Abstract
Background/objectives
Ectopic accumulation of lipids in skeletal muscle and the formation of deleterious lipid intermediates is thought to contribute to the development of insulin resistance and type 2 diabetes mellitus (T2DM). Similarly, impaired fat oxidation (metabolic inflexibility) are predictors of weight gain and the development of T2DM; however, no study has investigated the relation between muscle ceramide accumulation and 24-hour macronutrient oxidation. The purpose of this study was to retrospectively explore the relationships between whole body fat oxidation and skeletal muscle ceramide accumulation in obese non-diabetic individuals (ND) and in people with obesity and T2DM.
Methods
Daily substrate oxidation was measured in a respiratory chamber and skeletal muscle ceramides were measured using liquid chromatographyelectrospray ionization tandem-mass spectrometry.
Results
After adjusting for sex, age, and BMI no differences existed between the groups for fat oxidation or 24-h RQ. However, ceramides C18:1, C:20, C:22, C:24 and C:24:1 were significantly higher in people with T2DM compared to ND whereas no differences existed for C:16 and C:18. Despite low amounts of muscle ceramides, fat oxidation rates were positively associated with ceramide species concentration in ND only. Our data suggests that ceramides do not interfere with whole-body fat oxidation in ND individuals whereas a persistent lipid oversupply results in excessive ceramide muscle accumulation in people with T2DM.
Keywords: lipotoxicity, energy expenditure, type 2 diabetes
1. Introduction
Lipotoxicity interferes with insulin signaling and plays an important role in the development of metabolic disorders, particularly Type 2 diabetes mellitus (T2DM) [1]. Various lipid species have been measured in obese and TD2M individuals and ceramides have been proposed as one of the culprits for the induction of metabolic disorders [2]. Ceramides are bioactive precursors acting as central metabolic points in sphingolipid biosynthesis and breakdown [3] and increased muscle accumulations in obese [4] and insulin resistant [5] individuals have been hypothesized to trigger insulin resistance, even if the exact mechanisms have yet to be fully elucidated. Substrate competition between fat and glucose has long been thought to play a central role in the development of insulin resistance [6]. Dysregulation of fatty acid metabolism is a hallmark of the progression towards T2DM starting with a diminished capacity to oxidize fatty acids [7] leading with increased lipids stores and further insulin resistance [8]. Such oversupply of lipids in skeletal muscle could lead to an increased amount of ceramides and mechanistically explain the observed impaired insulin action in obese and insulin resistant subjects. We therefore conducted this cross-sectional analysis to investigate whether the variability in 24-hour substrate oxidation is associated to the variability in the concentration of ceramide species in obese individuals with or without T2DM.
2. Material and Methods
2.1 Subjects
Muscle samples primarily for the determination of the insulin signaling cascade and gene expression from three studies conducted at Pennington Biomedical Research Center from 2007–2013 were analyzed for ceramides species. Data were obtained from 106 research participants with obesity and T2DM (N=44) or obesity and non-diabetic patients (ND) (N=62) from Chromium II (NCT00398853), EAT (NCT01672632) and BARIA (NCT00936130) studies. T2DM was confirmed at screening. Only subjects on lifestyle therapy or whose fasting glucose was < 180 mg/dL were included in the T2DM group. All procedures were approved by the Pennington Biomedical Institutional Review board, and all participants provided written, informed consent.
2.2 Body Composition
Fat-free mass, fat mass and percent body fat were measured by dual-energy X-ray absorptiometry (DXA, QDR 4500A; Hologics, Bedford, MA).
2.3 Blood Analytes
Glucose was measured using a glucose oxidase electrode on a Beckman Coulter DXC600 (Brea, CA) instrument whereas insulin was assayed by enzyme immunoassay on a Siemens 2000 (Los Angeles, CA) instrument with an intra and inter-rate CV of 3.8% and 4.2%. Triglycerides were measured using an enzymatic method on a Beckman Coulter Synchron CX7 (Brea, CA).
2.4 Magnetic Resonance Spectroscopy
Intraymyocellular lipid (IMCL) and intrahepatic lipid (IHL) content were measured using 1H magnetic resonance spectroscopy (1H-MRS) on a 3.0T whole body imaging and spectroscopy system (General Electric Medical Systems, Milwaukee, WI) as previously described [9, 10].
