Abstract
Background
Whole-grain intake is associated with lower risk of type 2 diabetes but mechanisms are unclear.
Purpose
We tested the hypothesis that a WG diet reduces insulin resistance and improves glucose use in individuals at risk for type 2 diabetes compared with an isocaloric-matched refined-grain diet.
Methods
A double-blind, randomized, controlled, crossover trial of 14 moderately obese adults (Age, 38±2 yrs; BMI, 34.0±1.1 kg/m2). Insulin resistance and glucose metabolism was assessed using an oral glucose tolerance test combined with isotopic tracers of [6,6-2H2]-glucose and [U-13C]-glucose, and indirect calorimetry. Peripheral and hepatic insulin resistance was assessed as 1/(rate of disposal/insulin), and endogenous glucose rates of appearance (Ra) iAUC60–240 × insulin iAUC60–240, respectively. Both diets met ADA nutritional guidelines and contained either whole-grain (50 g per 1000 kcal) or equivalent refined-grain. All food was provided for 8 wk with an 8–10 wk washout period between diets.
Results
Post-prandial glucose tolerance, peripheral insulin sensitivity, and metabolic flexibility (insulin-stimulated – fasting carbohydrate oxidation) improvements were greater after whole-grain compared to the refined-grain diet (P<0.05). Compared to baseline, body fat (~2 kg) and hepatic Ra insulin resistance was reduced by both diets, while fasting glucose and exogenous glucose-meal were unchanged after both interventions. Changes in peripheral insulin resistance and metabolic flexibility correlated with improved glucose tolerance (P<0.05).
Conclusion
Whole-grains reduced diabetes risk and the mechanisms appear to work through reduced post-prandial blood glucose and peripheral insulin resistance that were statistically linked to enhanced metabolic flexibility.
Keywords: alkylresorcinols, low-glycemic diet, glucose tolerance, obesity, insulin
1.1. INTRODUCTION
Epidemiological data consistently find that dietary whole-grain intake is associated with a lower incidence of type 2 diabetes [1, 2]. These observations underlie AACE/ACE dietary guidelines that recommend whole-grains for people with, or at risk for type 2 diabetes [3]. Despite clear associations between higher whole-grain intake and reduced type 2 diabetes risk in population based studies [4], there is mixed evidence that whole-grain consumption improves glycemic control in adults at risk for type 2 diabetes [5–11]. Moreover, none of these studies used advanced techniques to assess multi-organ insulin resistance, and thus, important changes to glucose metabolism have not yet been measured in controlled long term whole grain intervention studies.
Previous research has shown that whole-grain intake increases clamp-derived insulin sensitivity in overweight adults with or without metabolic syndrome, suggesting that whole-grains may lower blood glucose by affecting skeletal muscle glucose metabolism [8]. Diets low in whole-grains are also associated with higher plasma glucose concentrations that, in turn, impair skeletal muscle insulin sensitivity. However, the mechanism linking whole-grains to improved skeletal muscle glucose uptake is currently unknown. Abnormalities in substrate metabolism, including reduced fasting fat oxidation and blunted metabolic flexibility (i.e. the switch from predominantly fasting fat oxidation to insulin-stimulated carbohydrate utilization), have been implicated in skeletal muscle insulin resistance in obese adults at risk for diabetes following weight loss induced by exercise plus diet [12, 13]. Indeed, recent work has raised the possibility that changes in fuel selection contribute to the insulin-sensitizing effects of whole-grains [14, 15]. To date, the current literature provides little physiologic insight to the glucoregulatory determinants of glycemic regulation after whole-grain intake, and there are no randomized controlled trials determining the effect of whole-grains on glucose regulation in obese adults at risk for diabetes when compared with an isocaloric control diet. In addition, the role of gut absorption and hepatic glucose production on blood glucose following whole-grain intake is unclear [5], and this is important to know because changes in meal appearance from the gastrointestinal system or release of liver glucose may significantly impact blood glucose concentrations. This knowledge gap is clinically relevant as glucose is an important determinant of cardiovascular disease (CVD) risk. We recently examined the effect of whole-grains comprised of mainly wheat, oats and rice on the primary outcome of body composition and CVD risk in overweight and obese adults compared with refined grain intake [16]. Our results suggest that whole-grains improved blood pressure and maintained adiponectin concentration to a greater degree than refined grain intake [16]. Whether this cardio-metabolic benefit of whole-grains extends to glucose metabolism and insulin resistance remains unknown. Thus, we used an isotopic tracer approach to test the hypothesis that whole-grains would lower blood glucose by improving insulin-stimulated peripheral and hepatic glucose kinetics in obese adults, thus reducing insulin resistance via greater metabolic flexibility.
