Abstract
Exposure to early life adversity may disrupt the development and maturation of neurons and brain circuits, which, in turn, underlie neurodevelopment and mental illnesses. During fetal life, maternal adversity is conveyed to the developing brain via several molecular signals, including the stress hormone corticotropin releasing hormone (CRH). Employing a large well characterized prospective cohort, we find that fetal exposure to placental-origin CRH levels predicts structural and functional brain outcomes in children. Specifically, elevated placental CRH levels portend thinning of selective cortical regions of exposed individuals, with commensurate cognitive and emotional deficits. Notably, the relations of placental-origin CRH to cortical thinning and childhood symptoms are sex-specific. In view of the established effects of CRH on survival and arborization of cortical neurons, these findings position placental CRH as an important mediator of the consequences of early-life adversity on neuropsychiatric outcomes.
Introduction
Exposure to early life adversity disrupts brain development resulting in altered brain networks and structure (1–3). These structural changes are associated with functional alterations that are often maintained throughout the lifespan, mediated by enduring epigenetic modifications of gene expression (2–4). Indeed, enduring alterations of brain structure and function at molecular, cellular and circuit levels (5,6) are considered fundamental mechanisms of how early-life experiences influence health and disease (1–5). In the aggregate, exposures to intrauterine and neonatal insults (7) and consequent altered brain anatomy (8,9) and connectivity (10) contribute significantly to the global burden of mental illness.
Specifically, early-life exposure to maternal stress and trauma is linked to subsequent depression (11–13), posttraumatic stress disorder (PTSD), panic disorder, substance abuse, abnormal stress response (13,14) and other serious disorders (2,3,11). Emerging evidence suggests that exposure to early-life adversity may be causally related to brain changes underlying the risk for psychopathology (7,15,16). Because the fetal period is unmatched by any other in growth and development, this stage in the human life span is the most vulnerable to both organizing and disrupting maternal signals.
Among the most salient signals shaping the human fetus is the stress hormone, corticotropic-releasing hormone (CRH). CRH, a 41-amino acid neuropeptide, is normally synthesized primarily in the paraventricular nucleus of the hypothalamus and has a major role in regulating pituitary-adrenal function and the physiological response to stress (17,18). CRH synthesized and released in other brain regions such as hippocampus (19) and cortex (20,21) contributes to the sculpting of neuronal dendritic development and maturation via its actions on specific receptors that are located on dendritic spines Indeed, nanomolar concentrations of CRH can excessively prune dendritic trees of developing rodent cortical neurons (22). CRH of brain origin is not detectable in the circulation (23). However, during human pregnancy, the CRH gene, located on the long arm of chromosome 8 (24), is expressed in the human placenta and amniotic membrane (25,26). Placental CRH (pCRH) is released into the maternal and fetal compartments as early as the eighth week of gestation and increases exponentially across gestation to regulate fetal maturation (27), metabolic functions (28,29) and the timing of birth (30).
Placental CRH expression is responsive to a range of maternal stress signals, including increased cortisol, norepinephrine and epinephrine, reduced uterine blood flow, and infection (31–33). Thus, placental CRH represents an integrative pathway through which diverse prenatal stressors inform the fetus of the state of its environment and shape fetal developmental trajectories in preparation for life after birth (34,35). There are several reports linking elevated human fetal exposure to pCRH including to decreased fetal startle and habituation (27,36), delayed neonatal neuromotor development (37); increased infant fear and distress (38); and prodromal markers of increased risk for affective disorders in young children (39). Here, we demonstrate the potential consequences of fetal exposure to elevated levels of CRH and identify putative mechanisms. Specifically, we find that higher levels of placental CRH are associated with cortical thinning in selective brain regions and with decreased cognitive and emotional function in preadolescents.
Methods
All methods, human procedures and protocols were approved by the Institutional Review Boards of the Universities of California-Irvine and Los Angeles, and Cedars-Sanai Hospital, Los Angeles. Parents and children gave informed (or affirmed) consent for all aspects of the protocol.
Participants
Ninety–seven mother/child dyads consented to participate in a longitudinal study of fetal exposures to maternal stress hormones on child MRI (see Consort Diagram, Figure S1; Tables S1A-1C). Women provided informed consent to provide a blood sample at five intervals during gestation; 13.5–16.6 (M=15.3), 17.8–20.5 (M=19.2), 23.7–26.5 (M=24.9), 29.9–32.3 (M=30.9) and 34.6–38.1 (M=35.9). All women were English-speaking, healthy adult (>18 years of age) pregnant women with singleton, intrauterine pregnancies. Subjects were excluded if they had (i) multiple births, (ii) tobacco, alcohol, or other drug use in pregnancy, (iii) uterine or cervical abnormalities, or (iv) presence of any conditions associated with dysregulated neuroendocrine function. Their children (48 boys, 49 girls) were enrolled at 6-9 years of age (M=7.3 ± 0.91). All participants provided written informed consent after receiving a complete description of the study.
Assessments of pCRH in pregnant women
Gestational age at testing was determined by last menstrual period and was confirmed by obstetric ultrasonographic biometry before 20 weeks. Maternal blood samples (20/ml) were collected serially at five intervals throughout pregnancy by antecubital venipuncture into siliconized ethylenediaminetetraacetic acid (EDTA) (purple top) vacutainers and then immediately chilled to 60C. Samples were centrifuged at 2,000 g for 15 minutes, decanted into polypropylene tubes prepared with aprotinin (Sigma Chemical, St. Louis, MO; 500 KIU/ml blood) and stored at −80°C until assayed.
pCRH determination
Following previously reported methods (40), the concentration of total maternal pCRH was determined by radio-immunoassay (RIA; Bachem Peninsula Laboratories, San Carlos, CA). The CRH assay had less than 0.01% cross-reactivity with ovine CRH, 36% cross-reactivity with bovine CRH and non-detectable reactivity with human ACTH. The intra- and inter-assay coefficient of variance ranged from 5 to 15%, respectively. The minimum detectable dose of the assay is 2.04 pg/ml (95% confidence interval; See Supplement for additional assay details).