2.5 Metabolic Chamber
Twenty-four-hour energy expenditure and substrate oxidation were measured in a whole-room indirect calorimeter as previously described [11]. Energy intake was provided to match energy expenditure according to predictive equations based on fat-free mass, fat mass, age, and sex [11]. Concentrations of O2 and CO2 in the chamber were measured throughout the chamber stay, from which oxygen consumption, carbon dioxide production, and therefore energy expenditure and substrate oxidation were calculated every 10 s and the values were plotted at 10-min intervals. Energy expenditure was calculated from VO2, VCO2, and 24-hour urinary nitrogen excretion [12].
2.6 Quantification of Lipid Metabolites
Skeletal muscle tissue was collected using the technique described by Bergstrom [13], flash frozen and stored at −80 °C. Muscle tissue (~25 mg) was homogenized in deionized water and protein was determined by Bio-Rad protein assay kit (Bio-Rad laboratories, Inc. Hercules, CA). Using the Folch extraction method under acidified conditions, extraction standards (unnatural derivatives) C17:0 ceramide, dC17:1 sphingosine, and C17:0 sphingomyelin were added and each sample was subjected to a double extraction for lipids. Liquid chromatographyelectrospray ionization tandem-mass spectrometry (LC-MSMS) was used to measure intracellular levels of sphingolipids on a Waters Acquity UPLC using a Waters Aquity Xevo triple quadruple MS/MS detector with an ion source ESI operated in the positive mode. According to the retention times of standards, common product ion and ions reflecting fatty acid substituents, all target compounds and ceramides were quantified as previously described [14]. Sphingosine was quantified as a ceramide catabolism product. All values were standardized by protein level in the muscle.
2.7 Statistical analyses
All analyses were performed using SAS/STAT® software, Version 9.4 of the SAS System for Windows (Cary, NC, USA) with a significance level set at α=0.05. Participants’ characteristics were summarized as Mean ± SD for each group and group comparison was done by two-sample t-tests. All outcomes related to fat oxidation, respiratory quotient (RQ), and ceramide species content were compared between T2DM and ND groups via two-sample t-tests of least squares means from linear fixed effects models incorporating covariates for sex, age, and BMI. Additionally, within-group Spearman correlations of ceramide content with substrate oxidation and RQ were estimated and tested by one-sample t-tests.
3. Results
Subjects’ characteristics are listed in Table 1. T2DM and ND groups were balanced for gender, and not significantly different in weight, BMI, and percent body fat (p=0.74, p=0.63, p=0.52, respectively). Participants with T2DM were older than ND (p<0.001). FFA and triglycerides were higher in T2DM than ND (p=0.0004 & p<.0001). As expected glucose, insulin, and subsequently HOMA-IR were higher in T2DM than ND (p<.0001, p=0.01, p=0.002, respectively). In a subset (two of the three studies that had this data) of 42 non-diabetic patients and 25 T2DM patients, although trending to be higher in T2DM than ND, similar amounts of IMCL in the soleus (p=0.34) and the tibialis (p=0.16) and IHL were present.
Table 1.
Subjects’ characteristics, respiratory chamber, and magnetic resonance imaging data.
