Abstract
Behavioral and mental health risk factors are prevalent among primary care patients and contribute substantially to premature morbidity and mortality and increased health care utilization and costs. Although prior studies have found most adults screen positive for multiple risk factors, limited research has attempted to identify factors that most commonly co-occur, which may guide future interventions. The purpose of this study was to identify subgroups of primary care patients with co-occurring risk factors and to examine sociodemographic characteristics associated with these subgroups. We assessed 12 behavioral health risk factors in a sample of adults (n = 1628) receiving care from nine primary care practices across six U.S. states in 2013. Using latent class analysis, we identified four distinct patient subgroups: a ‘Mental Health Risk’ class (prevalence = 14%; low physical activity, high stress, depressive symptoms, anxiety, and sleepiness), a ‘Substance Use Risk’ class (29%; highest tobacco, drug, alcohol use), a ‘Dietary Risk’ class (29%; high BMI, poor diet), and a ‘Lower Risk’ class (27%). Compared to the Lower Risk class, patients in the Mental Health Risk class were younger and less likely to be Latino/Hispanic, married, college educated, or employed. Patients in the Substance Use class tended to be younger, male, African American, unmarried, and less educated. African Americans were over 7 times more likely to be in the Dietary Risk versus Lower Risk class (OR 7.7, 95% CI 4.0–14.8). Given the heavy burden of behavioral health issues in primary care, efficiently addressing co-occurring risk factors in this setting is critical.
Keywords: behavioral health, multiple risk factors, primary care, latent class analysis
Introduction
Poor health behaviors, in particular tobacco use, poor diet, and physical inactivity, are major causes of morbidity and mortality.1,2 Alcohol misuse, drug use, sleep disorders, depression, and stress also significantly contribute to poor health and are associated with high health care utilization and health care costs.3 These health risks have been shown to co-occur, 4–6 potentially acting synergistically, resulting in greater negative health consequences including higher chronic disease incidence and severity.7–9 Thus, research is needed to understand the interrelationship between risk factors.
Primary care represents a key setting for addressing unhealthy behaviors and mental health risk factors, but methods for routinely assessing these factors are underdeveloped. The majority of the adult population in the U.S. has received primary care services in the past year, and prior research has demonstrated that primary care providers can play a critical role in addressing behavioral issues.10 Nevertheless, few practices systematically assess health risks. Among practices that do, most do not comprehensively assess these risks.11 Instead, many routinely assess only isolated risk factors (e.g., tobacco) or assess risks only in subpopulations (e.g., diabetics). Additionally, assessments are often not pragmatic, yielding results that are not actionable.12
Further, many existing interventions focus solely on one health risk. However, interventions targeting multiple behavioral health risks have been increasingly recognized as integral to efforts to improve population health.5,6,11,13 Emerging evidence suggests interventions may be as effective when they address co-occurring behavioral health risk factors as when addressing single risk factors.14–16 A number of multiple risk factor interventions have addressed secondary prevention among patients with existing disease, but few have focused on primary prevention.11,13 In primary care, addressing each risk factor separately may not be as practical or efficient as multiple risk factor interventions given time and resource constraints. Gaining a better understanding of the types of patients who typically present with co-occurring behavioral risk factors and identifying which risk factors tend to cluster is important for the development of interventions targeting multiple behavioral health needs.
The purpose of this study is to identify subgroups of primary care patients with co-occurring behavioral health risk factors, to ascertain sociodemographic characteristics associated with these subgroups, and to explore clustering of patient subgroups by clinic type. We anticipated that study results could be used to guide population-level interventions addressing commonly co-occurring risk factors. The study sample comprised adults receiving care from nine primary care practices that participated in the My Own Health Report (MOHR) project, funded by the National Cancer Institute (NCI) and the Agency for Healthcare Research and Quality (AHRQ).17–19 While several studies have examined the co-occurrence of health risk factors in primary care, 20–22 these studies assessed a limited number of domains. The present study assessed a wider range of health domains. Furthermore, the study sample, which includes patients recruited from urban, rural, suburban, and safety net clinics across the country, is more diverse than prior studies.
Methods
Study Design and Setting
A detailed description of the MOHR study has been previously published.17–19,23 The study was a pragmatic cluster randomized trial to evaluate the effect of the MOHR feedback system on goal setting in nine primary care practices including five AHRQ-funded Practice Based Research Network and four Federally Qualified Health Centers sites. The MOHR assessment was administered to patients electronically to patients presenting for chronic disease management and wellness visits as part of routine care and data were used to generate individualized feedback reports for patients and providers. The assessment was completed by 1,707 patients between March and December of 2013.18 We excluded 79 patients (<5%) due to missing data on sociodemographics, resulting in a final analytic sample of 1,628. The study was approved by the Institutional Review Boards of participating sites.