Data reduction for the RIA was conducted with a computer-assisted four-parameter logistics program (41). Values exhibiting greater than 25% error (deviation from the standard curve) were not included in the analyses. A subset of samples (n = 60) were sent to a clinical laboratory (Quest Diagnostics) for further validation. The correlation between the two sets of data was 0.87 (p < .01). Shared variance between 19 and 31 weeks CRH samples was a modest 14% (r=0.38). Mother/child dyads were recruited from two separate studies with identical prenatal assessments. To ensure comparability between the cohorts for analysis, the pCRH values were standardized within the two groups and then combined for statistical analysis. (see Supplement for details). As expected, pCRH levels increased geometrically as gestation advanced (Figure S4; Table S9).
Assessments in children
All children (ages 87.3 ± 10.9 mo) had a stable neonatal course (Median Apgar = 9, Range 8 to 10; GA at birth=39.2 ± 1.5 wks) and were without known neonatal illness or congenital, chromosomal, or genetic anomalies. Participants had no evidence of neurological abnormalities in the newborn period. Children’s structural MRI (sMRI) images were assessed for normal anatomical appearance. Seven children with motion artifacts and three children with abnormal scans were not included in the final sample (N=97). These ten subjects did not differ from subjects providing useable scans (Table S8). At 6-9 years of age, no physical conditions were reported by the parents in a structured interview format of the MacArthur Health and Behavior Questionnaire (42). The majority (88%) of children were right hand dominant (Edinburgh Handedness Inventory) (43).
Structural MRI (sMRI) Acquisition
The sMRI scan was acquired with a 3-T Philips Achieva system. Children were provided earplugs and watched a movie while in the scanner to increase compliance and minimize movement. A high resolution T1 anatomical scan was acquired in the sagittal plane with 1mm3 isotropic voxel dimensions. An Inversion-Recovery Spoiled Gradient Recalled Acquisition (IR-SPGR) sequence with optimal parameters was applied: repetition rate (TR)= 11ms, echo time (TE)= 3.3ms, inversion time (TI)= 1100ms, turbo field echo factor (TFE)= 192, number of slices: 150, no SENSE acceleration, flip angle=180°, shot interval (time from inversion pulse to the center of acquisition) = 2200ms. The images were reviewed by the MRI operator (who was unaware of any of the study parameters) immediately after the scan was completed. If there were visible signs of motion artifacts, the subject was asked to stay for an additional scan. If the subject agreed, a second scan was acquired.
Processing of MRI data
Cortical surface reconstruction and volumetric segmentation was performed with the FreeSurfer image analysis software suite (http://surfer.nmr.mgh.harvard.edu/). Streamlined image processing procedures included; application of intensity normalization prior to segmentation to minimize errors in identifying the boundaries (44); removal of non-brain tissues (45); and transforming images into the Talairach space. Pial and white matter surfaces were located by finding the highest intensity gradient. Surface inflation was applied to each individual brain (46) and the inflated brains were registered to a spherical atlas. Cortical thickness was the closest distance from the gray matter/white matter surface to the pial surface at each vertex on the tessellated surface (47).
Assessment of percent of cortical areas affected by fetal exposure to pCRH
Smoothing of images was done prior to regression. The command line interface was used for FDR corrections and to accommodate more than one nuisance variable. Gestational age at birth, birth weight, age of the child at testing, sex and handedness were included as covariates. A threshold of p<0.05 was used for all statistical tests and outcomes were corrected for multiple comparisons using False Discovery Rate (FDR).
After FDR corrections and spatial normalization, the number of vertices that were significantly associated with pCRH was determined in each region of the cortex. The number of significant vertices for each area was added and divided by the total number of vertices in that area to provide the percentage of the vertices that were significantly associated with pCRH concentrations. The same procedure was computed for the number of significant vertices in each lobe. For hemispheric and whole brain percentages, the procedure was the same except the total number of subcortical vertices was subtracted from the total.
Child Behavioral Analyses
We conducted a battery of tests that interrogate several brain regions and circuits, to obtain a relatively broad assessment of cognitive and emotional function.
The Child Behavior Checklist (CBCL) (48) is one of the most widely used parental interviews for identifying affective and conduct disorder problems in children. Structured interviews (rather than a questionnaire) were administered to mothers about the behavior of their children. We focused on two inclusive scales; one that assessed Internalizing problems (sum of anxious-depressed, withdrawn-depressed, and somatic-complaints scores) and the other that evaluated externalizing problems (sums rule-breaking and aggressive behavior problem scores). Responses were recorded on a Likert scale ranging from 0 = Not True, to 2 = Very True or Often True. Scores are summed and standardized scores are computed that are age and sex-specific.
