Abstract
Background
The relationship between plasma concentration of sedatives and delirium is unknown.
Objective
We hypothesized that higher plasma concentrations of lorazepam are associated with increased delirium risk, whereas higher plasma concentrations of dexmedetomidine are associated with reduced delirium risk.
Methods
This prospective cohort study was embedded in a double-blind randomized clinical trial, where ventilated patients received infusions of lorazepam and dexmedetomidine. Plasma concentrations of these drugs and delirium assessments were measured at least daily. A multivariable logistic regression model accounting for repeated measures was used to analyze associations between same-day plasma concentrations of lorazepam and dexmedetomidine (exposures) and the likelihood of next-day delirium (outcome), adjusting for same-day mental status (delirium, coma, or normal) and same-day fentanyl doses.
Results
This critically ill cohort (n = 103) had a median age of 60 years (IQR: 48-66) with APACHE II score of 28 (interquartile range [IQR] = 24–32), where randomization resulted in assignment to lorazepam (n = 51) or dexmedetomidine (n = 52). After adjusting for same-day fentanyl dose and mental status, higher plasma concentrations of lorazepam were associated with increased probability of next-day delirium (comparing 500 vs 0 ng/mL; odds ratio [OR] = 13.2; 95% CI = 1.4–120.1; P = 0.02). Plasma concentrations of dexmedetomidine were not associated with next-day delirium (comparing 1 vs 0 ng/mL; OR = 1.1; 95% CI = 0.9–1.3; P = 0.45).
Conclusions
In critically ill patients, higher lorazepam plasma concentrations were associated with delirium, whereas dexmedetomidine plasma concentrations were not. This implies that the reduced delirium risk seen in patients sedated with dexmedetomidine may be a result of avoidance of benzodiazepines, rather than a dose-dependent protective effect of dexmedetomidine.
Keywords: sedatives, analgesics, assays, drug safety, critical care, drug trials, pharmacodynamics, toxicity
Introduction
One-third to three-quarters of critically ill patients develop delirium, a type of acute brain dysfunction characterized by inattention and disorganized thinking, during their stay in the intensive care unit (ICU).1,2 Patients who develop delirium in the ICU are more likely to have worse clinical outcomes, including increased risk of mortality, longer ICU and hospital stays, and cognitive impairment.1–3 To combat these poor outcomes, multiple studies have focused on the role of neurotropic agents, such as ICU sedatives, and the occurrence of delirium.4–6
Multiple investigations have shown benzodiazepine administration to be an independent risk factor for delirium during critical illness,7,8 but one study found that neither the dose nor the plasma concentration of the benzodiazepine used—midazolam—was associated with delirium.9 Sedation with the α2-adrenoreceptor agonist dexmedetomidine has been associated with reduced prevalence and duration of acute brain dysfunction in mechanically ventilated patients,10,11 but it is unclear whether these reductions were attributable to avoidance of benzodiazepines or whether these reductions were attributable to a dose-dependent and plasma concentration–dependent protective effect of dexmedetomidine.
To advance the understanding of sedative pharmacodynamics in relation to delirium, we measured plasma concentrations of lorazepam and dexmedetomidine from patients enrolled in the double-blind, randomized clinical phase 3 trial, Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction Study (MENDS),10 which compared lorazepam with dexmedetomidine for sedation of mechanically ventilated ICU patients. We hypothesized a priori that higher plasma concentrations of lorazepam would be associated with increased delirium risk and that higher plasma concentrations of dexmedetomidine would be associated with reduced delirium risk.
Methods
Eligibility
We performed this prospective cohort study during the previously published MENDS trial (ClinicalTrials.gov NCT00095251), which occurred over a 2-year period at 2 centers, where relevant institutional review boards approved the study protocol.10 Patients met inclusion criteria for the MENDS study if they were adult medical or surgical patients in an ICU and required mechanical ventilation for at least 24 hours. We excluded patients with severe neurological disease (eg, prior stroke, cerebral palsy), active seizures, severe liver disease, moribund state and/or planned withdrawal of life support, family and/or physician refusal, alcohol abuse, active myocardial ischemia, second- or third-degree heart block, severe dementia, benzodiazepine dependence, pregnant or breastfeeding, or significant limitations to communication such as severe hearing loss or inability to understand English. Informed consent was obtained from authorized surrogates and again from patients when deemed competent. Patients were randomized to receive either lorazepam or dexmedetomidine, with fentanyl administered for pain for up to 120 hours after randomization, as long as they still required mechanical ventilation. Further details about enrollment, blinding, randomization, sample size calculation, study drug administration, analgosedative protocol, and outcome assessments are available in the original publication.10
Plasma Concentrations of Sedatives
Blood samples were collected on enrollment and up to 3 times per day (at 05:00, 10:00, and 16:00 hours) for up to 6 days postrandomization while on sedation. The plasma was immediately separated by centrifugation at 3000 rpm for 10 minutes and then stored frozen at −80°C in 500-μL aliquots until batched analysis in 2010.