| Non-Diabetics Patients | Type 2 Diabetics Patients | |
|---|---|---|
| Gender (Male/Female) | 31/31 | 23/21 |
| Race (%White/Black/Other) | 47/50/3 | 84/16/0 |
| Age (years) | 32.7 ± 10.80 | 57.7 ± 7.9* |
| Weight (kg) | 97.8 ± 31.8 | 95.9 ± 23.4 |
| Height (cm) | 171.4 ± 9.9 | 170.2 ± 10.0 |
| BMI (kg/m2) | 33.6 ± 11.8 | 32.7 ± 7.2 |
| Fat mass (kg) | 31.6 ± 22.7 | 33.0 ± 14.7 |
| Fat-free mass (kg) | 59.3 ± 11.0 | 61.8 ± 11.8 |
| Percent body fat (%) | 32.2 ± 15.6 | 34.0 ± 9.2 |
| Free fatty acids (mmol/L) | 0.421 ± 0.241 | 0.578 ± 0.142* |
| Triglycerides (mg/dL) | 93.0 ± 51.4 | 179.0 ± 123.0* |
| Total Cholesterol (mg/dL) | 184.0 ± 29.4 | 170.2 ± 10.0 |
| Glucose (mg/dL) | 94.0 ± 8.3 | 116.6 ± 22.2* |
| Insulin (mU/L) | 11.8 ± 10.7 | 19.4 ± 18.3* |
| HOMA-IR | 2.8 ± 2.6 | 5.9 ± 6.4* |
| 24 hour RQ | 0.889 ± 0.045 | 0.865 ± 0.032 |
| Sleep RQ | 0.874 ± 0.055 | 0.850 ± 0.038 |
| Fat oxidation (g/24hr/kgFFM) | 0.95 ± 0.87 | 1.29 ± 0.48 |
| Carbohydrate oxidation (g/24hr/kgFFM) | 5.15 ± 1.51 | 4.41 ± 1.11 |
| Soleus IMCL (% of oil signal) | 0.46 ± 0.25 | 0.71 ± 0.60 |
| Anterior Tibialis IMCL (% of oil signal) | 0.38 ± 0.20 | 0.79 ± 1.01 |
| Intrahepatic lipids (% of oil signal) | 0.02 ± 0.03 | 0.09 ± 0.12 |
Absolute unadjusted values are shown in the table. For statistical analyses, all estimates were adjusted for sex, age, and BMI and presented as mean ± SD. G=grams, hr=hour. kgFFM= kilograms of fat-free mass. IMCL = intramyocellular lipids. Data presented as mean ± standard deviation.
p<0.05
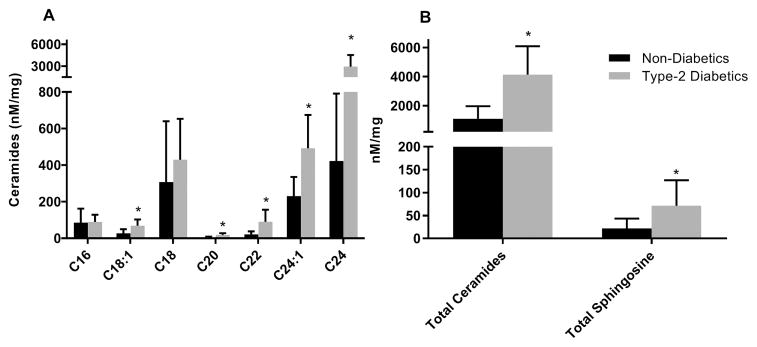
Group differences in ceramide species after adjusting for sex, age, and BMI are shown in Figure 1. In Figure 1 Panel A, T2DM had significantly higher amounts of species C18:1 (p=0.002), C20, C22, C24:1, C24, (all p<0.0001) than ND but not for species C16 and C18 (p=0.09 and p=0.83, respectively). In Figure 1 Panel B, total ceramide content was higher in T2DM than ND by two-fold as well as total sphingosine (p<0.0001 and p=0.003, respectively).
Figure 1. Comparison of ceramides between non-diabetic (black) and type 2 diabetic patients (gray) (mean ± SD).
Panel A: Comparison of ceramide species between diabetics and non-diabetics. Panel B: Comparison of total ceramides and total sphingosine between diabetics and non-diabetics. All values were normalized for protein content. Statistical significance (*= p<0.05) was assessed after adjustment for sex, age, and BMI. FFA = free-fatty acid, IMCL = intramyocellular lipids.