Reaction time to incongruent stimuli
The “Flanker” is an executive function task that requires the ability to resolve conflicts when competing information is present (49). Participants view five arrows arrayed horizontally on a screen and are instructed to press a left or right response button based on the direction of the center arrow (target). They are instructed to ignore the surrounding arrows which are either congruent (all aligned in the same direction) or incongruent with the center arrow. This task consists of 24 congruent trials and 24 incongruent trials. Each set of arrows is presented until the child responds (maximum of 5000 msec) with a 750 msec inter-trial interval. Among the scores median reaction time to targets with incongruent distractors correlates most highly with other indexes of performance derived from this test (all r’s >0.70). (The association between externalizing scores on the CBCL and the median reaction time on the flanker test was not significant (r= 0.09, p=.41)
RESULTS
Elevated CRH levels throughout gestation are associated with cortical thinning
Significant associations between fetal exposure to pCRH averaged across gestation and areas of cortical thinning in children were found as illustrated in the pial maps (Figure 1). Children exposed to high levels of pCRH throughout fetal life exhibited significant thinning in 12% of the whole cortical mantle (Table S2A). The anatomical distribution of the cortical thinning associated with CRH exposure involved equally the left (LH, 12%) and right (RH, 11%) hemispheres (Table S2A). Regional analyses indicated that cortical thinning associated with pCRH levels was primarily in the temporal (15%) and the frontal (16%) regions (Table 1). Figures 1B-D are illustrative scatterplots of the associations between total fetal exposure to pCRH across gestation and cortical thinning in the frontal and temporal areas (all significant p< 0.01-0.001). The representative scatterplots coupled with Table 1, highlight the widespread association between fetal exposures to pCRH and regional cortical thinning, which are most notable in temporal and frontal areas. Average concentrations of pCRH across gestation or levels at any gestational interval did not associate with increased cortical thickness either globally or in any cortical region (Table S2b). Focusing on two gestational ages, 19 weeks (early, when pCRH production begins to accelerate) and 31 weeks (late, the time strongly associated with preterm birth), we found that maternal levels of pCRH were significantly associated with cortical thinning.
FIGURE 1.
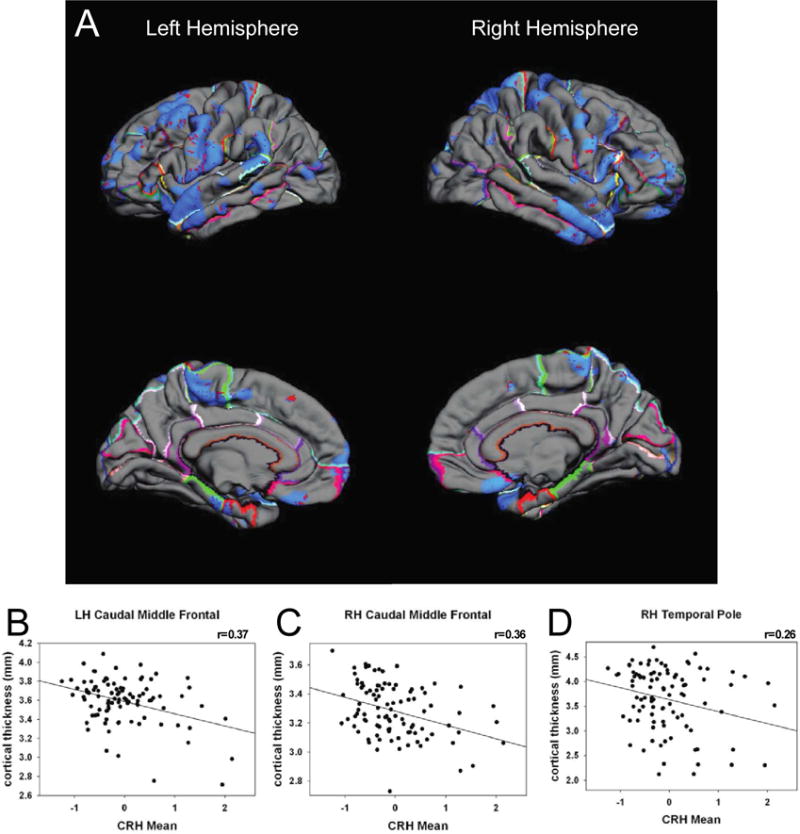
Pial maps (A) illustrating statistically significant areas of cortical thinning associated with average prenatal levels of pCRH measured throughout gestation. Representative scatterplots (B) r=0.37; (C) r=0.36; (D) r=0.26, all significant (p< 0.01-0.001), revealing the associations between placental corticotropin releasing hormone (CRH) and cortical thinning in specific cortical regions
Table 1.
Percentage of structures of the frontal and temporal lobes that are thinner in children exposed to placental CRH averaged across gestation. The results are presented for the left and right hemisphere.