Plasma lorazepam concentrations were measured with a previously described method.12 After the addition of internal standard (300 μL of a 1-μg/mL solution of diazepam) and 200 μL saturated sodium borate (pH ~9.4), plasma (0.5 mL) was extracted by shaking for 30 minutes with 7 mL of ethyl acetate. The organic extract was dried down at 40°C under nitrogen, and the residue was dissolved in 200 μL of high-performance liquid chromatography (HPLC) mobile phase. After centrifugation and filtration through a 0.22-μm nylon filter, a 50-μL aliquot was subjected to HPLC-UV analysis with separation on a 5-μm Ultrasphere C18 column with a mobile phase of 70/30/0.1, methanol/water/acetic acid at a flow rate of 1 mL/min and UV detection at 230 nm. Calibration curves were linear over the range of 50 to 1000 ng/mL.
Plasma dexmedetomidine concentrations were measured by reversed-phase HPLC with tandem mass spectrometric detection (HPLC-MS/MS; SCIEX API 365 instrument, Foster City, CA). The method is a modification from a published procedure.13 The lower limit of quantitation of the assay was 0.02 ng/mL. The within- and between-run precision of the assay (coefficient of variation) was within 8% in the relevant concentration range.
Delirium
The outcome for the current study was the occurrence of delirium on the next day following sedative plasma concentration measurement. Research personnel blinded to treatment group assignment assessed patients for delirium once daily for up to 12 days (or until hospital discharge) using the Confusion Assessment Method for the ICU (CAM-ICU) when Richmond Agitation-Sedation Scale (RASS) scores were ≥(−3).14
Statistical Analysis
We used multivariable Markov logistic regression models to examine the relationship between highest daily plasma levels of lorazepam and dexmedetomidine (exposures or independent variables) and delirium status on the next day (outcome or dependent 2-tiered variable represented by delirium or nondelirium), adjusting for a 3-tiered mental status (normal, delirious, or comatose) and 24-hour fentanyl dose on the same day of plasma level measurement. All patient-days with available plasma levels and an observed mental status of delirium or normal the next day were included in the model; we used Huber-White sandwich estimation, clustered by patient, to account for within-patient correlation of observations. We did not include patient-days when death or coma precluded delirium assessment on the day after measurement of sedative plasma concentration.
We used R version 3.3.2 for all statistical analyses and considered P <0.05 to indicate statistical significance. Descriptive data are presented as medians with interquartile ranges (IQRs). Odds ratios (ORs) with 95% CIs compare the 75th versus 25th percentiles (or clinically relevant maxima/minima when percentiles are equivalent) of the observations, using a previously reported statistical approach.1,15,16(p568)
Results
In the MENDS study, 51 patients were randomized to the lorazepam group and 52 patients were randomized to the dexmedetomidine group. All patients completed the study protocol and were followed until death, hospital discharge, or up to 21 days after randomization. Of this group, 85 patients completed the study and had at least 1 sedative plasma level measured among 302 patient-days. After excluding persistently comatose and missing exposure/outcomes, the number of evaluable patients was 62, leaving a total of 152 days where the patient had a plasma level measured and had at least 1 delirium assessment in the study period.