Measures
The MOHR assessment consisted of 20 items measuring 13 domains. Items were selected through a national consensus building process.12 Respondents were considered to have scored “positive” for a domain/risk factor based on the following thresholds: Overweight/Obese: self-reported height and weight corresponding to a BMI value > 25 kg/m2; Inadequate physical activity: < 150 minutes of physical activity per week; Inadequate fruit and vegetable consumption: < 5 servings of fruits and vegetables per day; Excessive fast food consumption: 1 or more times per week; Excessive sugar-sweetened beverage consumption: 1 or more times per day; Sleep disturbance: “sometimes/often/always” experiencing daytime sleepiness; Tobacco use: use of tobacco or smokeless tobacco in the past 30 days; Excessive alcohol intake: 1 or more binge episodes in the past year (≥4 drinks/day for women, ≥5 drinks/day for men); Illicit drug use/inappropriate prescription use: 1 or more times in the past year; Anxiety: score of 4 or more on two PHQ items; Depression: score of 4 or more on two PHQ items; Stress: stress level of > 5 out of 10 in the past week.18 These definitions resulted in binary indicator variables for 12 factors. Nine sociodemographic characteristics were assessed: gender, age, race, ethnicity, English proficiency, employment status, marital status, nativity, and education. A one-item measure of perceived health was omitted from the present analysis.
Statistical Analysis
We conducted latent class analysis (LCA) using the LCA procedure in SAS 9.4 (SAS Institute, Cary, NC).24 LCA is a statistical method used to identify subgroups of individuals in a population who share important characteristics or behaviors, as indicated by their responses to a set of observed categorical variables.25 The subgroups are unobserved and are therefore considered latent classes. For our analyses, the observed variables characterizing the latent classes included the 12 behavioral and mental health risk factor indicators. LCA differs from cluster analysis in that it fits a statistical model to the data rather than finding clusters based on an arbitrary distance measure. Because LCA is based on a statistical model, model selection and goodness of fit procedures are available, and the model can be extended to include covariates to predict individuals’ latent class membership.26
To fit a LCA model, one specifies the number of latent classes, k. Then, using maximum likelihood, the procedure finds the best k-class solution and estimates the response probability on each indicator for each class, and the probability that each individual in the sample belongs to each class. Typically, models specifying an increasing number of latent classes (e.g., k = 2, 3, 4, 5) are fit and compared with the goal of identifying the best k. Selection of the number of latent classes is made by taking into account both model fit and interpretability. Following recommendations for LCA models,27,28 we used the Bayesian Information Criterion (BIC) and adjusted BIC to compare models with different k on model fit, where lower values indicate a better fit. For model interpretability, we were guided by the following considerations: each class should be distinguishable from the others based on the item response probabilities, no class should be trivial in size, and it should be possible to give each class a meaningful label.24
After identifying the best k, we assessed whether model fit could be improved by allowing different class solutions by sex and/or racial/ethnic groups and confirmed that this modification was unnecessary. We then assigned each individual to a latent class based on their class of highest posterior probability and tabulated the frequencies of the 12 health risk behaviors within each class.
Next, we extended the model to include demographic covariates to predict class membership. This extension, also performed using the LCA procedure, involved fitting a multinomial logistic regression model in which the multinomial outcome variable was latent class membership. These analyses resulted in odds ratios for membership in one class compared to a reference class based on covariates. We also tabulated demographic characteristics by latent class.
Results
Comparing Class Solutions
Table 1 displays model fit indices for LCA models specifying 2, 3, 4 or 5 latent classes. BIC favored the three-class solution while adjusted BIC favored the four-class solution. In terms of interpretability, the three-class solution had a normative class, a dietary risk class, and a class for mental health and substance abuse risk. The four-class solution separated the last class into two more clearly delineated classes. By comparison, the five-class solution resulted in worse fit indices and was less interpretable. Consequently, the four-class solution was selected. Using posterior probabilities of class membership, individuals were then assigned to one of the four latent classes.
Table 1.
Model fit criteria for latent class models with varying numbers of latent classes
| Number of latent classes specified | BIC | Adjusted BIC |
|---|---|---|
| 2 | 1956 | 1877 |
| 3 | 1775* | 1654 |
| 4 | 1787 | 1625* |
| 5 | 1847 | 1643 |
BIC and adjusted BIC are criteria for selecting the best model from among a set of possible models; lower values indicate better models.
Lowest value
Four Latent Class Model
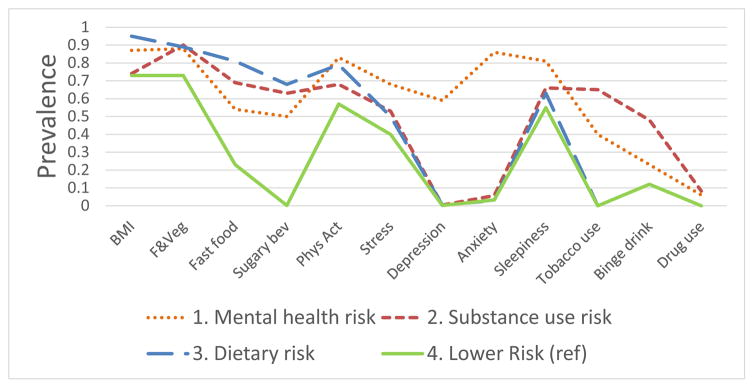
Table 2 and Figure 1 present the proportion of the sample assigned to each of the four classes and the prevalence of the 12 risk factors within each class. Class 1 had the highest rates of stress, anxiety, and worry of the four clusters and was consequently labeled “Mental Health Risk.” This class comprised 14% of the sample. Class 1 also had the highest rates of physical inactivity and daytime sleepiness among all classes. Class 2 had the highest rates of tobacco use, binge drinking, and drug use and was subsequently labeled the “Substance Use Risk” class. This group, which comprised 29% of the sample, also had high rates of inadequate fruit/vegetable intake and sugar sweetened beverage intake. Class 3, which also encompassed 29% of the sample had the highest rates of overweight/obese BMI, fast food intake, sugary beverage intake, and nearly the highest rate of inadequate fruit and vegetable intake and was therefore labeled the “Dietary Risk” class. This group also had among the lowest rates of anxiety, depression, tobacco, binge drinking, and drug use, which is underscored by Figure 1. Class 4 had the lowest rates of 8 of the 12 risk factors and did not have the highest rate of any risk factor, as illustrated in Figure 1, and was given the label of “Lower Risk.” This class comprised 27% of the sample. Close to three-quarters of Lower Risk class patients were overweight/obese. However, consumption of fast food and sugar-sweetened beverages was relatively infrequent, and tobacco and drug use was non-existent in this group.