| AVERAGE ACROSS GESTATION | |||
|---|---|---|---|
| LH Medial Surface Frontal | Percent of Structure | RH Medial Surface Frontal | Percent of Structure |
| Orbital Frontal | 11 | Orbital Frontal | 19 |
| Paracentral | 43 | Paracentral | 14 |
| LH Lateral Surface Frontal | Percent of Structure | RH Lateral Surface Frontal | Percent of Structure |
|---|---|---|---|
| Superior Frontal | 13 | Superior Frontal | 44 |
| Rostral Middle Frontal | 12 | Rostral Middle Frontal | 14 |
| Frontal Pole | 41 | Frontal Pole | 33 |
| Pars Triangularis | 16 | Pars Triangularis | 21 |
| Parsorbitalis | 6 | Parsorbitalis | 5 |
| Lateral Orbital Frontal | 15 | Lateral Orbital Frontal | 25 |
| Parsopercularis | 9 | Parsopercularis | 22 |
| Caudal Middle Frontal | 4 | Caudal Middle Frontal | 23 |
| Precentral | 18 | Precentral | 20 |
| Insula | 11 | Insula | 8 |
| LH Lateral Surface Temporal | Percent of Structure | RH Lateral Surface Temporal | Percent of Structure |
|---|---|---|---|
| Superior Temporal | 39 | Superior Temporal | 12 |
| Transverse Temporal | 0 | Transverse Temporal | 0 |
| Middle Temporal | 22 | Middle Temporal | 24 |
| Post Sup Temp sulcus | 16 | Post Sup Temp sulcus | 0 |
| Inferior Temporal | 3 | Inferior Temporal | 12 |
| Fusiform | 7 | Fusiform | 2 |
| Parahippocampal | 11 | Parahippocampal | 0 |
| Entorhinal | 31 | Entorhinal | 0 |
| Temporal Pole | 4 | Temporal Pole | 70 |
CRH levels early in gestation: regional cortical thinning and behavioral outcomes
Fetal exposure to pCRH early in gestation (19 weeks) was associated with significant thinning of the frontal poles. The significant association between pCRH and cortical thinning was bilateral, but strongest in the right (B=0.14, p<0.001; 78% of the structure) compared with the left frontal pole (B=−6.88, p<0.05; 69% of the structure; Table S3). Pial maps depict areas of significant thinning linked with fetal pCRH exposure early in gestation (Figure 2A) and the scatterplots illustrate the regions of the frontal cortex with the strongest association (Figures 2B-D). (Findings were essentially unchanged with log transformed pCRH values, Table S5). Nearly identical associations were observed in a smaller subsample (N=57) of children exposed to high pCRH levels measured even earlier, at 15 weeks of fetal life (Figures S3A-D).
FIGURE 2.
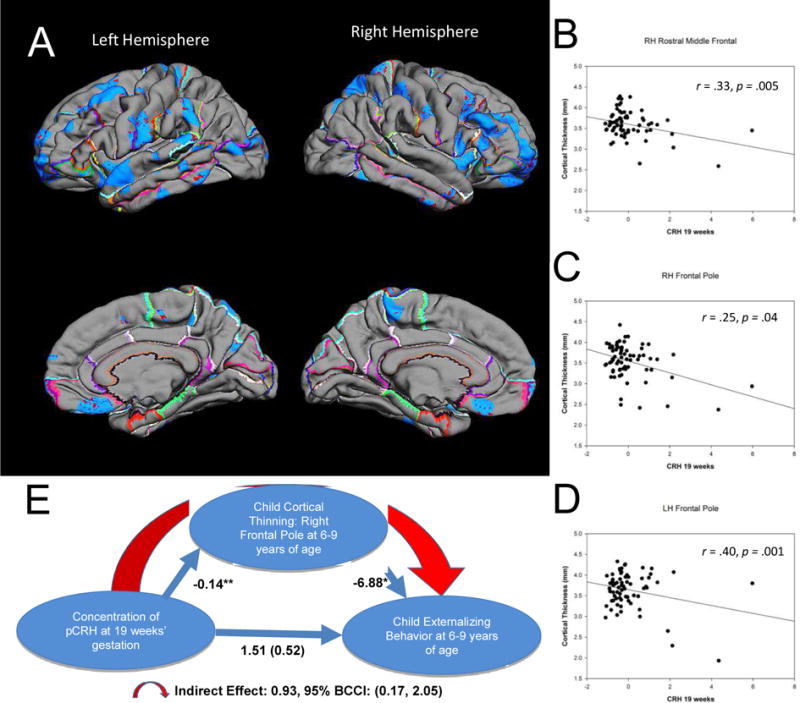
Pial maps (A) illustrating statistically significant areas of cortical thinning associated with prenatal levels of pCRH at 19 weeks gestation. Representative scatterplots (B [r=.33, p=.005] C [r=.25, p=.04], D [r=.40, p=.001]) of the significant associations between pCRH and cortical thinning in cortical subregions. Model (E) of the indirect significant association among prenatal concentrations of pCRH, child cortical thinning in the frontal pole and child internalizing behavior. The values correponding to each path in the model are unstandardized regression coefficients. The indirect effect was estimated with bootstrapping (1000 samples with replacement).
Thinning of the frontal pole has been associated with externalizing behaviors (50) defined as actions that direct energy outward and tend to harm others (51). We tested if the effects of pCRH exposure on cortical thinning contributed to the development of these behaviors (Figure 2E). Our model supported the belief that reduced cortical volume in the frontal pole, associated with elevated fetal exposure to concentrations of pCRH at 19 weeks gestation, contribute to externalizing symptoms in 6-9 year old children (indirect effect: 0.93; 95% BCCI 0.17 to 2.05; p < .05). Neither internalizing problems nor reaction time on the flanker task were associated with cortical thinning in this region.
CRH levels in late gestation: regional cortical thinning and cognitive outcomes
Exposure to elevated levels of pCRH later in gestation (31 weeks) was associated with cortical thinning in the lateral temporal and paracentral regions (Figure 3A). The effect was localized to the left paracentral region and bilateral in the temporal cortex. Remarkably, 98% of the lateral surface of the right temporal pole and 66% of the left temporal pole were significantly thinner in children exposed to high levels of pCRH at 31 weeks gestation (Table S4). Scatterplots (Figure 3B-D) illustrate these regional associations (and similar associations were observed at 25 weeks gestation in a smaller subsample [N=50]).
FIGURE 3.