Both groups were similar at baseline (Table 1) with respect to demographics, severity of illness, comorbid conditions, and ICU admission diagnoses. The median age for this critically ill cohort was 60 years (IQR = 48–66), with a median APACHE II score of 28 (IQR = 24–32), evenly split gender (female 49%, n = 50; male 51%, n = 53), and requiring admissions either to the surgical ICU (30%, n = 31) or medical ICU (70%, n = 72). Of the 103 patients who were included in this analysis, about one-third (n = 36, 35%) had sepsis or acute respiratory distress syndrome as a primary diagnosis. Median lorazepam or dexmedetomidine use prior to enrollment was similar and equal to zero; 50 patients did receive lorazepam prior to enrollment, and 6 patients received dexmedetomidine. Baseline mental status was delirious (29%, n = 30), comatose (53%, n = 54), and normal (18%, n = 18), with lower baseline delirium in the lorazepam versus the dexmedetomidine group (24% vs 35%).
Table 1.
Baseline Characteristics for Sedative Drug Levels and ICU Delirium.a
| Baseline Characteristicsb | Dexmedetomidine (n = 52) | Lorazepam (n = 51) | Combined (n = 103) |
|---|---|---|---|
| Age | 60 (49–65) | 59 (45–67) | 60 (48–66) |
| Female gender | 42% (22) | 55% (28) | 49% (50) |
| Medical ICU | 71% (37) | 69% (35) | 70% (72) |
| Surgical ICU | 29% (15) | 31% (16) | 30% (31) |
| APACHE II score | 29 (24–32) | 27 (24–32) | 28 (24–32) |
| Lorazepam given | 0.2 (0.0–4.2) | 0.0 (0.0–3.0) | 0.0 (0.0–4.0) |
| Pre-enrollment (mg) | 5.1 ± 10.7 | 3.6 ± 6.8 | 4.3 ± 8.9 |
| Dexmedetomidine given | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Pre-enrollment (μg) | 16 ± 66 | 122 ± 600 | 69 ± 426 |
| Mental statusc | |||
| Coma | 43% (22) | 63% (32) | 53% (54) |
| Delirium | 35% (18) | 24% (12) | 29% (30) |
| Normal | 22% (11) | 14% (7) | 18% (18) |
| RASSc | |||
| −1 | 8% (4) | 6% (3) | 7% (7) |
| −2 | 15% (8) | 4% (2) | 10% (10) |
| −3 | 17% (9) | 16% (8) | 17% (17) |
| −4 | 35% (18) | 48% (24) | 41% (42) |
| −5 | 4% (2) | 6% (3) | 5% (5) |
| 0 | 13% (7) | 10% (5) | 12% (12) |
| 1 | 6% (3) | 6% (3) | 6% (6) |
| 2 | 0% (0) | 0% (0) | 0% (0) |
| 3 | 0% (0) | 4% (2) | 2% (2) |
| 4 | 2% (1) | 0% (0) | 1% (1) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II Score; ICU, intensive care unit; RASS, Richmond Agitation-Sedation Scale.
All values are presented as percentage (n) or median (interquartile range) or mean ± SD.
All combined n = 103, except where specified.
n = 102.
As previously reported,10 the median dexmedetomidine rate was 0.74 μg/kg/h (IQR = 0.39–1.04) and the median lorazepam rate was 3 mg/h (IQR = 2.2–6). The median administered fentanyl dose was 575 μg/d in the dexmedetomidine group and 150 μg/d in the lorazepam group. For those with plasma levels, the dexmedetomidine group had mean levels of 0.46 ng/mL, and the lorazepam group had mean levels of 167 ng/mL.
After adjusting for the same day’s fentanyl dose and mental status, higher plasma concentrations of lorazepam were associated with a nonlinear increased risk of delirium on the next day after plasma concentration measurement (see Figure 1 for entire association, comparing 500 ng/mL of lorazepam vs 0 ng/mL; OR = 13.2; 95% CI = 1.4–120.1; P = 0.02). This means for a lorazepam plasma concentration of 500 ng/mL, there is a 13-fold higher odds of delirium as compared with a plasma concentration of 0 ng/mL. At a lorazepam plasma concentration >750 ng/mL, the predicted risk of next-day delirium approached 100%. Within the same model adjusting for the same-day covariates, higher plasma concentrations of dexmedetomidine were associated with neither an increase nor a decrease in the risk of delirium on the next day (Figure 2, comparing 1 ng/mL of dexmedetomidine vs 0 ng/mL; OR = 1.1; 95% CI = 0.9–1.3; P = 0.45). The fentanyl dose administered on the same day as the plasma concentration measurements was nonlinearly associated with the development of delirium on the next day, after adjustment for both sedative drug levels (Figure 3, P < 0.001). In general, the probability of delirium increased as fentanyl doses rose from 0 to 2500 μg/d and then fell, although the number of patient-days during which very high doses were received was so small that the CI in this range was wide.