Table 2.
Prevalence of behavioral health risk factor for the overall sample and by latent class
| Class 1 Mental Health Risk (n=232) |
Class 2 Substance Use Risk (n=474) |
Class 3 Dietary Risk (n=480) |
Class 4 Lower Risk (n=442) |
Overall (n=1628) | |
|---|---|---|---|---|---|
| % | % | % | % | % | |
| Class prevalence | 14 | 29 | 29 | 27 | 100 |
| Risk Factors | |||||
| Body mass index (≥25 kg/m2) | 87 | 74 | 95 | 73 | 82 |
| Fruits and vegetables (<5 servings/week) | 88 | 90 | 89 | 73 | 85 |
| Fast food (≥1 time/week) | 54 | 69 | 81 | 23 | 58 |
| Sugary beverages (≥1 time per day) | 50 | 63 | 68 | 0.2 | 46 |
| Physical activity (<150 minutes/week) | 83 | 68 | 79 | 57 | 70 |
| Stress (>4 stress/week) | 68 | 53 | 50 | 40 | 51 |
| Depression (Score ≥4) | 59 | <1 | <1 | <1 | 9 |
| Anxiety or worry (Score ≥4) | 86 | 6 | 3 | 3 | 16 |
| Sleepiness (Sometimes/Often/Always) | 81 | 66 | 63 | 55 | 64 |
| Tobacco use (Use in past 30 days) | 40 | 65 | 0.0 | 0.0 | 25 |
| Binge drinking (≥1 binge episode/year) | 23 | 48 | 12 | 12 | 24 |
| Drug use (≥1 time/year) | 6 | 8 | 0 | 0 | 3 |
Shading is provided for interpretation and indicates risk factors that have high prevalence within each class.
Reference group
Figure 1.
Relationship between Demographic Characteristics and Class Membership
Table 3 shows the sociodemographic characteristics of each class. Table 4 displays the results from the multinomial logistic regression model predicting class membership based on sociodemographic characteristics. The odds ratios in Table 4 compare the Lower Risk class (Class 4) to all other classes. Compared to individuals in the Lower Risk class, patients in the Mental Health Risk class were significantly less likely to be older (≥ 65 years), Latino/Hispanic, married, college educated, or employed full time. Patients in the Substance Use Risk class had over 2 times greater odds of being male (OR 2.8, 95% CI: 1.9, 4.2), African American (OR 2.3, 95% CI: 1.2, 4.3), and speaking English well (OR 2.7, 95% CI: 1.4, 5.2). They were also more likely to be younger and less likely to be Latino/Hispanic, married, or college-educated compared to the Lower Risk class. Patients in the Dietary Risk class had 7.7 times greater odds of being African American (95% CI: 4.0, 14.8) and were less likely to be married (OR 0.6, 95% CI: 0.4, 0.9) or have a college education (OR 0.3, 95% CI: 0.2, 0.5) compared to the Lower Risk class.
Table 3.
Demographic characteristics of the overall sample and by latent class
| Demographic Characteristic | Class 1 Mental Health Risk (n=232) |
Class 2 Substance Use Risk (n=474) |
Class 3 Dietary Risk (n=480) |
Class 4 Lower Risk (n=442) |
Overall (n=1628) |
|---|---|---|---|---|---|
|
| |||||
| % | % | % | % | % | |
|
| |||||
| Mean age, years (sd) | 47.2 (12.8) | 43.8 (14.9) | 51.3 (14.0) | 54.3 (14.8) | 49.3 (14.9) |
| < 30 | 9 | 26 | 7 | 7 | 13 |
| 30 to < 50 | 45 | 32 | 35 | 27 | 33 |
| 50 to < 65 | 39 | 35 | 40 | 38 | 38 |
| ≥ 65 | 7 | 7 | 18 | 28 | 16 |
|
| |||||
| Male | 21 | 53 | 19 | 35 | 34 |
|
| |||||
| Race/ethnicity | |||||
| White | 62 | 53 | 27 | 53 | 46 |
| Latino/Hispanic | 21 | 16 | 28 | 35 | 25 |
| African American | 13 | 25 | 44 | 5 | 24 |
| Other | 4 | 6 | 1 | 8 | 5 |
|
| |||||
| Married | 41 | 39 | 56 | 70 | 53 |
|
| |||||
| At least some college | 34 | 36 | 30 | 65 | 44 |
|
| |||||
| Employed | 25 | 50 | 47 | 44 | 44 |
|
| |||||
| Speaks English very well/well | 89 | 97 | 79 | 80 | 86 |
Table 4.