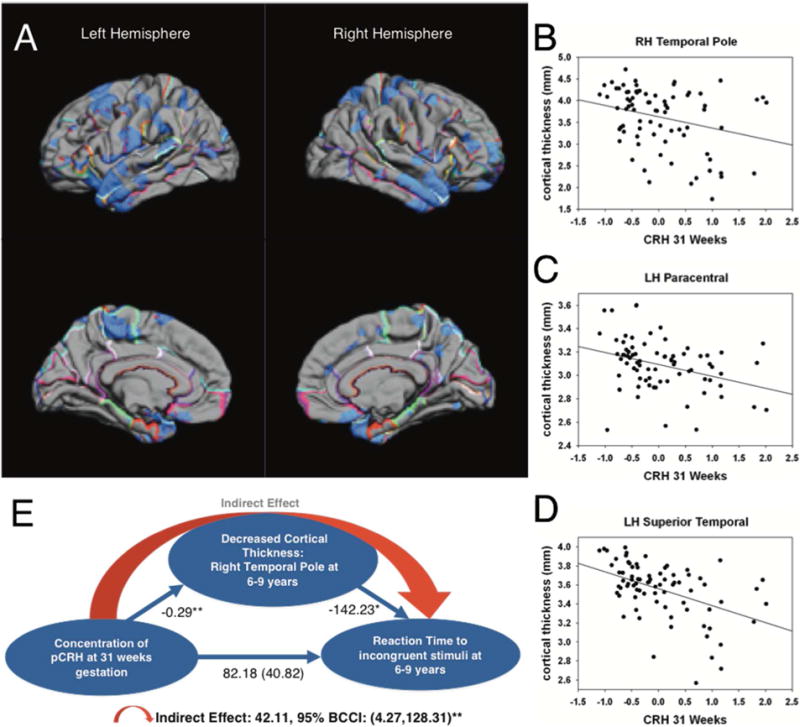
Pial maps (A) from FreeSurfer illustrating statistically significant areas of cortical thinning associated with prenatal levels of pCRH at 31 weeks gestation. Representative scatterplots (B [r=.29, p=.01] C [r=.37, p=.001], D [r=.48, p=.001]) of the significant associations between pCRH and cortical thinning in cortical subregions. Model (E) of the indirect association among prenatal concentrations of pCRH, child cortical thinning in the right temporal pole and child reaction time to a behavioral challenge. The values correponding to each path in the model are unstandardized regression coefficients. The indirect effect was estimated with bootstrapping (1000 samples with replacement).
Structures comprising the temporal cortex subserve numerous cognitive and emotional functions. Temporal cortical thinning has been reported in individuals with attentional deficits (52), especially in the right temporal pole (53) which is active in tasks requiring attention to relevant stimulation (54). We employed a statistical model (55) to test if the effects of exposure to pCRH late in prenatal life on cortical thinning contributed to impairment of attention in 6-9 year olds. The model (Figure 3E) indicated that the reduced right temporal pole volume associated with exposure to pCRH at 31 week gestation may partially account for poorer performance on a visual processing and sustained attention test (indirect effect: 42.11; 95% BCCI 4.27 to 128.31; p <0.01) (56,57).
Sex differences
Exploratory analyses suggest significant sex differences in the nature and degree of association between pCRH levels and global as well as regional cortical thinning. At both 19 and 31 weeks gestation, the associations were stronger in girls (Figure S2). The association between fetal exposure to pCRH at 19 weeks gestation and cortical thinning involved most cortical areas in girls (Figure S2A) but minimal in boys (Figure S2B). The fetal exposure to pCRH at 31 weeks affected cortical thinning globally in boys (Figure S2C) but locally in the temporal pole in girls (Figure S2D; similar to the findings for the combined sexes).
DISCUSSION
The principal and novel findings in these series of studies are that human fetal exposure to pCRH, even at concentrations which are insufficient to initiate labor or early delivery, is associated with regional cortical thinning and commensurate cognitive and emotional problems in a sex-specific manner in school-age children. Notably, such problems often are prodromal events that are associated with eventual neuropsychiatric outcomes.
The human placenta expresses pCRH by the eighth week of gestation and concentrations of pCRH increase geometrically as pregnancy advances to regulate the timing and onset of labor and delivery (30,58). Indeed, extremely high levels of placental CRH, often a result of adverse events during pregnancy, stimulate a cascade of events that result in pre-term labor and delivery. Premature birth is a significant contributing factor to impaired neurological and psychiatric outcomes. However, pCRH levels also rise in response to a variety of maternal stresses, and the novel finding here is the profound consequence of fetal exposure to pCRH at levels that are not associated with early delivery, in full-term school-age children. Specifically, we identify pronounced thinning in discrete cortical areas with implications for emotional and cognitive functions. Pronounced cortical thinning was associated with fetal exposure to pCRH across gestation with some evidence of localization in prefrontal and temporal poles. Examination of discrete gestational intervals clarified that prefrontal thinning was associated with exposure at early mid-gestation and thinning of the temporal pole was associated with exposure later in gestation.