Figure 1.
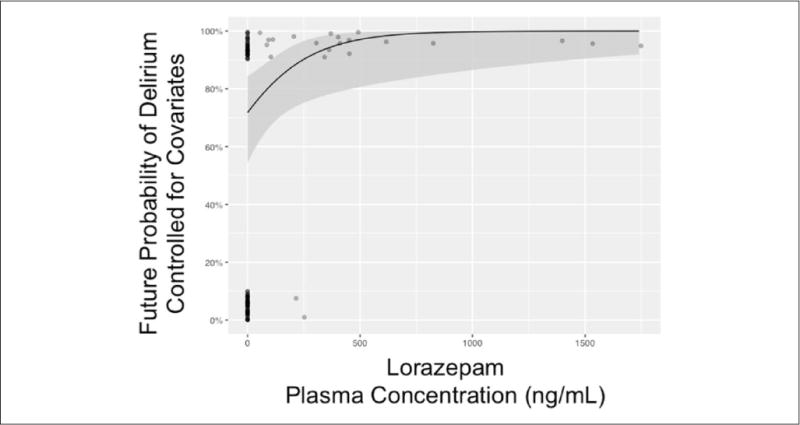
Plasma concentrations of lorazepam in critically ill patients and probability of delirium on the next day: The solid black line represents the relationship between the future (ie, next-day) probability of delirium (vs nondelirious) with plasma lorazepam concentration in nanogram per milliliter, adjusted for the mode/median of other covariates in the single multivariable logistic regression model. Covariates for our single model included fentanyl dose and mental status (eg, normal, coma, or delirium), same-day plasma dexmedetomidine concentration, and same day of lorazepam concentration analysis. The gray area represents the 95% CI for this relationship (see also Figures 2 and 3). The number of evaluable patients was 62, given that persistently comatose and missing exposure/outcomes were disallowed.
Figure 2.
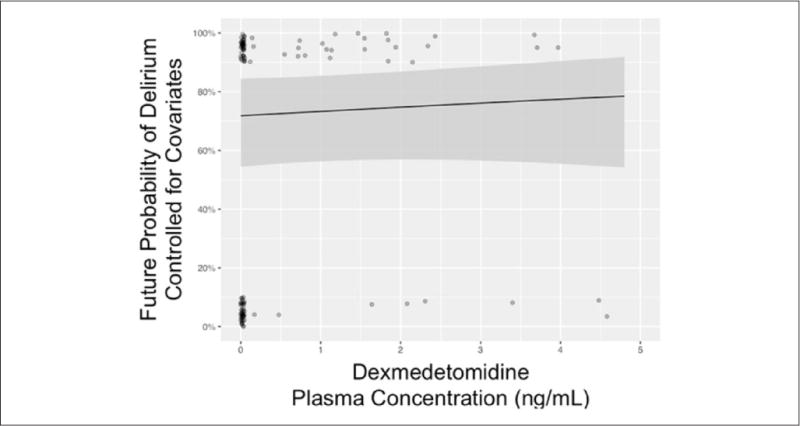
Plasma concentrations of dexmedetomidine in critically ill patients and probability of delirium on the next day: The solid black line represents the relationship between the future (ie, next-day) probability of delirium (vs nondelirious) with plasma dexmedetomidine concentration in nanogram per milliliter, adjusted for the mode/median of other covariates in the single multivariable logistic regression model. Covariates for our single model included fentanyl dose and mental status (eg, normal, coma, or delirium), same-day plasma dexmedetomidine concentration, and same day of lorazepam concentration analysis (see also Figures 1 and 3). The gray area represents the 95% CI for this relationship. The number of evaluable patients was 62, given that persistently comatose and missing exposure/outcomes were disallowed.
Figure 3.