2.1. MATERIALS AND METHODS
2.2. Subjects and Design
This was a randomized, double-blind, controlled crossover trial involving fourteen middle-aged, obese adults at risk for diabetes (Table 1). The subjects were part of a larger study on body composition and CVD risk who underwent additional testing to evaluate glucose metabolism [16]. Randomization occurred prior to metabolic testing. Subject numbers were placed in sequentially numbered, sealed envelopes. Only the study dietitian and statistical consultant (see Acknowledgements) were aware of subject assignments. Blinding of subjects and investigators was achieved by packaging meals into identical containers so the visual appearance of the food was similar for whole-grain and the control, refined-grain diets. Subjects were excluded if >50 y, weight unstable (>2 kg in prior 6 m), physically active (>60 min/wk), BMI >40 kg/m2, on anti-diabetic medications, or had diagnosed chronic disease (i.e. renal, hepatic, type 2 diabetes, cardiovascular, etc.). Women were pre-menopausal and studied during the mid-follicular phase (i.e. 5–10 d post menses). All adults underwent a medical examination that included a resting ECG, urine analysis, and blood biochemistry. Subjects were verbally briefed about the study and signed informed consent documents approved by the Cleveland Clinic Institutional Review Board (ClinicalTrials.gov registration # NCT01411540).
Table 1.
Comparison of body composition and blood glucose factors between Whole-Grain and Refined-Grain Interventions.
| Whole-Grain | Refined-Grain | ANOVA | |||
|---|---|---|---|---|---|
|
| |||||
| Pre | Δ | Δ vs. Δ | |||
| n (M,F) | 14 (11F, 3M) | - | - | - | - |
| Age (years) | 37.9 ± 1.8 | - | - | - | |
| Weight (kg) | 97.9 ± 3.8 | −2.8 ± 0.7 | 97.9 ± 3.1 | −2.5 ± 0.6 | 0.80 |
| BMI (kg/m2) | 33.8 ± 1.3 | −1.0 ± 0.2 | 33.9 ± 1.0 | −0.9 ± 0.2 | 0.71 |
| Fat Mass (kg) | 41.4 ± 2.6 | −2.2 ± 0.5 | 42.1 ± 2.1 | −2.7 ± .54 | 0.46 |
| Body fat (%) | 43.5 ± 1.9 | −0.8 ± 0.2 | 44.5 ± 1.8 | −1.5 ± 0.3 | 0.06 |
| FFM (kg) | 56.3 ± 2.9 | −1.0 ± 0.2^ | 55.9 ± 2.9 | −0.2 ± 0.3 | 0.05 |
| VAT (g) | 1027.4 ± 126.2 | −95.4 ± 59.9 | 1002.9 ± 120.4 | −161.8 ± 42.6 | 0.40 |
| SAT (g) | 2782.1 ± 252.5 | −144.1 ± 62.7 | 2732.3 ± 240.0 | −117.9 ± 109.8 | 0.82 |
| FPG (mg/dl) | 93.8 ± 2.7 | −1.6 ± 2.2 | 94.5 ± 2.2 | −1.4 ± 1.2 | 0.92 |
| 2-hr PG (mg/dl) | 152.0 ± 11.5 | −2.2 ± 6.9 | 152.2 ± 9.7 | 2.9 ± 3.5 | 0.53 |
| Peak PG (mg/dl) | 184.6 ± 10.1 | −2.7 ± 6.4 | 189.7 ± 10.1 | 1.8 ± 4.1 | 0.49 |
| FPI (µU/ml) | 19.2 ± 2.5 | −3.5 ± 1.9 | 20.9 ± 3.3 | −3.5 ± 1.7 | 0.98 |
| 2-hr PI (µU/ml) | 147.7 ± 20.0 | −27.3 ± 13.5^ | 133.8 ± 18.2 | 30.7 ± 11.0 | 0.001 |
| Peak PI (µU/ml) | 183.8 ± 18.3 | −7.7 ± 9.9 | 184.8 ± 16.0 | 18.7 ± 9.8 | 0.07 |
| PI iAUC (µU/ml*240min) | 18458.6 ± 2168.3 | −824.1 ± 929.8 | 18407.7 ± 1939.4 | 1435.6 ± 1457.5 | 0.23 |
Data are reported as mean ± SEM. Compared to Refined-Grain diet,
P < 0.05 using ANCOVA.
BMI = body mass index. FFM = fat-free mass. VAT = visceral fat. SAT = subcutaneous fat. FPG = fasting plasma glucose. FPI = fasting plasma insulin. iAUC = incremental area under the curve.
2.3. Diet Intervention
Participants were provided either whole-grain or refined-grain diets for 8 weeks with an 8–10 week washout period between diets. During the washout, subjects were instructed to resume their usual diets. Subjects were provided the alternate diet for the 2nd phase. A registered dietitian monitored the diets, which were isocaloric to the individual requirements (i.e. resting metabolic rate × 1.3 activity factor). Resting metabolic rate was determined before each dietary condition using indirect calorimetry (Vmax Encore, Viasys, Yorba Linda, CA) as previously described [12]. The macronutrient composition of the diets was matched and consisted of 50 g per 1000 kcal of whole-grain or refined-grain, respectively. Whole-grains in this study comprised of mainly wheat, oat and rice. All meals and fluids were provided throughout the study, and recipes were identical between diets, with only frozen ready meals and breakfast cereals differing in the source of carbohydrate (refined grain or whole grain). Importantly, ready meals were specifically designed for this study to ensure adequate blinding as to carbohydrate source. Dietary compliance was estimated by weekly food container weigh backs, and calculated as the percent difference between prescribed and actual caloric intake. Alkylresorcinols, a biomarker of whole-grain wheat and rye intake, were used to objectively confirm diet adherence [17]. Diet analysis was performed using ESHA Food Processor Pro v.10.80 (Salem, OR).