Association between demographic characteristics and class membership based on results of multinomial logistic regression
| Demographic Characteristic | Class 1 vs Class 4 (Mental Health Risk vs. Lower Risk) | Class 2 vs Class 4 (Substance Use Risk vs. Lower Risk) | Class 3 vs Class 4 (Dietary Risk vs. Lower Risk) |
|---|---|---|---|
|
| |||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
|
| |||
| Age (years) | |||
| < 30 (Ref) | 1.0 | 1.0 | 1.0 |
| 30 to < 50 | 1.5 (0.7, 3.1) | 0.4 (0.2, 0.7) | 0.9 (0.4, 1.8) |
| 50 to < 65 | 0.6 (0.3, 1.4) | 0.2 (0.1, 0.4) | 0.7 (0.3, 1.4) |
| ≥ 65 | 0.1 (0.1, 0.4) | 0.1 (0.0, 0.1) | 0.6 (0.3, 1.3) |
|
| |||
| Male | 0.8 (0.5, 1.2) | 2.8 (1.9, 4.2) | 0.7 (0.5, 1.1) |
|
| |||
| Race/ethnicity | |||
| White (Ref) | 1.0 | 1.0 | 1.0 |
| Latino/Hispanic | 0.3 (0.1, 0.5) | 0.3 (0.2, 0.5) | 0.8 (0.5, 1.5) |
| African American | 0.9 (0.4, 1.9) | 2.3 (1.2, 4.3) | 7.7 (4.0, 14.8) |
| Other | 0.4 (0.2, 1.0) | 0.8 (0.4, 1.4) | 0.5 (0.2, 1.2) |
|
| |||
| Married | 0.3 (0.2, 0.5) | 0.4 (0.2, 0.5) | 0.6 (0.4, 0.9) |
|
| |||
| At least some college | 0.2 (0.1, 0.4) | 0.2 (0.1, 0.3) | 0.3 (0.2, 0.5) |
|
| |||
| Employed | 0.4 (0.3, 0.7) | 1.2 (0.8, 1.7) | 1.4 (0.9, 2.0) |
|
| |||
| Speaks English very well/well | 1.8 (0.9, 3.7) | 2.7 (1.4, 5.2) | 1.0 (0.5, 1.8) |
Clinic Characteristics by Class Membership
The multinomial logistic regression model including clinic as a predictor was significant (p<0.001), indicating that the proportions of patients in each class varied by clinic. We examined class distribution by clinic location (urban, n = 3; suburban, n = 1; rural, n = 5) and type (FQHC, n = 6; non-FQHC, n = 3). The suburban clinic had a larger Lower Risk class (67% of sample), relative to urban (27% of sample) and rural clinics (22% of sample). The Dietary Risk class was larger in the FQHC clinics (36%) compared to non-FQHC clinics (16%). The Lower Risk class was larger in non-FQHC clinics (37%) compared to FQHC clinics (23%). We attempted to fit a multivariable model to examine the effect of clinic characteristics on class membership after controlling for individual sociodemographics. However, the model would not converge due to high collinearity between patient and clinic characteristics. For example, 93% of Latino/Hispanic patients were from three of the nine clinics. Thus, it was not possible to determine whether the variation in class size by clinic was due to clinic-level practices or sociodemographic differences in clinic populations.
Discussion
The LCA identified four meaningful latent subgroups of patients, each with distinct clusters of behavioral risks. No class represented more than 30% of the sample. By contrast, two prior studies that conducted LCA to examine the co-occurrence of behavioral risk factors identified solutions where classes represented substantially larger proportions. One study conducted among primary care patients in the VA study identified only three classes and found that 89% could be classified into a “healthier” class.20 Another conducted with National Epidemiologic Survey of Alcohol and Related Conditions (NESARC) data identified five classes and found 50% of the sample belonged to the “inactive, non-substance abuser” class.22 The results of these prior studies may differ from ours due to their assessment of fewer behavioral risk factor domains or their relatively more homogenous populations.
We found a low proportion of primary care patients fit into the lower risk class, which is consistent with prior descriptive research from the MOHR study.18 Prior analyses of MOHR data found that patients on average screened positive for six risk factors. Even though the group labeled “Lower Risk” given they had the lowest prevalence for most risk factors compared to the other three classes, it is important use caution when interpreting this group. No members used tobacco or drugs and few consumed sugar-sweetened beverages, consistent with healthy behavior. However, most “lower risk” patents were still overweight/obese, consumed inadequate servings of fruits and vegetables per day, and reported low physical activity levels, likely putting them at elevated risk for metabolic syndrome and chronic disease. These findings confirm the importance of population-wide efforts to support healthier eating, increased physical activity, and weight management, given the ubiquity of these issues in the population.