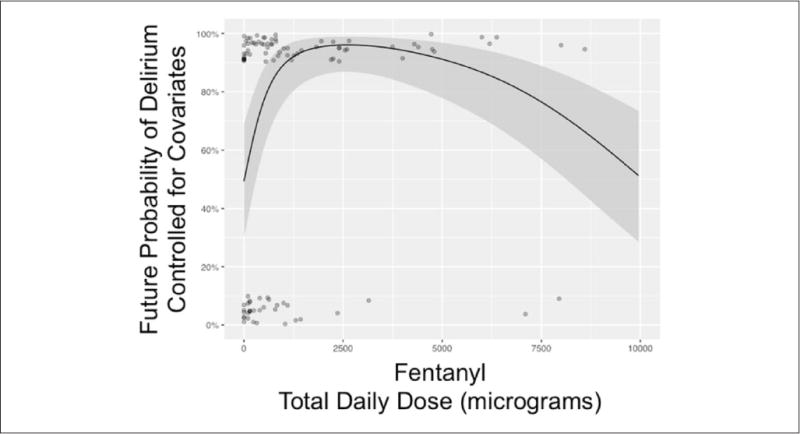
Next-day probability of delirium versus same-day cumulative dose of fentanyl in critically ill patients: The solid black line represents the nonlinear relationship between the future (ie, next day) probability of delirium (vs nondelirious) with the cumulative same-day dose of fentanyl in micrograms, adjusted for the mode/median of other covariates in the single multivariable logistic regression model. Covariates for our single model included fentanyl dose and mental status (eg, normal, coma, or delirium), same-day plasma dexmedetomidine concentration, and same day of lorazepam concentration analysis (see also Figures 1 and 2). The gray area represents the 95% CI for this relationship. The number of evaluable patients was 62, given that persistently comatose and missing exposure/outcomes were disallowed.
Discussion
In this study of mechanically ventilated ICU patients randomized to one of two intravenous sedation strategies, higher lorazepam plasma concentrations were associated with a higher probability of delirium on the following day after adjusting for concurrent mental status and fentanyl dose. In contrast, the plasma concentrations of dexmedetomidine were not associated with either an increased or decreased probability of delirium on the following day. The total dose of fentanyl was associated with delirium risk on the following day, even after adjusting for doses of dexmedetomidine and lorazepam.
Plasma concentration of lorazepam, a more accurate approximation of drug exposure than drug dose (since it accounts for pharmacokinetic variability), was associated with delirium risk. A high predicted probability of delirium was observed when high plasma concentrations of lorazepam were reached, however limited by very few observations and moderately wide CIs. We could have cube-root transformed these drug concentrations to limit the effect of these extreme values, however that would be less interpretable to the readership. Still, these findings build on numerous previous investigations that have shown a temporal association between the administration of benzodiazepines (both lorazepam and midazolam) and the increased probability of daily delirium.7,8
Our results, however, differ from the study by Skrobik et al,9 who found no association between plasma levels of midazolam and delirium. Also, important differences in their study methodology may explain these discordant findings. The authors categorized enrolled ICU patients into 3 mutually exclusive groups for the purpose of the logistic regression analysis; those who only were comatose (n = 24), those who only had delirium (n = 22), and those who had neither (n = 12). In the small subset of patients with only delirium, they were unable to show statistical significance between sparsely but concomitantly drawn plasma levels of midazolam and delirium. The majority of patients in this study (and frequently seen in clinical practice) who had some days of coma and some days of delirium (n = 42) were excluded from this regression analysis, which limits the generalizability of the findings. Additionally, concomitantly determined plasma levels do not allow the analysis of temporal relationships, especially because we know that benzodiazepines may have prolonged effects on the brain.
In contrast, we studied the association of the highest benzodiazepine plasma level on each day (from 1 to 3 samples per patient per day) on the probability of delirium (or nondelirious status) on the next day, accounting for the administered opiates (fentanyl) and the mental status (normal, coma, or delirium) on the previous day. Our methodology facilitates a temporal evaluation and interpretation that is independent of the current mental status. Specifically, an increased plasma level of lorazepam (eg, reflective of present and cumulative drug dose and pharmacokinetic modifiers) increases the probability of either persistent or new delirium on the next day, rather than having a nondelirious mental status.