In ND only, there were significant positive correlations between fat oxidation and species C16 (ρ=0.54, p<.0001), C18 (ρ=0.56, p<.0001), C18:1 (ρ=0.48, p=0.0001), C:20 (ρ=0.44, p=0.001), C:22 (ρ=0.35, p=0.01), C24 (ρ =0.43, p=0.001) and total ceramides (ρ=0.48, p=0.0002), but not 24:1 (ρ=0.11, p=0.42) whereas none existed for C16 (ρ=0.11, p=0.48), C18 (ρ=0.20, p=0.21), C18:1 (ρ=0.06, p=0.71), C:20 (ρ=0.02, p=0.92), C:22 (ρ=0.01, p=0.94), C24 (ρ=0.004, p=0.98), C24:1 (ρ= −0.16, p=0.32) and total ceramides (ρ=0.002. p=0.99) in T2DM. Similarly, significant inverse relationships existed between 24-hour RQ and C16 (ρ= −0.54, p<.0001), C18 (ρ= −0.50, p<.0001), C18:1 (ρ= −0.49, p=<.0001), C:20 (ρ= −0.43, p=0.0004), C:22 (ρ= −0.30, p=0.02), C24 (ρ =−0.41, p=0.001) and total ceramides (ρ= −0.44, p=0.0003) but not C24:1 (ρ= −0.07, p=0.61) in ND, but not C16 (ρ= −0.18, p=0.23), C18 (ρ= −0.22, p=0.15), C18:1 (ρ= −0.02, p=0.91), C:20 (ρ= −0.10, p=0.50), C:22 (ρ= −0.17, p=0.26), C24 (ρ =−0.20, p=0.19), C24:1 (ρ= −0.11, p=0.49) and total ceramides (ρ= −0.19, p=0.22) in T2DM.
4. Discussion
Ceramides are thought to be involved in the development of insulin resistance and have been shown to be increased in obesity [4] and T2DM [5]. Impairments in fat oxidation are present during the development of obesity and T2DM and may contribute to lipid accumulation [7, 15]. No one has yet explored whole-body substrate oxidation in relation to muscle ceramide species accumulation. Here for the first time, we report positive associations between ceramides and whole-body oxidation in ND individuals but not in those with T2DM.
Our data supports higher amounts of total ceramides in T2DM including all species except for C16 and C18. Although we did not observe any differences in C16 and C18 in our cohort, we have previously reported higher amounts of both C16 and C18 in myotubes of T2DM donors [16]. Recently, Bergman et al [17] reported higher levels of C16 and C18 in T2DM versus obese although these were measured in serum and not muscle. The longer chain ceramides, which were all higher in T2DM, may be the ones implicated with the development of insulin resistance and diabetes. This is supported from cross-sectional studies in humans linking longer chain ceramides as culprits for development of insulin resistance [4, 18]. Impaired skeletal muscle fat oxidation has been often described in obese and diabetic patient populations [19, 20]. In presence of established whole body insulin resistance, lipolysis remains elevated resulting in higher plasma FFA. Such excess FFA supply induces ceramide synthesis via the de novo pathway [21]. However, a previous study has noted a role for only palmitate (C16) to serve as a substrate for de novo ceramide synthesis and C16 was not different between our groups [22]. In the present study, T2DM participants had higher plasma FFA concentrations possibly leading to a higher amount of muscle ceramides. However, storage of IMCL was similar between groups with only a trend to be higher in T2DM. It is possible that in addition to the elevated FFA in T2DM, the higher levels of glucose may have be synergistically harmful, a concept known as glucolipotoxicity, which is still debated [23].
This study is not without limitations. T2DM participants were older than their ND counterparts. A strong positive relationship (r=.76, p<0.0001) existed between age and total ceramides (data not shown). For that reason, we adjusted our results for age in all statistical analyses. It has been suggested that insulin resistance is not only associated with age but importantly with obesity and physical inactivity [24]. Although no measurements of physical activity were performed, both groups were matched in terms of adiposity. Another explanation could be the transition of type 2 to type 1 fibers as one ages [25], which could explain the higher amounts of lipid in the muscle of the T2DM group, although it is also reported that type 1 fibers are lower in T2DM individuals [26]. Unfortunately, this study did not include fiber type data or direct measurements of cellular skeletal muscle fatty acid oxidation; thus, an association between whole body and muscle fat oxidation cannot be deciphered or explain the interindividual differences in fat balance.
5. Conclusion
Higher amounts of skeletal muscle ceramides, individuals with T2DM showed no associations between ceramides and whole-body 24-hour fat oxidation whereas a positive association was present in ND participants. Together, our data suggests that the elevated ceramides present in T2DM are not from hindrance of fat oxidation, at least at the whole-body level, but rather could be from a more persistent lipid oversupply. Further research is thus warranted to elucidate differences in the relationship between lipid species with whole-body and cellular fat oxidation.
