophylaxis with fluconazole and trimethoprim/sulfamethoxazole, and followed as an outpatient given intact family structure and lack of other children in the household.
Figure 1.
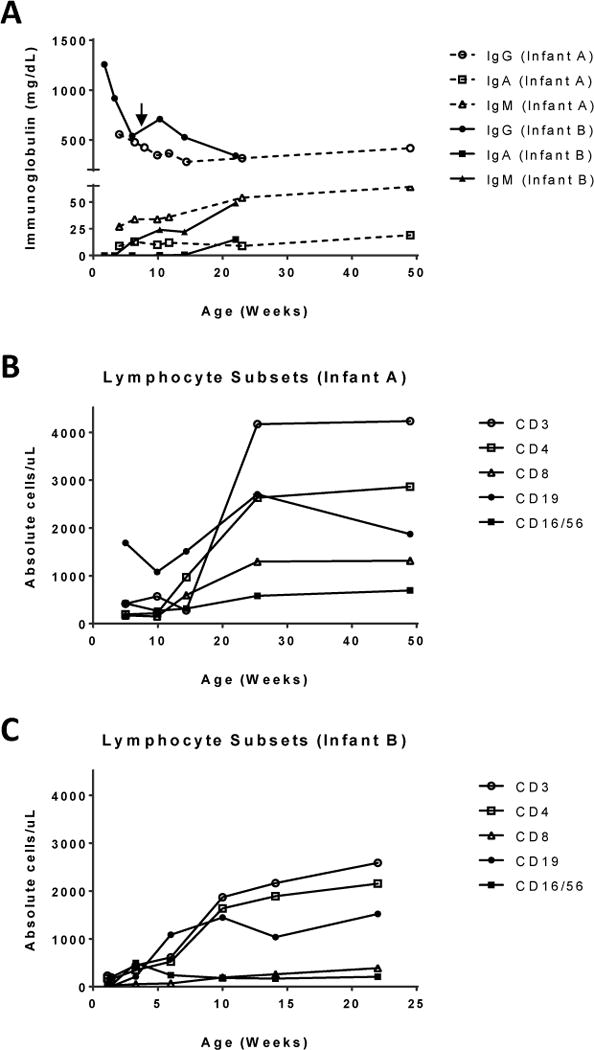
A) Immunoglobulin levels for Infant A (dotted line) and Infant B (solid line). Infant A maintained normal immunoglobulin levels for age without intervention. Infant B received a single dose of IVIG at 47 days of life (arrow) with normal IgA and IgM production. B & C) Normalization of lymphocyte counts for both infants over time.
The second, unrelated, infant (Infant B) was a female of Northern European descent, admitted at 10 days of life for presumed SCID due to critically low TRECs through the state of Utah NBS program (6 TREC/μL). She was born at term without complications except for mild hyperbilirubinemia. Lymphocyte subsets demonstrated a low T cell count of 177 cells/μL, near absent B cells (1 cell/μL), and low NK cells (21 cells/μL) at 8 days of life (Figure 1C). Antimicrobial prophylaxis was initiated for presumed SCID, and a dose of intravenous immunoglobulin was administered at 47 days of life. The family history was significant for Crohn’s disease in the mother. A 2-year-old full male sibling (subject C) presented with intermediately low TRECs (Table 1) on NBS that normalized within the first month of life. The mother was treated with 6-mercaptopurine (6-MP) and allopurinol throughout pregnancy, and her 6-MP 25mg daily dose had been increased to 50mg daily during the second and third trimesters. Thiopurine metabolite levels were monitored during the pregnancy and were within normal range, and infant B had normal thiopurine S-methyltransferase (TPMT) activity levels. Interestingly, the mother took 6-MP at a dose of 25mg daily during her previous pregnancy with subject C. Infant B was eventually discharged without further intervention after T cell counts spontaneously increased to >500 cells/μL.
Table 1.
Clinical laboratory evaluation
| Infant A | Infant B | Subject C | |
|---|---|---|---|
| TREC copies/μl (age) | 9 (2 days) | 12 (6 days) 18 (5 weeks) 52 (7 weeks) |
113 (1 day) 146 (11 days) |
| Lymphocyte subsets (cells/μl) | 12 weeks: CD4+ 199 (9%) CD4+CD45RA+ 478 (62%) CD4+CD45RA+CD62L+ 563 (73%) CD4+CD45RO+ 177 (23%) CD8+ 155 (7%) CD8+CD45RA+ 422 (86%) CD8+CD45RA+CD62+L 391 (76%) CD3+ 419 (16%) CD19+ 1689 (64%) CD15/56+ 427 (16%) |
8 days: CD4+ 177 (69%) CD4+CD45RA+ 148 (58%) CD4+CD45RO+ 25 (10%) CD8+ 24 (9%) |
24 months: CD4+ 2186 (69%) CD4+CD45RA+ 1849 (35.7%) CD4+CD45RO+ 337 (6.5%) CD8+ 746 (14.4%) CD19 1844 (35.6%) NK cells 181 (3.5%) |
| Lymphocyte proliferation | 5 weeks: Normal to PHA, Con-A, and PWM | 15 days: Normal to PHA, Con-A, and PWM | ND |
| Mutation Testing | Negative for CD3D/E/Z, IL2RG, IL7R, JAK3, PNP, PTPRC, RMRP, ZAP70 | Negative for ADA, AK2, CD3D/E/Z, CORO1A, DCLRE1C, FOXN1, IL2RG, IL7R, JAK3, LIG4, NHEJ1, PNP, PRKDC, PTPRC, RAG1/2, RMRP | ND |
| Vaccine Responses | Protective for (23 weeks): Tetanus S. pneumoniae (13/13 pneumococcal conjugate serotypes) H. influenzae B |
ND | ND |
| Thymic Shadow | Present | Present | ND |
| Other | HIV negative CMV PCR negative |
HIV negative TPMT normal ADA and PNP normal Maternal chimerism absent T cell clonality by PCR not detected |
Both infants remained clinically well with normal weight gain. Aside from lymphopenia, laboratory evaluation including blood count and differential, complete metabolic panel, inflammatory markers (ESR/CRP), and chest x-ray were normal for age. T cell counts in Infants A and B normalized by 5 to 6 months of age. Additional immune evaluation and imaging/gene studies are shown in Table 1.