This is also the first report to our knowledge examining the association between dexmedetomidine plasma concentrations and delirium during critical illness. After controlling for effects of time-varying exposure to fentanyl, which was administered more often to patients receiving dexmedetomidine than to those receiving lorazepam in the MENDS Study, we found that—contrary to our hypothesis—dexmedetomidine plasma concentrations were not associated with altered delirium risk. This finding supports the hypothesis that the reductions in delirium observed in dexmedetomidine-treated patients during MENDS and other trials10,11,17 may have been attributable to avoidance of delirium-provoking sedative agents—namely, benzodiazepines. For example, there are data that suggest that dexmedetomidine may not improve patient outcomes after optimizing sedation and delirium metrics.18,19 An alternative explanation is that dexmedetomidine does have protective efficacy against delirium, as suggested by the recent placebo-controlled Dexmedetomidine to Lessen ICU Agitation (DahLIA) study,20 but that this effect is not dose dependent within the range of doses used in the MENDS Study (0.15–1.5 μg/kg/h).
Our work also accounted for one the most common ICU narcotics, intravenous fentanyl, whose doses are potentially inversely affected by the analgesic properties of dexmedetomidine.21 Similar to a prior report,22 we found that, with the exception of extremely high doses, higher fentanyl doses were associated with higher probability of delirium development on the following day, after adjustment for concomitant sedatives. Fentanyl is the most frequently used opioid analgesic in the ICUs of the United States and many European nations.23 Previous studies investigating fentanyl’s association with delirium have yielded conflicting results. Fentanyl was shown to be associated with delirium in surgical and trauma ICU patients who were mechanically ventilated.7,9 However, in a study of burn ICU patients, a population with high levels of pain, opioids (fentanyl and morphine) decreased the risk of developing delirium by half.24 Thus, the findings of this study along with those previously published suggest that fentanyl likely contributes to delirium when used for sedation but may be protective in patients with high pain levels.
Our study has some important limitations. For example, analyzing the highest daily plasma concentration of both drugs may or may not erroneously presume that these are at steady-state levels; hence, depending on the time and frequency of dosage adjustments, the plasma drug concentrations may not be in equilibrium between plasma and the central nervous system. Also, although maximum daily plasma concentrations provide the greatest chance of signal in this pilot work, this method risks a serious type I error given that we did not have reliably consistent multiple concentrations to create area-under-the-curve analyses. Measuring plasma concentrations of intravenously infused sedatives at 3 or fewer time points per day is a limited method for capturing the total daily drug exposure. More frequent measurements of sedative plasma concentrations may have led to more refined models of the relationships between these exposures and delirium. Additionally, there are many factors that modify an individual’s pharmacodynamic response to a specific plasma concentration of sedative, including age, illness severity, comorbid illness, additional sedative and analgesic agents, and possibly pharmacogenetic factors.25–29 Measuring each of these factors was beyond the scope of our investigation. Even if acute kidney injury should not have affected our analysis, given that the clearance of neither dexmedetomidine nor lorazepam involves renal excretion of unchanged drug,30 the lack of adjustment for other potential confounders, including prior exposure, tolerance, daily severity of illness, consciousness levels, and pain scores, as well as systems factors such as ICU protocols and sedative stewardship initiatives—all may have biased this analysis.8,31–34 Furthermore, our evaluations for our outcome of delirium were restricted to RASS ≥(−3) and higher, which have been shown to be reliable and valid. Still, there was a possibility that oversedation may have resulted in rapidly reversible delirium, known to possibly affect up to 12% of patients that are similar to those in our cohort.35,36 Similarly, our CAM-ICU tool for delirium in these patients could have been confounded by the presence of elevated lorazepam concentrations, which could be a surrogate for the presence of deeper sedation levels.37–39 We acknowledge that these were other limitations of our study, which we were underpowered to adjust for in our models. Finally, guidelines from critical care authorities have changed in regard to sedation and recently recommend nonbenzodiazepine sedative strategies.40
Conclusion
This prospective cohort analysis of patients enrolled in the MENDS trial found that higher lorazepam plasma concentrations were associated with increased delirium risk, whereas dexmedetomidine plasma concentrations were not associated with increased or decreased probability of delirium. As our knowledge of ICU delirium has deepened, the importance of its prevention and treatment has become more apparent, and this study adds to the growing body of literature supporting the use of sedation strategies that minimize benzodiazepine exposure.