Acknowledgments
The authors thank the dedicated staff of the Pennington Biomedical Research Center inpatient and outpatient units and imaging center for their contributions.
Funding
Supported in part by 1U54GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases R55DK060126 and R01DK060126 (to WTC) and R01DK060412 (to E.R.). This work was also supported by Ethicon-Endo Surgery Inc. and partially supported by the infrastructure of Nutrition Obesity Research Center Grant P30DK072476 (to E.R.)
List of Abbreviations
- FFA
Free fatty acids
- IHL
Intrahepatic lipids
- IMCL
Intramyocellular lipids
- RQ
Respiratory Quotient
- T2DM
Type 2 Diabetes Mellitus
Footnotes
Clinical Trials: NCT00398853, NCT01672632 and NCT00936130
Conflicts of Interest
The authors have no conflicts of interest to declare.
Author Contributions
N.T.B. researched data and wrote the manuscript. D.N.O. researched data and reviewed the manuscript. J.H.B. provided statistical support. W.T.C. researched data and reviewed/edited the manuscript. E.R. researched data and reviewed/edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–70. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 2.Chaurasia B, Summers SA. Ceramides - Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol Metab. 2015;26:538–50. doi: 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 4.de la Maza MP, Rodriguez JM, Hirsch S, Leiva L, Barrera G, Bunout D. Skeletal muscle ceramide species in men with abdominal obesity. J Nutr Health Aging. 2015;19:389–96. doi: 10.1007/s12603-014-0548-7. [DOI] [PubMed] [Google Scholar]
- 5.Coen PM, Dube JJ, Amati F, Stefanovic-Racic M, Ferrell RE, Toledo FG, et al. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes. 2010;59:80–8. doi: 10.2337/db09-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–9. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 7.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–6. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 9.Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008;16:1355–62. doi: 10.1038/oby.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14:73–87. doi: 10.1038/oby.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam YY, Redman LM, Smith SR, Bray GA, Greenway FL, Johannsen D, et al. Determinants of sedentary 24-h energy expenditure: equations for energy prescription and adjustment in a respiratory chamber. Am J Clin Nutr. 2014;99:834–42. doi: 10.3945/ajcn.113.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–16. [PubMed] [Google Scholar]
- 14.Obanda DN, Hernandez A, Ribnicky D, Yu Y, Zhang XH, Wang ZQ, et al. Bioactives of Artemisia dracunculus L. mitigate the role of ceramides in attenuating insulin signaling in rat skeletal muscle cells. Diabetes. 2012;61:597–605. doi: 10.2337/db11-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259:E650–7. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 16.Bajpeyi S, Myrland CK, Covington JD, Obanda D, Cefalu WT, Smith SR, et al. Lipid in skeletal muscle myotubes is associated to the donors’ insulin sensitivity and physical activity phenotypes. Obesity (Silver Spring) 2014;22:426–34. doi: 10.1002/oby.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, et al. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab. 2015;309:E398–408. doi: 10.1152/ajpendo.00134.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coen PM, Hames KC, Leachman EM, DeLany JP, Ritov VB, Menshikova EV, et al. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity (Silver Spring) 2013;21:2362–71. doi: 10.1002/oby.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colberg SR, Simoneau JA, Thaete FL, Kelley DE. Skeletal muscle utilization of free fatty acids in women with visceral obesity. J Clin Invest. 1995;95:1846–53. doi: 10.1172/JCI117864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2349–56. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(Suppl):S91–6. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem. 2003;278:30015–21. doi: 10.1074/jbc.M302548200. [DOI] [PubMed] [Google Scholar]
- 23.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontes G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta. 2010;1801:289–98. doi: 10.1016/j.bbalip.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amati F, Dube JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care. 2009;32:1547–9. doi: 10.2337/dc09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):11–6. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 26.Stuart CA, McCurry MP, Marino A, South MA, Howell ME, Layne AS, et al. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J Clin Endocrinol Metab. 2013;98:2027–36. doi: 10.1210/jc.2012-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]