The Dietary Risk and Substance Use risk classes were the largest, each accounting for 29% of the sample. The size of the Dietary Risk class is not surprising; substantial prior research has documented the high prevalence of obesity and poor dietary and physical activity levels among primary care populations in the U.S.29–31 Although the link between overweight/obesity and negative health consequences is well documented,32 given the potential for misclassification,33 the value of BMI in predicting risk for chronic disease is enhanced when considered in combination with other measures such as blood pressure, triglycerides, and insulin resistance, when available. Perhaps more surprising was the equivalent size of the Substance Use Risk and Dietary Risk classes, despite relatively lower prevalence of substance abuse risk factors in the general population.34 The inclusion of prescription drug misuse as well as illicit drug use in our “substance use” measure may have contributed to this finding. Among patients in the Substance Use Risk class, tobacco was the most common risk factor, followed by binge drinking and drug use. It should be noted that reported illicit drug use/prescription drug misuse was relatively low in our sample compared to rates observed in other primary care samples.35,36 Although at present addressing tobacco use is strongly emphasized in primary care, binge drinking and illicit drug use are infrequently addressed in this setting.37 Co-occurrence of these risk factors in our primary care sample suggests a bundled intervention approach may be warranted. This type of approach may be more feasible if clinics’ existing infrastructure for tobacco control can be leveraged to address binge drinking and drug use. Despite well-documented associations between mental health conditions and substance use,38 few patients in the substance use class screened positive for anxiety or depressive symptoms. This could suggest underreporting of mental health symptoms in this group or that the substance use class is currently experiencing less anxiety and depressive symptoms, potentially due to self-medicating.39,40
The Mental Health Risk class was the smallest, accounting for 14% of the sample. In addition to high rates of the risk factors that earned it the label of “Mental Health Risk” (e.g., stress, anxiety, depression), this class also showed the highest rates of sleeplessness and inadequate physical activity, plus very low levels of fruit and vegetable intake. Prior research has found that interventions aimed at promoting physical activity may be effective in reducing depressive symptoms.41,42 Studies have also documented the importance of addressing sleep disorders simultaneously with depression and anxiety. Our findings further underscore the potential value of goal-setting for physical activity and sleep habits with primary care patients who are experiencing depression, stress, or anxiety.
Examples of prior multiple risk factor interventions include those that simultaneously target nutrition and physical inactivity, smoking cessation and weight gain, and various, multiple substance abuse behaviors.11,13,43,44 Such interventions have often targeted populations with specific chronic conditions such as cardiovascular disease,11,13,45 diabetes,11 and cancer.13,46–48 Although more research is needed, there is some evidence to support that implementing interventions that simultaneously address multiple risk factors may be as or more effective than interventions targeting single risk factors or behaviors.14,15,49,50 Our results underscore the potential value of bundled approaches and provide guidance for the direction such interventions. Adaptive interventions applying the Multiphase Optimization Strategy (MOST) or Sequential Multiple Assignment Randomized Trial (SMART) designs emphasize tailoring intervention types, intensity, and sequence at multiple stages based on individual progress, which offers a promising approach to increasing efficiency and effectiveness of interventions.51 For example, Strecher and colleagues (2008) evaluated the effect of a web-based smoking cessation program that used a MOST design to compare the results of varying five intervention aspects. Study results revealed that a higher level of tailoring for two aspects resulted in greater tobacco abstinence at 6-month follow-up compared to less tailoring.52 “SMART” electronic health record-based interventions may present opportunities for providers to monitor patient progress over time (e.g., blood pressure, BMI, etc.) and develop customized treatment plans designed to give a patient the right type and dose of an intervention at the right time.
Examining the relationship between demographics and class membership may be useful when choosing among intervention strategies. The Mental Health Risk class tended to be younger, less ethnically-diverse, unmarried, and less educated compared to other classes. However, these associations may be reflective of demographic differences in who is willing to report symptoms of mental illness, rather than the prevalence of those symptoms. Prior research has found greater mental health-related stigma among ethnic minorities compared to whites.53,54 The Dietary Risk class included a substantially greater proportion of African Americans, unmarried patients, and less educated patients compared to patients in the Lower Risk class. The Substance Use class tended to be younger, male, African American, and were less likely to be married or have a college education compared to the Lower Risk class. Prior research has observed higher rates of tobacco use, binge drinking, and illicit drug use among younger males with low levels of education, compared to other segments of the population, which is consistent with our findings.55–57 It should be noted that our substance use results may not reflect the growing opioid epidemic among non-Latino whites, given few participating clinic sites were located in high burden areas.58
The study has limitations. We are only able to classify people based on risk factors measured. Sun exposure, non-aerobic physical activity, and more nuanced aspects of diet, although relevant were not measured. The MOHR assessment relied on self-report, which could lead to under-reporting, particularly for sensitive behaviors. The tool was designed to screen for risk factors, not to estimate the proportion of patients with diagnosable mental health or substance use conditions. Although we sought to engage a diverse set of primary care clinics, we did not intend to recruit a nationally representative sample, which limits generalizability of findings. Nevertheless, strengths of the study include a large and diverse sample, assessment of a wider variety of domains than prior studies, and use of LCA methods.20–22,59,60
Although it is important to understand the co-occurrence of risk factors among primary care patients, it remains a tremendous challenge to address these risk factors within a primary care setting.61–63 Addressing multiple risk factors simultaneously may seem even more challenging than addressing risk factors one-by-one. Providers are unlikely to be able to address more than one risk factor in any single visit. Given this context, significant attention has focused on integration of technology that may reduce provider burden.