Acknowledgments
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Hughes has been supported by a Foundation for Anesthesia Education and Research (Rochester, MN) Mentored Research Training Grant, American Geriatrics Society (New York, NY) Jahnigen Career Development Award, and National Institutes of Health HL111111, AG045085, and GM120484 (Bethesda, MD). Dr Girard received support from the National Institutes of Health AG034257 and HL135144 (Bethesda, MD). Dr Ely has been supported by the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (Nashville, TN). Dr Ely has also been supported by the VA Clinical Science Research and Development Service (Washington, DC) and the National Institutes of Health AG027472, AG035117, and HL111111 (Bethesda, MD). Dr Pandharipande has been supported by the National Institutes of Health AG027472, GM120484, HL111111 (Bethesda, MD). Dr Patel has been supported by National Institutes of Health HL111111 and GM120484 (Bethesda, MD).
Footnotes
Posthumous authorship has been given to Grant R. Wilkinson for outstanding contribution. ClinicalTrials.gov Registration: NCT00095251.
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Pandharipande has received grant support from Hospira Inc. Drs Hughes and Ely have received research grant support from Dr Franz Kohler Chemie GMBH. Dr Scheinin has carried out contract research for Hospira Inc and Orion Pharma Corporation.
ORCID iDs
Mika Scheinin https://orcid.org/0000-0001-7579-9126
Mayur B Patel https://orcid.org/0000-0001-5230-0871
References
- 1.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salluh JI, Wang H, Schneider EB, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350:h2538. doi: 10.1136/bmj.h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38:2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 4.Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38:419–427. doi: 10.1097/CCM.0b013e3181b9e302. [DOI] [PubMed] [Google Scholar]
- 5.Girard TD, Pandharipande PP, Carson SS, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38:428–437. doi: 10.1097/ccm.0b013e3181c58715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1:515–523. doi: 10.1016/S2213-2600(13)70166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Skrobik Y, Leger C, Cossette M, Michaud V, Turgeon J. Factors predisposing to coma and delirium: fentanyl and midazolam exposure; CYP3A5, ABCB1, and ABCG2 genetic polymorphisms; and inflammatory factors. Crit Care Med. 2013;41:999–1008. doi: 10.1097/CCM.0b013e318275d014. [DOI] [PubMed] [Google Scholar]
- 10.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 11.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 12.Gunawan S, Treiman DM. Determination of lorazepam in plasma of patients during status epilepticus by high-performance liquid chromatography. Ther Drug Monit. 1988;10:172–176. doi: 10.1097/00007691-198802000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Ji QC, Zhou JY, Gonzales RJ, Gage EM, El-Shourbagy TA. Simultaneous quantitation of dexmedetomidine and glucuronide metabolites (G-Dex-1 and G-Dex-2) in human plasma utilizing liquid chromatography with tandem mass spectrometric detection. Rapid Commun Mass Spectrom. 2004;18:1753–1760. doi: 10.1002/rcm.1548. [DOI] [PubMed] [Google Scholar]
- 14.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 15.Hughes CG, Patel MB, Jackson JC, et al. Surgery and anesthesia exposure is not a risk factor for cognitive impairment after major noncardiac surgery and critical illness. Ann Surg. 2017;265:1126–1133. doi: 10.1097/SLA.0000000000001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrell FE. Regression Modeling Strategies, With Applications to Linear Models, Logistic Regression, and Survival Analysis. Berlin, Germany: Springer Verlag; 2001. [Google Scholar]
- 17.Mo Y, Zimmermann AE. Role of dexmedetomidine for the prevention and treatment of delirium in intensive care unit patients. Ann Pharmacother. 2013;47:869–876. doi: 10.1345/aph.1AR708. [DOI] [PubMed] [Google Scholar]
- 18.Deiner S, Luo X, Lin HM, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg. 2017;152:e171505. doi: 10.1001/jama-surg.2017.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawazoe Y, Miyamoto K, Morimoto T, et al. Effect of Dexmedetomidine on Mortality and Ventilator-Free Days in Patients Requiring Mechanical Ventilation With Sepsis: A Randomized Clinical Trial. JAMA. 2017;317:1321–1328. doi: 10.1001/jama.2017.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reade MC, Eastwood GM, Bellomo R, et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. 2016;315:1460–1468. doi: 10.1001/jama.2016.2707. [DOI] [PubMed] [Google Scholar]