Our findings raise the novel possibility that CRH is causally linked to cortical thinning, rather than merely signifying the presence of a harsh, stressful milieu to the developing fetal brain. A causal role for CRH in cortical thinning is supported by recent findings in an animal model (22). Cortical volume is largely comprised of dendritic trees of cortical neurons (22,59) and changes in dendritic arborization can be measured as volume loss in MR imaging (60). Our recent experiments demonstrate that exposure of developing cortical neurons to physiological levels of CRH results in a dose-dependent reduction and impoverishment of dendritic arborization (22). In addition, both exposure to early postnatal CRH in vivo (61) and to maternal stress signals (62,63) provokes similarly impoverished dendritic trees in the hippocampus. Indeed, exposure to nanomolar levels of CRH has been shown to reduce dendritic length and complexity via CRH receptor type 1 (64), which is expressed on the dendrites (65,66). Eliminating the actions of endogenous CRH led to exuberant dendritic trees both in transgenic mice lacking the CRH receptor and chronic exposure of organotypic slice cultures to CRH receptor blockers (64,65). Thus, it appears that the role of physiological levels of CRH is to modulate – perhaps in concert with glucocorticoids (67,68)– neuronal dendritic development in the perinatal hippocampus and neocortex.
The sex differences observed here are intriguing, and consistent with increased prevalence of stress-related disorders in women compared to men, tendencies observed also in prepubertal children (69). Our novel findings here suggest that sex-specific structural consequences of early-life adversity and potentially of CRH may be most apparent in females and are consistent with conclusions that females exposed to prenatal stress are more likely than males to exhibit increased levels of anxiety, impaired executive function and neurological markers associated with these behaviors (69).
In summary, our findings uncover a novel and unexpected result of prenatal elevation of pCRH: reduction in cortical volume in typically developing children, perhaps related to stunting of normal neuronal growth, and consequent commensurate subtle but significant emotional and cognitive impairments. The behavioral assessments were not a priori designed for assessing the unexpected cortical thinning observed in the temporal lobes, and this limitation should be addressed in future studies. Even when CRH levels are not sufficient to trigger premature birth and children are born at term, fetuses exposed to increased levels of pCRH carry less-well developed cortical neurons, apparent from extensive yet selective areas of cortical thinning. The cortical thinning is biologically significant because it is associated with both cognitive and emotional deficits.
Supplementary Material
Acknowledgments
Supported by awards NS41298, HD51852 and HD28413 (CAS); HD50662 andHD065823 (EPD); HD40967 (LMG); and P50 MH096889 (TZB). The assistance of Claudia Buss and Mariann Howland is gratefully acknowledged.
Footnotes
Disclosure:
Drs. Sandman, Davis, Glynn, Head, and Baram and Ms. Curran all have no competing financial interests to disclose.
Previous Presentation in Abstract form:
Curran MM, Sandman C, Davis E, Glynn L, Andres AL, Baram TZ. Chronic CRH stunts cortical neuron dendritic arborization: A basis for reduced cortical thickness in children exposed to high CRH in utero. Society for Neuroscience Conference; October 17-21, 2015; Chicago, IL.
Davis E, Baram TZ, Glynn L, Curran MM, Sandman C. Placental Corticotropin Releasing Hormone Influences Neurodevelopment in Rodents and Humans. International Conference on Infant Studies. 2016, May 26-28. New Orleans, LA, USA
Authorship:
CAS conceived and designed the studies including maternal/fetal monitoring, imaging in children and prepared the initial draft. MMC contributed to figures and sections of the manuscript and edited the final manuscript. EPD was involved in all phases of the studies including monitoring the maternal/fetal dyad, supervised the imaging studies in children and assisted in the preparation of the final draft. LMG focused on the maternal/fetal stage of the study, on the analysis of mediational effects, on the analysis of CRH and assisted in the final preparation of the manuscript. KH performed all imaging analysis with Freesurfer and statistical tests of significance. TZB contributed to the hypotheses guiding the study, and co-wrote and edited the manuscript throughout.
Transfer of Copyright:
The authors shall transfer copyright in accord with Journal and University policies.
References
- 1.McMullen S, Langley-Evans SC, Gambling L, Lang C, Swali A, McArdle HJ. A common cause for a common phenotype: The gatekeeper hypothesis in fetal programming. Med Hypotheses. 2012;78(1):88–94. doi: 10.1016/j.mehy.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnan V, Nestler EJ. Linking molecules to mood: New insight into the biology of depression. American Journal of Psychiatry. 2010:1305–20. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010 Aug 15;68(4):314–9. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClelland S, Korosi A, Cope J, Ivy A, Baram TZ. Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol Learn Mem. 2011 Jul;96(1):79–88. doi: 10.1016/j.nlm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh-Taylor A, Molet J, Jiang S, Korosi A, Bolton JL, Noam Y, et al. Mol Psychiatry. Nature Publishing Group; 2017. Jan 10, NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pena CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science. 2017;356(6343):1185–8. doi: 10.1126/science.aan4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mwaniki MK, Atieno M, Lawn JE, Newton CRJC. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. The Lancet. 2012:445–52. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetries: 2. Findings in neuropsychiatrie disorders. Mol Psychiatry. 2005;10(2):160–84. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- 9.Bellani M, Baiano M, Brambilla P. Brain anatomy of major depression II. Focus on amygdala. Epidemiol Psychiatr Sci. 2011 Jul;20:33–6. doi: 10.1017/s2045796011000096. [DOI] [PubMed] [Google Scholar]