2.4. Control Period
Metabolic testing was conducted during a 3-day inpatient stay at our Clinical Research Unit. Subjects were provided isocaloric mixed meals and were instructed to avoid strenuous physical activity for 48-hour prior to metabolic testing.
2.5. Anthropometrics
Height and weight were obtained in a standard hospital gown on a wall-mounted stadiometer (Veeder-Root, Elizabethtown, NC) and a calibrated scale. BMI was calculated as body mass (kg) divided by height (m)2. Total body fat, fat-free mass (FFM), and visceral fat (VAT) were assessed using dual-energy x-ray absorptiometry (DXA, Lunar Prodigy CORE Scan, Madison, WI). Subcutaneous fat (SAT) was estimated by subtracting VAT from total android body fat [18].
2.6. Glucose Regulation
After an overnight fast, a 75 gram oral glucose tolerance test (OGTT) was performed using isotopically labeled glucose [6,6-2H2] and [U-13C] to assess hepatic glucose production and exogenous glucose oxidation, respectively [19]. A polyethylene catheter was inserted into an antecubital vein and fasting blood samples were obtained (t = −120 min). Baseline breath samples were collected for the determination of expired CO2, glucose oxidation (Rox) and glucose appearance (Rameal). A primed (3.28 mg/kg) bolus of [6,6-2H2]-glucose plus a sodium bicarbonate prime (NaH13CO3; 0.2 mg/kg) was administered at t = −120 min. A constant infusion of [6,6-2H2]-glucose was used to determine total endogenous rates of glucose appearance (Ra), which primarily reflects hepatic glucose production (RaHGP). A retrograde indwelling catheter was also placed in a vein in the contralateral hand, which was warmed to 60°C for collection of arterialized blood samples. Exhaled air was collected for 20 min using a ventilated hood and indirect calorimetry to determine basal substrate oxidation prior to glucose ingestion containing 0.02 g/kg of [U-13C]-glucose for the determination of Rameal to the total Ra. The [6,6-2H2]-glucose tracer infusion rate was altered from 8 ml/hr to 12, 16, 12, and 8 ml/hr at 60, 120, 180 and 240 min, respectively, to mimic the corresponding changes in blood glucose and minimize artificially low Ra and rates of disposal (Rd) [20]. Breath samples for isotope enrichment and indirect calorimetry were also collected during the last 15 min of each hour up to 180 min. Plasma glucose was determined at minutes 0, 30, 60, 90, 120, 150, 180, 210 and 240, while insulin was determined at the same points with the exception of 150 and 210 minutes.
2.7. Calculations
Glucose and insulin incremental area under the curve (iAUC) during the OGTT were calculated using the trapezoidal rule. Total Ra, RaHGP, and Rd from 6,6-2H2 glucose were calculated based on the Steele non-steady state equations [21]. The Rameal of [13C] glucose into breath was determined by transposition of the Steele equation and the known 13C enrichment of the ingested glucose adapted for use with stable isotopes [21]. HGP was calculated as the difference between total Ra and Rameal. Hepatic insulin resistance was estimated as HGP iAUC60–240 × insulin iAUC60–240 and divided by 100,000 for data presentation. Peripheral insulin resistance, reflecting mostly skeletal muscle, was calculated as the inverse of Rd/insulinmean 0–240. Carbohydrate and fat oxidation were calculated from indirect calorimetry using standard equations [22]. Non-oxidative glucose disposal (NOGD) was calculated (NOGDmean 60–180 = Rd − total carbohydrate oxidationmean 60–180). Metabolic flexibility was defined as insulin-stimulated CHO oxidationmean 60–180 – fasting CHO oxidation. Rox was also determined from breath samples [23].
2.8. Biochemical Analysis
Plasma glucose was measured immediately after collection using the glucose oxidase method (YSI 2300 STAT Plus, Yellow Springs, OH). The remaining blood was centrifuged at 4°C for 10 minutes and frozen at −70°C until subsequent analysis. To minimize inter-assay variability, all frozen blood measurements pre- and post-intervention were analyzed on the same plate. Plasma insulin was assayed by radioimmunoassay (Millipore, St. Charles, MO). Alkylresorcinols were measured using gas chromatography-mass spectrometry (GC-MS) [24]. Glucose kinetic plasma samples were deproteinized, extracted, and derivatized before analysis by GC–MS [12]. Breath samples were analyzed for 13C/12C ratio by GC continuous flow isotope ratio MS [23].
2.9. Statistical Analysis
Data were assessed using R software (Vienna, Austria, 2011). Normally distributed data were analyzed by parametric tests. Paired t-tests were used to compare baseline differences. No baseline differences were observed for any outcome. Analysis of variance (ANOVA) with linear mixed-effects was used to compare between condition differences. In the event of significant group differences, analysis of co-variance (ANCOVA) with linear mixed-effects was used to compare between condition differences with the co-variates: order effect, period effect, age, sex, baseline levels of the respective outcome, and changes in body fat and dietary fiber. Pearson’s correlation was used to test associations between blood glucose and metabolic characteristics using delta-delta (i.e. whole-group) values. Significance was accepted as P<0.05 and data are reported as mean±SEM.