Prior studies conducted in the VA have demonstrated the feasibility of assessing multiple risk factors using electronic health records (EHRs), which may translate to other settings.20,59 There is also considerable interest in wearable health technology. To be most useful, data from wearable technologies could be automatically synced to the EHR and used to trigger EHR-guided interventions such as clinical decision prompts, patient-directed text messages, or patient portal alerts. However, to date these technologies have primarily been used to address physical activity. Additional research is needed to better understand how these technologies could be used to address other risk factors (e.g., diet, sleep, alcohol use, mood), perhaps by integrating self-report data. Given the heavy burden posed by behavioral health issues in primary care, effectively and efficiently addressing co-occurring risk factors in this setting is critical.
Study Highlights.
Using latent class analysis, we identified four distinct patient subgroups (i.e., classes)
The classes included Mental Health Risk, Substance Use Risk, Dietary Risk, and a Lower Risk class
Substance use and dietary risk classes were the largest, each representing 29% of the sample
The mental health risk was the smallest class, encompassing 14% of the sample
A number of demographic factors differentiated the lower risk class from the other three classes
Acknowledgments
Funding for the MOHR project was provided by the National Cancer Institute, Agency for Healthcare Research and Quality, Office of Behavioral and Social Sciences Research, and National Center for Advancing Translational Sciences (NCATS; CTSA Grant Number ULTR00058). Narissa Nonzee was also supported by NIH/NCATS UCLA CTSI Grant Numbers TL1TR000121 and TL1TR001883. The opinions expressed in this manuscript are those of the authors and do not necessarily reflect those of the funders. We would also like to thank L. Cindy Chang for her assistance with data cleaning and analysis.
Footnotes
Conflict of Interest: The authors declare there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004 Mar 10;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Fisher EB, Fitzgibbon ML, Glasgow RE, et al. Behavior matters. Am J Prev Med. 2011 May;40(5):e15–30. doi: 10.1016/j.amepre.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coups EJ, Gaba A, Orleans CT. Physician screening for multiple behavioral health risk factors. Am J Prev Med. 2004 Aug;27(2 Suppl):34–41. doi: 10.1016/j.amepre.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Poortinga W. The prevalence and clustering of four major lifestyle risk factors in an English adult population. Prev Med. 2007 Feb;44(2):124–128. doi: 10.1016/j.ypmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Noble N, Paul C, Turon H, Oldmeadow C. Which modifiable health risk behaviours are related? A systematic review of the clustering of Smoking, Nutrition, Alcohol and Physical activity (‘SNAP’) health risk factors. Prev Med. 2015 Dec;81:16–41. doi: 10.1016/j.ypmed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Loprinzi PD. Health behavior combinations and their association with inflammation. American Journal of Health Promotion. 2016;30(5):331–334. doi: 10.1177/0890117116646340. [DOI] [PubMed] [Google Scholar]
- 8.Mamudu HM, Paul TK, Wang L, et al. The effects of multiple coronary artery disease risk factors on subclinical atherosclerosis in a rural population in the United States. Prev Med. 2016 Jul;88:140–146. doi: 10.1016/j.ypmed.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Berrigan D, Dodd K, Troiano RP, Krebs-Smith SM, Barbash RB. Patterns of health behavior in U.S. adults. Prev Med. 2003 May;36(5):615–623. doi: 10.1016/s0091-7435(02)00067-1. [DOI] [PubMed] [Google Scholar]
- 10.Stange KC, Woolf SH, Gjeltema K. One minute for prevention: the power of leveraging to fulfill the promise of health behavior counseling. Am J Prev Med. 2002 May;22(4):320–323. doi: 10.1016/s0749-3797(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein MG, Whitlock EP, DePue J. Multiple behavioral risk factor interventions in primary care. Summary of research evidence. Am J Prev Med. 2004 Aug;27(2 Suppl):61–79. doi: 10.1016/j.amepre.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Estabrooks PA, Boyle M, Emmons KM, et al. Harmonized patient-reported data elements in the electronic health record: supporting meaningful use by primary care action on health behaviors and key psychosocial factors. J Am Med Inform Assoc. 2012 Jul-Aug;19(4):575–582. doi: 10.1136/amiajnl-2011-000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prochaska JJ, Prochaska JO. A review of multiple health behavior change interventions for primary prevention. American journal of lifestyle medicine. 2011;5(3):208–221. doi: 10.1177/1559827610391883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyman DJ, Pavlik VN, Taylor WC, Goodrick GK, Moye L. Simultaneous vs sequential counseling for multiple behavior change. Archives of internal medicine. 2007 Jun 11;167(11):1152–1158. doi: 10.1001/archinte.167.11.1152. [DOI] [PubMed] [Google Scholar]