- 10.Ryman SG, van den Heuvel MP, Yeo RA, Caprihan A, Carrasco J, Vakhtin AA, et al. Sex differences in the relationship between white matter connectivity and creativity. Neuroimage. 2014;101:380–9. doi: 10.1016/j.neuroimage.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Coffino B. The role of childhood parent figure loss in the etiology of adult depression: Findings from a prospective longitudinal study. Attach Hum Dev. 2009;11(5):445–70. doi: 10.1080/14616730903135993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48(8):778–90. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 14.Kajantie E, Räikkönen K. Early life predictors of the physiological stress response later in life. Neuroscience and Biobehavioral Reviews. 2010:23–32. doi: 10.1016/j.neubiorev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell KJ, Meaney MJ. Am J Psychiatry. 10. Vol. 174. American Psychiatric Association; Arlington, VA: 2017. Oct 1, Broader Focus Required to Understand the Effects of the Perinatal Environment on Child Neurodevelopment: Response to Bell and Chimata; pp. 999–1000. [DOI] [PubMed] [Google Scholar]
- 16.Davis EP, Stout SA, Molet J, Vegetabile B, Glynn LM, Sandman CA, et al. Exposure to unpredictable maternal sensory signals influences cognitive development across species. Proc Natl Acad Sci. 2017;201703444 doi: 10.1073/pnas.1703444114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(80)(4514):1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 18.Chrousos GP. Regulation and dysregulation of the hypothalamic-pituitary-adrenal axis. The corticotropin-releasing hormone perspective. Endocrinol Metab Clin North Am. 1992 Dec;21(4):833–58. [PubMed] [Google Scholar]
- 19.Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci. 2001;21(18):7171–81. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan XX, Baram TZ, Gerth A, Schultz L, Ribak CE. Co-localization of corticotropinreleasing hormone with glutamate decarboxylase and calcium-binding proteins in infant rat neocortical interneurons. Exp Brain Res. 1998;123(3):334–40. doi: 10.1007/s002210050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, et al. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature. 1995 Nov 16;378(6554):284–7. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 22.Curran MM, Sandman CA, Davis EP, Glynn LM. Abnormal dendritic maturation of developing cortical neurons exposed to corticotropin releasing hormone (CRH): Insights into effects of prenatal adversity? . PLoS One. 2017 doi: 10.1371/journal.pone.0180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King BR, Smith R, Nicholson RC. The regulation of human corticotrophin-releasing hormone gene expression in the placenta. Peptides. 2001 Nov;22(11):1941–7. doi: 10.1016/s0196-9781(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 24.Arbiser JL, Morton CC, Bruns GA, Majzoub JA. Human corticotropin releasing hormone gene is located on the long arm of chromosome 8. Cytogenet Cell Genet. 1988;47(3):113–6. doi: 10.1159/000132525. [DOI] [PubMed] [Google Scholar]
- 25.Grino M, Chrousos GP, Margioris AN. The corticotropin releasing hormone gene is expressed in human placenta. Biochem Biophys Res Commun. 1987;148(3):1208–14. doi: 10.1016/s0006-291x(87)80261-9. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto E, Takagi T, Azuma C, Kimura T, Tokugawa Y, Mitsuda N, et al. Expression of the corticotropin-releasing hormone (CRH) gene in human placenta and amniotic membrane. Horm Metab Res. 1990;22(7):394–7. doi: 10.1055/s-2007-1004930. [DOI] [PubMed] [Google Scholar]
- 27.Class QA, Buss C, Davis EP, Gierczak M, Pattillo C, Chicz-DeMet A, et al. Low levels of corticotropin-releasing hormone during early pregnancy are associated with precocious maturation of the human fetus. Dev Neurosci. 2008;30(6):419–26. doi: 10.1159/000191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power ML, Schulkin J. Functions of corticotropin-releasing hormone in anthropoid primates: From brain to placenta. Am J Hum Biol. 2006;18:431–47. doi: 10.1002/ajhb.20521. September 2005. [DOI] [PubMed] [Google Scholar]
- 29.Stout SA, Espel EV, Sandman CA, Glynn LM, Davis EP. Fetal programming of children’s obesity risk. Psychoneuroendocrinology. 2015 Mar;53:29–39. doi: 10.1016/j.psyneuen.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith R, Nicholson RC. Corticotrophin releasing hormone and the timing of birth. Front Biosci. 2007 Jan 1;12:912–8. doi: 10.2741/2113. [DOI] [PubMed] [Google Scholar]
- 31.Mesiano S. The endocrinology of human pregnancy and fetal-placental neuroendocrine development. In: Strauss J, Barbieri R, editors. Yen and Jaffe’s reproductive endocrinology: Physiology, pathophysiology, and clinical managemen. Philadelphia, PA: Saunders; pp. 243–271. [Google Scholar]
- 32.Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol. 1989;160(1):247–51. doi: 10.1016/0002-9378(89)90130-0. [DOI] [PubMed] [Google Scholar]
- 33.Wadhwa PD, Culhane JF, Rauh V, Barve SS. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J. 2001 Jun;5(2):119–25. doi: 10.1023/a:1011353216619. [DOI] [PubMed] [Google Scholar]
- 34.O’Donnell K, O’Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31(4):285–92. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- 35.Sandman CA, Davis EP, Buss C, Glynn LM. Prenatal Programming of Human Neurological Function. Int J Pept. 2011;2011:1–9. doi: 10.1155/2011/837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandman CA, Glynn L, Wadhwa PD, Chicz-DeMet A, Porto M, Garite T. Maternal hypothalamic-pituitary-adrenal disregulation during the third trimester influences human fetal responses. Dev Neurosci. 2003;25(1):41–9. doi: 10.1159/000071467. [DOI] [PubMed] [Google Scholar]