3.1. RESULTS
3.2. Diet
Food compliance was excellent at 91.3±2.1 vs. 90.4±1.8% of target intake for whole-grain and refined-grain conditions, respectively (P=0.70). There was no difference between whole-grain and refined-grain conditions for energy (2028±86 vs. 2016±83 kcal/d, P=0.84), total carbohydrate (54.6±0.7 vs. 53.7±0.7%, P=0.45), sugar (122±6 vs. 128±6 g/d, P=0.35), fat (28.4±0.3 vs. 28.7±0.5%, P=0.63), or protein intake (18.1±0.3 vs. 17.5±0.3%, P=0.12). However, as expected, whole-grain (90.5±4.5 vs. 0±0 g/d, P<0.001) and dietary fiber consumption (26.5 ± 1.4 vs. 20.1±1.3 g/d, P<0.001) was higher during the whole-grain compared with refined-grain period. In addition, plasma alkylresorcinols were significantly higher after whole-grain compared with refined-grain intake (208.3±50.3 vs. 7.0±5.5 nM, P<0.01). No difference in plasma alkylresorcinols were observed pre-intervention between whole-grain and refined-grain (44.2 ± 5.6 vs. 38.3 ± 3.9 nM, P=0.14), suggesting both groups commenced interventions having consumed similar grain filled diets.
3.3. Body Composition
Whole-grain and refined-grain interventions induced approximately 3–6% weight and fat loss (Table 1), but there no differences observed between conditions. While whole-grain intake was associated with approximately 1.9% loss of FFM compared with a 0.2% loss following refined-grains (P<0.05), both whole-grain and refined-grain reduced VAT and SAT comparably.
3.4. Glucose Kinetics and Substrate Oxidation
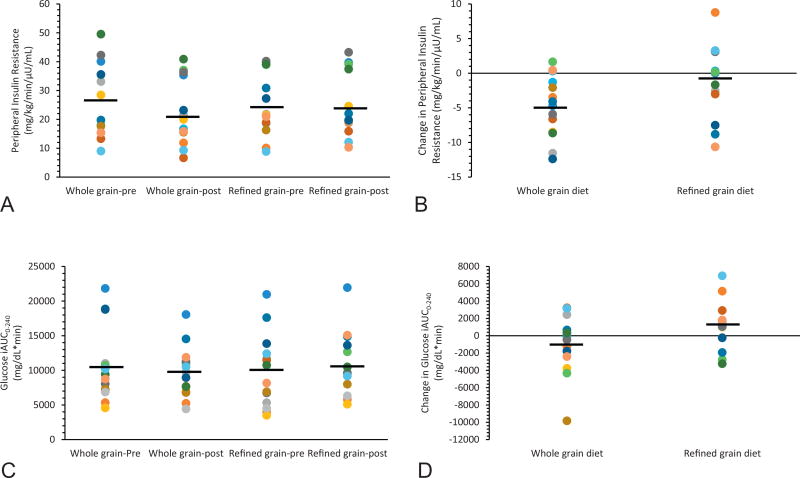
Whole-grain and refined-grain intake improved fasting and post-prandial hepatic insulin resistance, while Rameal did not change (Table 2). Whole-grain consumption reduced peripheral insulin resistance by approximately 18% compared with a 2% rise following refined-grain intake (P<0.03; Figure 1). In addition, whole-grains increased fasting fat oxidation compared to the refined-grain diet (P<0.01; Table 2). Although there was no group difference in NOGD and Rox (Table 2), metabolic flexibility was elevated after whole-grain compared with refined-grain intake (P<0.01).
Table 2.
Comparison of glucose kinetics and substrate oxidation between Whole-Grain and Refined-Grain Interventions.