- 15.James E, Freund M, Booth A, et al. Comparative efficacy of simultaneous versus sequential multiple health behavior change interventions among adults: A systematic review of randomised trials. Prev Med. 2016 Aug;89:211–223. doi: 10.1016/j.ypmed.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Nigg CR, Long CR. A systematic review of single health behavior change interventions vs. multiple health behavior change interventions among older adults. Translational behavioral medicine. 2012 Jun;2(2):163–179. doi: 10.1007/s13142-012-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krist AH, Phillips SM, Sabo RT, et al. Adoption, reach, implementation, and maintenance of a behavioral and mental health assessment in primary care. Ann Fam Med. 2014 Nov-Dec;12(6):525–533. doi: 10.1370/afm.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips SM, Glasgow RE, Bello G, et al. Frequency and prioritization of patient health risks from a structured health risk assessment. Ann Fam Med. 2014 Nov-Dec;12(6):505–513. doi: 10.1370/afm.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krist AH, Glasgow RE, Heurtin-Roberts S, et al. The impact of behavioral and mental health risk assessments on goal setting in primary care. Translational behavioral medicine. 2016 Jun;6(2):212–219. doi: 10.1007/s13142-015-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funderburk JS, Maisto SA, Sugarman DE, Wade M. The covariation of multiple risk factors in primary care: a latent class analysis. J Behav Med. 2008 Dec;31(6):525–535. doi: 10.1007/s10865-008-9176-1. [DOI] [PubMed] [Google Scholar]
- 21.Héroux M, Janssen I, Lee D-c, Sui X, Hebert JR, Blair SN. Clustering of unhealthy behaviors in the aerobics center longitudinal study. Prevention Science. 2012;13(2):183–195. doi: 10.1007/s11121-011-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leventhal AM, Huh J, Dunton GF. Clustering of modifiable biobehavioral risk factors for chronic disease in US adults: a latent class analysis. Perspect Public Health. 2014 Nov;134(6):331–338. doi: 10.1177/1757913913495780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krist AH, Glenn BA, Glasgow RE, et al. Designing a valid randomized pragmatic primary care implementation trial: the my own health report (MOHR) project. Implementation science : IS. 2013;8:73. doi: 10.1186/1748-5908-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: A SAS Procedure for Latent Class Analysis. Structural equation modeling : a multidisciplinary journal. 2007;14(4):671–694. doi: 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins LM, Lanza ST. Latent class and latent transition analysis for the social, behavioral, and health sciences. New York: Wiley; 2010. [Google Scholar]
- 26.Hagenaars JA, McCutcheon AL. Applied Latent Class Analysis. Cambridge, England: Cambridge University Press; 2009. [Google Scholar]
- 27.Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo Simulation Study. Structural Equation Modeling. 2007;14(4):535–569. [Google Scholar]
- 28.Yang C. Evaluating latent class analyses in qualitative phenotype identification. Computational Statistics & Data Analysis. 2006;50:1090–1104. [Google Scholar]
- 29.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016 Jun 07;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keadle SK, McKinnon R, Graubard BI, Troiano RP. Prevalence and trends in physical activity among older adults in the United States: A comparison across three national surveys. Prev Med. 2016 Aug;89:37–43. doi: 10.1016/j.ypmed.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014 Jul 05;384(9937):45–52. doi: 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- 32.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC public health. 2009 Mar 25;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomiyama AJ, Hunger JM, Nguyen-Cuu J, Wells C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005–2012. International journal of obesity. 2016 May;40(5):883–886. doi: 10.1038/ijo.2016.17. [DOI] [PubMed] [Google Scholar]
- 34.National Center for Chronic Disease Prevention and Health Promotion. Chronic Diseases: The Leading Causes of Death and Disability in the United States. 2016 https://www.cdc.gov/chronicdisease/overview/index.htm.