- 37.Ellman LM, Schetter CD, Hobel CJ, Chicz-DeMet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50(3):232–41. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA, et al. Dev Neurosci. 5. Vol. 27. Karger; Publishers: 2005. Jan, Corticotropin-releasing hormone during pregnancy is associated with infant temperament; pp. 299–305. [DOI] [PubMed] [Google Scholar]
- 39.Howland MA, Sandman CA, Glynn LM, Crippen C, Davis EP. Fetal exposure to placental corticotropin-releasing hormone is associated with child self-reported internalizing symptoms. Psychoneuroendocrinology. 2016;67:10–7. doi: 10.1016/j.psyneuen.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glynn LM, Sandman CA. Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med. 2014 Jun;76(5):355–62. doi: 10.1097/PSY.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 41.Rodbard D, Munson PJ, DeLean A. International symposium on radioimmunoassay and related procedures in medicine. Berlin, Germany; F.R: 1978. Improved curve-fitting, parallelism testing, characterisation of sensitivity and specificity, validation, and optimization for radioligand assays. [Google Scholar]
- 42.Boyce WT, Essex MJ, Woodward HR, Measelle JR, Ablow JC, Kupfer DJ, et al. The confluence of mental, physical, social, and academic difficulties in middle childhood. I: exploring the "head waters" of early life morbidities. J Am Acad Child Adolesc Psychiatry. 2002 May;41(5):580–7. doi: 10.1097/00004583-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 44.Sled JG, Zijdenbosa P, Evansa C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 45.Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically Parcellating the Human Cerebral Cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 46.Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 2000;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Achenbach TM, Rescorla L. Manual for the ASEBA School-Age Forms and Profiles: An Integrated System of Multi-Informant Assessment. University of Vermont Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 49.Eriksen BA, Eriksen CW. Percept Psychophys. 1. Vol. 16. Springer-Verlag; 1974. Jan, Effects of noise letters upon the identification of a target letter in a nonsearch task; pp. 143–9. [Google Scholar]
- 50.Levan A, Baxter L, Kirwan CB, Black G, Gale SD. Right frontal pole cortical thickness and social competence in children with chronic traumatic brain injury: cognitive proficiency as a mediator. 2015;30(2) doi: 10.1097/HTR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 51.Hinshaw SP. Process, mechanism, and explanation related to externalizing behavior in developmental psychopathology. J Abnorm Child Psychol. 2002 Oct;30(5):431–46. doi: 10.1023/a:1019808712868. [DOI] [PubMed] [Google Scholar]
- 52.Sasayama D, Hayashida A, Yamasue H, Harada Y, Kaneko T, Kasai K, et al. Neuroanatomical correlates of attention-deficit-hyperactivity disorder accounting for comorbid oppositional defiant disorder and conduct disorder. Psychiatry Clin Neurosci. 2010 Aug;64(4):394–402. doi: 10.1111/j.1440-1819.2010.02102.x. [DOI] [PubMed] [Google Scholar]
- 53.Fernández-Jaén A, López-Martín S, Albert J, Fernández-Mayoralas DM, Fernández-Perrone AL, Tapia DQ, et al. Cortical thinning of temporal pole and orbitofrontal cortex in medication-naïve children and adolescents with ADHD. Psychiatry Res. 2014 Oct 30;224(1):8–13. doi: 10.1016/j.pscychresns.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2008 Feb 1;18(2):262–71. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- 55.Hayes AF, Preacher KJ. Quantifying and Testing Indirect Effects in Simple Mediation Models When the Constituent Paths Are Nonlinear. Multivariate Behav Res. 2010 Aug 6;45(4) doi: 10.1080/00273171.2010.498290. [DOI] [PubMed] [Google Scholar]
- 56.Riccio CA, Reynolds CR, Lowe P, Moore JJ. The continuous performance test: a window on the neural substrates for attention? . Arch Clin Neuropsychol. 2002 Apr;17(3):235–72. [PubMed] [Google Scholar]
- 57.Posner MI, Inhoff AW, Friedrich FJ, Cohen A. Psychobiology. 2. Vol. 15. Springer-Verlag; Isolating attentional systems: A cognitive-anatomical analysis; pp. 107–21. [Google Scholar]
- 58.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. Nat Med. 5. Vol. 1. Nature Publishing Group; 1995. May, A placental clock controlling the length of human pregnancy; pp. 460–3. [DOI] [PubMed] [Google Scholar]
- 59.Paus T. Brain Development. In: Lerner RM, Steinberg L, editors. Handbook of Adolescent Psychology. Hoboken, NJ USA: John Wiley & Sons, Inc; 2009. pp. 95–115. [Google Scholar]
- 60.Molet J, Maras PM, Kinney-Lang E, Harris NG, Rashid F, Ivy AS, et al. MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus. 2016 Dec;26(12):1618–32. doi: 10.1002/hipo.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8856–61. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005 Oct 12;25(41):9328–38. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010 Sep 29;30(39):13005–15. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, et al. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci U S A. 2004 Nov 2;101(44):15782–7. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y, Dubé CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–11. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y, Andres AL, Frotscher M, Baram TZ. Tuning synaptic transmission in the hippocampus by stress: the CRH system. Front Cell Neurosci. 2012 Jan;6:13. doi: 10.3389/fncel.2012.00013. (April) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci. 2011 Sep 20;108(38):16074–9. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Y, Molet J, Lauterborn JC, Trieu BH, Bolton JL, Patterson KP, et al. Converging, Synergistic Actions of Multiple Stress Hormones Mediate Enduring Memory Impairments after Acute Simultaneous Stresses. J Neurosci. 2016;36(44) doi: 10.1523/JNEUROSCI.2542-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandman CA, Glynn LM, Davis EP. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res. 2013;75(4):327–35. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