| Whole-Grain | Refined-Grain | ANOVA | |||
|---|---|---|---|---|---|
|
| |||||
| Pre | Δ | Pre | Δ | Δ vs. Δ | |
| Fasted | - | ||||
| RaHGP (mg/kg/min) | 2.8 ± 0.3 | 0.5 ± 0.3 | 2.9 ± 0.2 | 0.3 ± 0.2 | 0.49 |
| RaHGP * FPI (mg/kg/min*µU/ml) | 61.1 ± 9.9 | −4.4 ± 6.0 | 61.8 ± 9.5 | −3.5 ± 4.8 | 0.87 |
| CHOox (mg/kg/min) | 0.81 ± 0.11 | −0.21 ± 0.14^ | 0.66 ± 0.12 | 0.24 ± 0.11 | 0.008 |
| Fatox (mg/kg/min) | 0.94 ± 0.07 | 0.06 ± 0.05^ | 0.99 ± 0.05 | −0.11 ± 0.04 | 0.009 |
| Insulin-Stimulated | |||||
| RaHGP (mg/kg/min) | 0.7 ± 0.1 | 0.2 ± 0.1 | 0.8 ± 0.1 | 0.1 ± 0.1 | 0.35 |
| HIR (mg/kg/min*µU/ml-240min) | −392.8 ± 40.3 | −298.9 ± 75.0 | 379.1 ± 28.2 | 296.7 ± 42.0 | 0.99 |
| Rameal (mg/kg/min*240min) | 1008.3 ± 67.0 | 75.6 ± 49.9 | 1028.8 ± 55.8 | 22.7 ± 54.4 | 0.72 |
| Rd (mg/kg/min) | 4.8 ± 0.3 | 0.7 ± 0.3 | 5.0 ± 0.3 | 0.4 ± 0.3 | 0.35 |
| Total CHOox (mg/kg/min) | 1.9 ± 0.1 | 0.01 ± 0.08 | 1.9 ± 0.1 | −0.02 ± 0.11 | 0.85 |
| NOGD (mg/kg/min) | 2.9 ± 0.3 | 0.67 ± 0.25 | 3.1 ± 0.3 | 0.38 ± 0.31 | 0.40 |
| Exogenous Rox (mg/kg/min) | 3.8 ± 0.3 | 0.5 ± 0.2 | 3.8 ± 0.3 | 0.4 ± 0.2 | 0.56 |
| Metabolic Flexibility (a.u.) | 0.98 ± 0.12 | 0.20 ± 0.13^ | 1.07 ± 0.12 | −0.10 ± 0.13 | 0.03 |
Data are reported as mean ± SEM. Compared to Refined-Grain diet,
P < 0.05 using ANCOVA.
Ra = rate of glucose appearance. HGP = hepatic glucose production. Rd = rate of glucose disappearance. CHO = carbohydrate. Ox = oxidation. iAUC = incremental area under the curve. HIR = hepatic insulin resistance (RaHGP * insulin iAUC60–240min). a.u. = arbitrary units.
Figure 1.
Effect of Whole-Grain and Refined-Grain Diet on Glucose and Peripheral Insulin Resistance. Data are reported as mean ± SEM. Compared to Refined-Grain diet, ^P < 0.05 using ANCOVA. WG = Whole-Grain. RG = Refined-Grain. iAUC = incremental area under the curve.
3.5. Glucose and Insulin Metabolism
The intervention had no effect on fasting glucose, however, whole-grains lowered 2-hour glucose by approximately 2.2 mg/dl, although this did not reach statistical significance (Table 1, P=0.53). This improved clinical outcome was corroborated by significant reductions in glucose iAUC of approximately 5% by whole-grains compared with a 23% rise following refined-grains (P<0.02; Figure 1). Fasting insulin was reduced by both whole-grain and refined-grain diets. However, 2-hour insulin levels were reduced by 14% after whole-grain intake compared with a 39% rise with refined-grains (P<0.001, Table 1). Whole-grains also lowered insulin iAUC compared with refined-grains, but this was not statistically significant (P=0.27).
3.6. Correlation Analysis
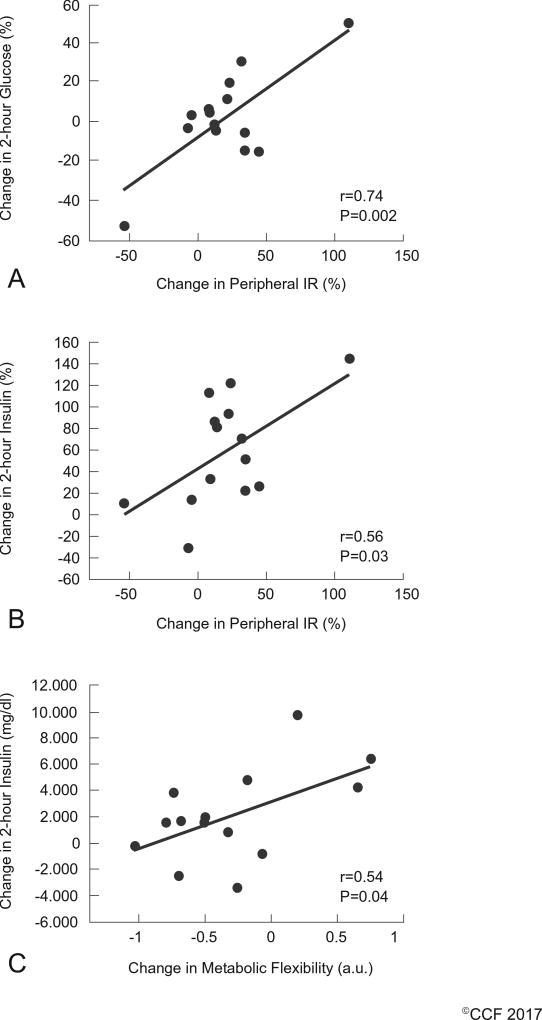
After the intervention, reduced peripheral insulin resistance correlated with decreased 2-hour circulating glucose (r=0.74, P=0.002; Figure 2a) and insulin (r=0.56; P=0.03; Figure 2b). Lower glucose iAUC0–240 was significantly associated with increased metabolic flexibility (r=0.54, P=0.04; Figure 2c).
Figure 2.