- 35.McNeely J, Cleland CM, Strauss SM, Palamar JJ, Rotrosen J, Saitz R. Validation of Self-Administered Single-Item Screening Questions (SISQs) for Unhealthy Alcohol and Drug Use in Primary Care Patients. J Gen Intern Med. 2015 Dec;30(12):1757–1764. doi: 10.1007/s11606-015-3391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilowsky DJ, Wu LT. Screening for alcohol and drug use disorders among adults in primary care: a review. Substance abuse and rehabilitation. 2012 Apr;3(1):25–34. doi: 10.2147/SAR.S30057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramsay PP, Shortell SM, Casalino LP, Rodriguez HP, Rittenhouse DR. A Longitudinal Study of Medical Practices’ Treatment of Patients Who Use Tobacco. Am J Prev Med. 2016 Mar;50(3):328–335. doi: 10.1016/j.amepre.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of general psychiatry. 2004 Aug;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 39.Bolton JM, Robinson J, Sareen J. Self-medication of mood disorders with alcohol and drugs in the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of affective disorders. 2009 Jun;115(3):367–375. doi: 10.1016/j.jad.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Gehricke JG, Loughlin SE, Whalen CK, et al. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2007 Nov;9( Suppl 4):S523–536. doi: 10.1080/14622200701685039. [DOI] [PubMed] [Google Scholar]
- 41.Catalan-Matamoros D, Gomez-Conesa A, Stubbs B, Vancampfort D. Exercise improves depressive symptoms in older adults: An umbrella review of systematic reviews and meta-analyses. Psychiatry research. 2016 Oct 30;244:202–209. doi: 10.1016/j.psychres.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 42.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. The Cochrane database of systematic reviews. 2013 Sep 12;(9):CD004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellegrini CA, Steglitz J, Johnston W, et al. Design and protocol of a randomized multiple behavior change trial: Make Better Choices 2 (MBC2) Contemporary clinical trials. 2015 Mar;41:85–92. doi: 10.1016/j.cct.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spring B, Howe D, Berendsen M, et al. Behavioral intervention to promote smoking cessation and prevent weight gain: a systematic review and meta-analysis. Addiction. 2009 Sep;104(9):1472–1486. doi: 10.1111/j.1360-0443.2009.02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebrahim S, Beswick A, Burke M, Smith DG. Multiple risk factor interventions for primary prevention of coronary heart disease (Review) John Wiley & Sons, Ltd; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amireault S, Fong AJ, Sabiston CM. Promoting healthy eating and physical activity behaviors: A systematic review of multiple behavior change interventions among cancer survivors. American Journal of Lifestyle Medicine. 2016:1–15. doi: 10.1177/1559827616661490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green AC, Hayman LL, Cooley ME. Multiple health behavior change in adults with or at risk for cancer: a systematic review. American journal of health behavior. 2015 May;39(3):380–394. doi: 10.5993/AJHB.39.3.11. [DOI] [PubMed] [Google Scholar]
- 48.Stacey FG, James EL, Chapman K, Courneya KS, Lubans DR. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. Journal of cancer survivorship : research and practice. 2015 Jun;9(2):305–338. doi: 10.1007/s11764-014-0413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prochaska JJ, Prochaska JO. A Review of Multiple Health Behavior Change Interventions for Primary Prevention. Am J Lifestyle Med. 2011 May;5(3) doi: 10.1177/1559827610391883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prochaska JJ, Velicer WF, Prochaska JO, Delucchi K, Hall SM. Comparing intervention outcomes in smokers treated for single versus multiple behavioral risks. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2006 May;25(3):380–388. doi: 10.1037/0278-6133.25.3.380. [DOI] [PubMed] [Google Scholar]
- 51.Collins LM, Nahum-Shani I, Almirall D. Optimization of behavioral dynamic treatment regimens based on the sequential, multiple assignment, randomized trial (SMART) Clin Trials. 2014 Aug;11(4):426–434. doi: 10.1177/1740774514536795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strecher VJ, McClure JB, Alexander GL, et al. Web-based smoking-cessation programs: results of a randomized trial. Am J Prev Med. 2008 May;34(5):373–381. doi: 10.1016/j.amepre.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nadeem E, Lange JM, Edge D, Fongwa M, Belin T, Miranda J. Does stigma keep poor young immigrant and U.S.-born Black and Latina women from seeking mental health care? Psychiatr Serv. 2007 Dec;58(12):1547–1554. doi: 10.1176/ps.2007.58.12.1547. [DOI] [PubMed] [Google Scholar]
- 54.Conner KO, Copeland VC, Grote NK, et al. Mental health treatment seeking among older adults with depression: the impact of stigma and race. Am J Geriatr Psychiatry. 2010 Jun;18(6):531–543. doi: 10.1097/JGP.0b013e3181cc0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centers for Disease C, Prevention. Vital signs: binge drinking prevalence, frequency, and intensity among adults - United States, 2010. MMWR. Morbidity and mortality weekly report. 2012 Jan 13;61(1):14–19. [PubMed] [Google Scholar]
- 56.Centers for Disease C, Prevention. Current cigarette smoking among adults - United States, 2011. MMWR. Morbidity and mortality weekly report. 2012 Nov 9;61(44):889–894. [PubMed] [Google Scholar]
- 57.Substance Abuse and Mental Health Services Administration. Age- and genderbased Populations. 2017 https://www.samhsa.gov/specific-populations/age-gender-based.
- 58.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. [Accessed 11/30/17];Opioid Overdose. 2016 https://www.cdc.gov/drugoverdose/data/statedeaths.html.
- 59.Funderburk JS, Kenneson A, Maisto SA. Identifying classes of veterans with multiple risk factors. Military medicine. 2014 Oct;179(10):1119–1126. doi: 10.7205/MILMED-D-14-00119. [DOI] [PubMed] [Google Scholar]
- 60.Rebholz CE, Rueegg CS, Michel G, et al. Clustering of health behaviours in adult survivors of childhood cancer and the general population. British journal of cancer. 2012 Jul 10;107(2):234–242. doi: 10.1038/bjc.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995 Nov;24(6):546–552. doi: 10.1006/pmed.1995.1087. [DOI] [PubMed] [Google Scholar]
- 62.Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: patient and provider factors. Diabetes research and clinical practice. 2011 Jul;93(1):1–9. doi: 10.1016/j.diabres.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? American journal of public health. 2003 Apr;93(4):635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]