Both SLE and inflammatory bowel disease (IBD) can affect women of child-bearing age, and require careful consideration with regards to therapy during pregnancy. Disease flare-ups increase the risk of adverse pregnancy outcomes, and these risks must be weighed against the risks of treatment.3,4 AZA and its metabolite, 6-MP, have been used in the management of autoimmune disease and post-transplant rejection for more than three decades. These agents suppress immune responses partly by interfering with nucleic acid synthesis in cells undergoing rapid cell division, including T cells. Although both drugs are classified as pregnancy FDA category D, their use during gestation has been deemed relatively safe.
In utero exposure to AZA and 6-MP has been associated with an increased risk of prematurity and low birth weight. Profound lymphopenia and/or immunodeficiency have been considered to be very rare. Our findings and those of others5 would suggest that maternal immunosuppression with thiopurines has a negative impact on fetal lymphopoiesis and that, in this setting, neonatal lymphopenia may be more common than previously anticipated. Monitoring of maternal thioguanine metabolites may minimize this risk.6
The correlation between maternal leukocyte counts in the third trimester and cord blood leukocyte counts at delivery in renal allograft patients on AZA7 suggests that the degree of lymphopenia may be dose-dependent. This can explain the more severe and prolonged T cell lymphopenia in infant B compared to her older male sibling, subject C. Further, in infant B the effects of 6-MP may have been enhanced by concomitant allopurinol use. Both infant B and her mother had normal thiopurine metabolite and/or TPMT levels, and thus overt medication toxicity could not account for the infant’s lymphopenia.
Standard care for neonates with abnormal TREC results and severe T cell lymphopenia can include the initiation of prophylactic antibiotics and antifungals, discontinuation of breastfeeding until maternal/infant CMV status is known, and protective isolation and potential hospitalization.8 These interventions, while important in SCID, have many medical and socioeconomic consequences for the patient and family. Our results suggest that maternal immunosuppression should be added to the list of non-SCID conditions that could present with marked lymphopenia and abnormal TRECs. In some instances, a careful maternal medication history may question the necessity to perform extensive confirmatory tests for presumed SCID and re-direct medical management. These lymphopenic newborn can be followed on an outpatient basis in certain settings.
The long-term effects of in utero exposure to thiopurines on immune development and function are not known. Ultimately, more thorough and long-term follow-up of these infants is needed to ascertain whether transient lymphopenia and low TRECs caused by in utero exposure to purine analogues has implications later in life.
References
- 1.Kwan A, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: Results of the first 2 years. J Allergy Clin Immunol. 2013;132:140–150.e7. doi: 10.1016/j.jaci.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pai SY, et al. Transplantation Outcomes for Severe Combined Immunodeficiency, 2000-2009. N Engl J Med. 2014;371:434–446. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buyon JP, et al. Predictors of pregnancy outcomes in patients with lupus: A cohort study. Ann Intern Med. 2015;163:153–163. doi: 10.7326/M14-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornish J, et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut. 2007;56:830–837. doi: 10.1136/gut.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Felipe B, et al. Prospective neonatal screening for severe T- and B-lymphocyte deficiencies in Seville. Pediatr Allergy Immunol. 2015;27:70–77. doi: 10.1111/pai.12501. [DOI] [PubMed] [Google Scholar]
- 6.Jharap B, et al. P385 Intrauterine exposure and pharmacology of conventional thiopurine therapy in pregnant inflammatory bowel disease patients. J Crohn’s Colitis. 2012;6:S163. doi: 10.1136/gutjnl-2012-303615. [DOI] [PubMed] [Google Scholar]
- 7.Davison JM, Dellagrammatikas H, Parkin JM. Maternal azathioprine therapy and depressed haemopoiesis in the babies of renal allograft patients. Br J Obstet Gynaecol. 1985;92:233–9. doi: 10.1111/j.1471-0528.1985.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 8.Rivers L, Gaspar HB. Severe combined immunodeficiency: recent developments and guidance on clinical management. Arch Dis Child. 2015;100:667–72. doi: 10.1136/archdischild-2014-306425. [DOI] [PubMed] [Google Scholar]