Correlations between Insulin Resistance, Metabolic Flexibility and Circulating Blood Glucose. Data are reported as delta-delta (Δ-Δ, i.e. the RG-WG difference between Post-Pre). WG = whole-grain. RG = Refined-Grain. IR = insulin resistance and calculated as 1 divided by Rd/insulin. HIR = hepatic insulin resistance and calculated as iAUC HGP 60–240 min of the OGTT.
4.1. DISCUSSION
Whole-grain intake as part of a mixed-meal diet significantly improved post-prandial glucose metabolism in middle-aged obese adults. Importantly these effects were independent of fat loss, which suggests that glycemic control can be improved by dietary intervention beyond simply focusing on reducing body weight and body fat. Data on the effect of whole grains on glucose outcomes is controversial [11, 25–28]. It is important to note the heterogeneity of these studies in terms of population, study design, and types of grains tested. In our study, we recruited a relatively young and obese population who are at risk of developing type 2 diabetes, and we used a whole grain intervention based on commercially available meals and the commonly consumed grains wheat and rice as the main ingredients. Thus, finding that simple substitution of whole grains for refined grains in the diet at a level that was acceptable to this, at risk population, is of great clinical relevance when creating healthy eating messages for the prevention of type 2 diabetes.
Single meal studies comparing whole grains to refined grains show that whole grains reduce plasma glucose responses by 29% and 9% either after an OGTT [14], or a meal [29], respectively, supporting our findings, though these studies have suggested that fermentation of soluble fiber from whole grains mediates this improvement. Both of these studies fed barley-based meals rich in the soluble fiber β-glucan, whereas wheat and rice, the main grains used in our study, are low in β-glucan. Also, the overall difference in fiber intake of 8 g/d seen in our study is unlikely to lead to gut microbiota mediated changes in glucose metabolism. Similarly, this difference in fiber intake is unlikely to lead to changes in gastric emptying, and no difference in stool frequency or amount was found (data not shown). Other factors must be at play, and while we can all but rule out overall weight loss, fat loss and change in FFM, there is no clear explanation for the differences beyond the nutrient and phytochemical differences between whole- and refined grains.
While many studies examining the link between whole grains and biomarkers of health have been carried out on apparently healthy populations, our study focused on obese subjects who were at-risk for type 2 diabetes and CVD. One other intervention study found that eating 80 g whole grains/d over 12 weeks led to normoglycemia in prediabetic subjects [11]. An observational study also found that a difference in whole grain intake of 30 g/d reduced the risk of becoming prediabetic or diabetic by 34%, even after correction for major risk factors [30], thereby supporting our findings in relation to glucose tolerance.
Since fasting hyperglycemia is due primarily to hepatic insulin resistance [12], we addressed the effects of whole-grains on endogenous glucose production by using isotopic tracer dilution experiments. Our results suggest no difference in fasting glucose or hepatic glucose production between the two diets. These findings are consistent with some [8, 14], but not all [29], prior work showing that a single whole-grain enriched meal has no effect on endogenous glucose production during a standard OGTT. In fact, the reduction in hepatic glucose production observed by Thorburn et al. [29] was thought to be the result of gut fermentation, which is unlikely to be a major factor in our intervention given the relatively small difference in fiber intake. Thus, our results suggest that reductions in blood glucose in whole-grain vs. refined grain intake are unlikely to be the result of hepatic glucose production in obese individuals. However, we suggest that further work is required on this topic given that hepatic steatosis is prevalent in the U.S. and few data exist characterizing the effects of whole-grains in people with non-alcoholic fatty liver disease.
Alternatively, the observation that whole-grains improved peripheral insulin resistance suggests that skeletal muscle, and possibly adipose tissue, improved glucose metabolism. Indeed, skeletal muscle is an important site for insulin mediated glucose metabolism, and previous work reports that whole-grains increase clamp-derived insulin sensitivity [8]. Although clamp data are the gold-standard for insulin-mediated glucose metabolism, the utility of the data from a clinical perspective is limited. Moreover, prior whole grain diet studies did not include stable isotopes as part of the clamp approach, thereby limiting our understanding of the contribution of skeletal muscle to improved glucose control. Prior acute meal studies using whole-grains, nevertheless, suggest that skeletal muscle glucose uptake is increased compared with refined-grain carbohydrates [14]. Our study extends these findings by investigating the effects of whole-grains in the diet on postprandial like nutrient stimulation, and demonstrates that the reduction of peripheral insulin resistance was independent of changes in FFM. Indeed, while additional research is required to test the mechanism by which whole-grains potentially influence FFM, prior work suggests that whole-grains generally have no effect on muscle mass despite loss of fat mass [31, 32]. Why FFM may decline with whole-grains is unclear from the current study design, but our data suggest that greater muscle mass alone is not required for the whole grain related improvement in peripheral glucose uptake. This is clinically meaningful as well since the change in peripheral insulin resistance was correlated with lower 2-hour glucose levels, suggesting that skeletal muscle metabolism per se, not muscle mass, is important for the regulation of post-prandial circulating glucose [33]. Herein metabolic flexibility was also significantly correlated with lower glucose iAUC, which is consistent with enhanced metabolic flexibility being linked to improved skeletal muscle insulin action [34]. Interestingly, to our knowledge this is the first study to systematically assess fasting and insulin-stimulated substrate oxidation during an OGTT with isotopic tracers following a whole-grain intervention. These data are consistent with Robertson et al. who performed skeletal muscle biopsies following resistant starch intake for 4-weeks and reported no effect on GLUT-4 protein levels or insulin signaling gene expression [35]. Their data suggest that alternative pathways likely contributed to the effects of whole-grains on skeletal muscle glucose metabolism. Given that whole-grains are known to elevate gut-derived short-chain fatty acids (SCFAs), which alter fuel utilization through AMPK activation and PGC1-α protein expression, we speculate that the increased reliance on energy from fat in the fasted state following whole-grain intake may have alleviated deleterious effects of lipid species on insulin-stimulated GLUT-4 translocation for enhanced carbohydrate uptake and utilization [14, 15]. It is not possible from this study to discern whether compounds within whole-grains (e.g. vitamins, phenolic compounds, magnesium, and phytoestrogens) either in isolation or in combination produced the glucose lowering effect in muscle [36], but future work is warranted to isolate the effects of particle size, grain type, and encapsulation of whole-grains to glucose metabolism and insulin sensitivity. One avenue of interest for future work is the possibility that alkylresorcinols may lead to improved insulin sensitivity [37], as plasma concentrations of these compounds were much higher during the whole-grain diet.