- 21.Youngblood FE, Knaak E, Rose J, Malesker MA. Use of dexmedetomidine to discontinue high-dose fentanyl. Ann Pharmacother. 2011;45:1589–1590. doi: 10.1345/aph.1Q389. [DOI] [PubMed] [Google Scholar]
- 22.Pandharipande PP, Morandi A, Adams JR, et al. Plasma tryptophan and tyrosine levels are independent risk factors for delirium in critically ill patients. Intensive Care Med. 2009;35:1886–1892. doi: 10.1007/s00134-009-1573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wunsch H, Kahn JM, Kramer AA, Rubenfeld GD. Use of intravenous infusion sedation among mechanically ventilated patients in the United States. Crit Care Med. 2009;37:3031–3039. doi: 10.1097/CCM.0b013e3181b02eff. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal V, O’Neill PJ, Cotton BA, et al. Prevalence and risk factors for development of delirium in burn intensive care unit patients. J Burn Care Res. 2010;31:706–715. doi: 10.1097/BCR.0b013e3181eebee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung JY, Cho JY, Yu KS, et al. Effect of the UGT2B15 genotype on the pharmacokinetics, pharmacodynamics, and drug interactions of intravenous lorazepam in healthy volunteers. Clin Pharmacol Ther. 2005;77:486–494. doi: 10.1016/j.clpt.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Swart EL, Zuideveld KP, de Jongh J, Danhof M, Thijs LG, Strack van Schijndel RM. Comparative population pharmaco-kinetics of lorazepam and midazolam during long-term continuous infusion in critically ill patients. Br J Clin Pharmacol. 2004;57:135–145. doi: 10.1046/j.1365-2125.2003.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56:893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banasch HL, Dersch-Mills DA, Boulter LL, Gilfoyle E. Dexmedetomidine use in a pediatric intensive care unit: a retrospective cohort study [published online September 1, 2017] Ann Pharmacother. doi: 10.1177/1060028017734560. [DOI] [PubMed] [Google Scholar]
- 29.Humble SS, Wilson LD, Leath TC, et al. ICU sedation with dexmedetomidine after severe traumatic brain injury. Brain Inj. 2016;30:1266–1270. doi: 10.1080/02699052.2016.1187289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flexman AM, Wong H, Riggs KW, et al. Enzyme-inducing anticonvulsants increase plasma clearance of dexmedetomidine: a pharmacokinetic and pharmacodynamic study. Anesthesiology. 2014;120:1118–1125. doi: 10.1097/ALN.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 31.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 33.Stollings JL, Foss JJ, Ely EW, et al. Pharmacist leadership in ICU quality improvement: coordinating spontaneous awakening and breathing trials. Ann Pharmacother. 2015;49:883–891. doi: 10.1177/1060028015582050. [DOI] [PubMed] [Google Scholar]
- 34.Schickli MA, Eberwein KA, Short MR, Ratliff PD. Evaluation of pharmacy-driven dexmedetomidine stewardship and appropriate use guidelines in a community hospital setting. Ann Pharmacother. 2017;51:27–32. doi: 10.1177/1060028016666100. [DOI] [PubMed] [Google Scholar]
- 35.Barr J, Zomorodi K, Bertaccini EJ, Shafer SL, Geller E. A double-blind, randomized comparison of i.v. lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model. Anesthesiology. 2001;95:286–298. doi: 10.1097/00000542-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189:658–665. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
- 37.Haenggi M, Blum S, Brechbuehl R, Brunello A, Jakob SM, Takala J. Effect of sedation level on the prevalence of delirium when assessed with CAM-ICU and ICDSC. Intensive Care Med. 2013;39:2171–2179. doi: 10.1007/s00134-013-3034-5. [DOI] [PubMed] [Google Scholar]
- 38.Woien H, Balsliemke S, Stubhaug A. The incidence of delirium in Norwegian intensive care units; deep sedation makes assessment difficult. Acta Anaesthesiol Scand. 2013;57:294–302. doi: 10.1111/j.1399-6576.2012.02793.x. [DOI] [PubMed] [Google Scholar]
- 39.Wunsch H, Meltzer JS. Sedation with dexmedetomidine vs lorazepam in mechanically ventilated patients. JAMA. 2008;299:1540–1541. doi: 10.1001/jama.299.13.1540-b. author reply 1542. [DOI] [PubMed] [Google Scholar]
- 40.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:278–280. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]