Despite being one of the largest controlled feeding studies on whole-grains in obese adults at risk for type 2 diabetes and CVD, this study does have some limitations. The majority of participants were female, so it is not possible to determine if sex differences in glycemic responsiveness to whole-grain intake, as has previously been suggested [30]. Generally, females are characterized by impairments in post-prandial glucose metabolism, while men are more likely to have impaired fasting glucose [38]. However, statistical adjustments for sex suggest no such effect on glucose regulation in this study. No difference in gut absorption was observed in the current study based on Rameal, which represents the net effect of glucose appearing from the gut and/or liver release. Nonetheless, it remains possible that differences in hepatic glucose uptake contributed to differences in plasma glucose [29]. Moreover, we recognize that our study was conducted in a relatively small sample size and interpretation of condition and correlation analysis should be made with caution. Lastly, this study did not specifically test the effect of whole-grains in people with type 2 diabetes. Therefore, the lack of statistical significance between groups of secondary outcomes, may in part, relate to the relative normal glucose metabolism in these obese individuals. It is important to acknowledge though that 3 people were identified in this study population to have type 2 diabetes based on 2-hour glucose levels, and 5 people had prediabetes (n=0 fasting glucose 100–125mg/dl; n=3 2-hour glucose 140–200mg/dl; n=2 combined glucose intolerant). Therefore, future work should examine whole-grains in these clinical populations to confirm the current findings as well as elucidate mechanisms by which whole-grains act to lower circulating glucose. To that end, it should be noted that a major strength of the current study is that all subjects served as their own controls and were fed the entirety of their mixed-whole-grain meals, thereby strengthening the clinical application of our findings.
In conclusion, whole-grain intake improved glucose tolerance in obese adults at risk factor for diabetes, and this lower plasma glucose response was paralleled by reduced peripheral insulin resistance and improved metabolic flexibility, independent of body fat loss or dietary fiber. Together with elevated fasting fat oxidation and insulin-stimulated carbohydrate utilization, our data extend previous clinical research observations and demonstrate that whole-grain intake effectively promotes glycemic control by improving insulin action. Future work is necessary to address specific mechanisms whereby whole-grains lead to better glucose control in obese adults in order to optimize nutritional recommendations that may prevent and/or delay on the onset of type 2 diabetes and CVD.
Acknowledgments
We thank the CRU nursing staff, CORE lab, and participants for their outstanding efforts. In particular, we thank Kay Stelmach for CRU support, Marianne Fischer, RD, for dietetic support, Velma Stephens and Brenda Foley-Murray for excellent organization of food distribution and biospecimen collection, Teresa Markle for outstanding biospecimen organization, Jeff Hammel for statistical advice, Ciarán Fealy for technical support, Isabelle Breton, Anne-France Kapp, Corinne Ammon-Zufferey, Laurence Guignard, and Alicia Zangger for biochemical analyses, and the staff of Nestlé PTC Solon and Cereal Partners Worldwide for providing the study meals and foods.
Funding: Investigator-initiated trial from Nestlé (JPK); NIH T32 DK007319 (for SKM and ELK by support through JPK); and NIH Research Resources Grant UL1RR024989
ABBREVIATIONS
- AACE
American Association of Clinical Endocrinology
- ACE
American College of Endocrinology
- BMI
Body mass index
- ECG
Electrocardiogram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
JPG and SK are employed by Nestlé, while the remaining authors report no conflict of interest.
Trial Registration: NCT01411540
AUTHOR CONTRIBUTIONS
SKM and JPK had full access to all the data in the study, and SKM takes responsibility for the accuracy of the statistical analysis. JPK is the guarantor of this work. The study was conceived and designed by JPK and ABR. All authors contributed to data collection, data organization, or analysis. SKM drafted the manuscript and all other authors provided edits and approved the final manuscript. Nestlé marketing provided no input on the final data analysis, interpretation or writing of this work.
References
- 1.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr. 2012;142(7):1304–13. doi: 10.3945/jn.111.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik VS, Willett WC, Hu FB. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med. 2010;170(11):961–9. doi: 10.1001/archinternmed.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract. 2016;22(Suppl 3):1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 4.Liese A, Roach A, Sparks K, Marquart L, D'Agostino R, Mayer Davis E. Whole-grain intake and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Am J Clin Nutr. 2003;78(5):965–71. doi: 10.1093/ajcn/78.5.965. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MK, Koh-Banerjee P, Franz M, Sampson L, Gronbaek M, Rimm E. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation 1. Am J Clin Nutr. 2006;83(2):275–83. doi: 10.1093/ajcn/83.2.275. [DOI] [PubMed] [Google Scholar]
- 6.Rave K, Roggen K, Dellweg S, Heise T, tom Dieck H. Improvement of insulin resistance after diet with a whole-grain based dietary product: results of a randomized, controlled cross-over study in obese subjects with elevated fasting blood glucose. Br J Nutr. 2007;98(5):929–36. doi: 10.1017/S0007114507749267. [DOI] [PubMed] [Google Scholar]
- 7.Andersson A, Tengblad S, Karlstrom B, et al. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J Nutr. 2007;137(6):1401–7. doi: 10.1093/jn/137.6.1401. [DOI] [PubMed] [Google Scholar]
- 8.Pereira MA, Jacobs DR, Jr, Pins JJ, et al. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75(5):848–55. doi: 10.1093/ajcn/75.5.848. [DOI] [PubMed] [Google Scholar]
- 9.Laaksonen DE, Toppinen LK, Juntunen KS, et al. Dietary carbohydrate modification enhances insulin secretion in persons with the metabolic syndrome. Am J Clin Nutr. 2005;82(6):1218–27. doi: 10.1093/ajcn/82.6.1218. [DOI] [PubMed] [Google Scholar]
- 10.Juntunen KS, Laaksonen DE, Poutanen KS, Niskanen LK, Mykkanen HM. High-fiber rye bread and insulin secretion and sensitivity in healthy postmenopausal women. Am J Clin Nutr. 2003;77(2):385–91. doi: 10.1093/ajcn/77.2.385. [DOI] [PubMed] [Google Scholar]
- 11.Harris Jackson K, West SG, Vanden Heuvel JP, et al. Effects of whole and refined grains in a weight-loss diet on markers of metabolic syndrome in individuals with increased waist circumference: a randomized controlled-feeding trial. Am J Clin Nutr. 2014;100(2):577–86. doi: 10.3945/ajcn.113.078048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malin SK, Haus JM, Solomon TPJ, Blaszczak A, Kashyap SR, Kirwan JP. Insulin sensitivity and metabolic flexibility following exercise training among different obese insulin resistant phenotypes. Am J Physiol Endo Metabol. 2013;305(10):E1292–1298. doi: 10.1152/ajpendo.00441.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malin SK, Viskochil R, Oliver CO, Braun B. Mild fasting hyperglycemia shifts fuel reliance towards fat during exercise in adults with impaired glucose tolerance. J Appl Physiol. 2013;115(1):78–83. doi: 10.1152/japplphysiol.00084.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Priebe MG, Wang H, Weening D, Schepers M, Preston T, Vonk RJ. Factors related to colonic fermentation of nondigestible carbohydrates of a previous evening meal increase tissue glucose uptake and moderate glucose-associated inflammation. Am J Clin Nutr. 2010;91(1):90–7. doi: 10.3945/ajcn.2009.28521. [DOI] [PubMed] [Google Scholar]
- 15.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–17. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirwan JP, Malin SK, Scelsi AR, et al. A Whole-Grain Diet Reduces Cardiovascular Risk Factors in Overweight and Obese Adults: A Randomized Controlled Trial. J Nutr. 2016;146(11):2244–51. doi: 10.3945/jn.116.230508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross AB. Present status and perspectives on the use of alkylresorcinols as biomarkers of wholegrain wheat and rye intake. J Nutr Metab. 2012:462967. doi: 10.1155/2012/462967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaul S, Rothney MP, Peters DM, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity. 2012;20(6):1313–8. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirwan JP, O'Gorman DJ, Cyr-Campbell D, et al. Effects of a moderate glycemic meal on exercise duration and substrate utilization. Med Sci Sports Exerc. 2001;33(9):1517–23. doi: 10.1097/00005768-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Rodieux F, Giusti V, D'Alessio D, et al. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity. 2008;16(2):298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. Wiley-Liss; New York: 1992. pp. 119–144. [Google Scholar]
- 22.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 23.Jeukendrup AE, Raben A, Gijsen A, et al. Glucose kinetics during prolonged exercise in highly trained human subjects: effect of glucose ingestion. J Physiol (Lond) 1999;515(2):579–89. doi: 10.1111/j.1469-7793.1999.579ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landberg R, Man P, Kamal-Eldin A. A rapid gas chromatography-mass spectrometry method for quantification of alkylresorcinols in human plasma. Anal Biochem. 2009;385(1):7–12. doi: 10.1016/j.ab.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Rosen LA, Silva LO, Andersson UK, Holm C, Ostman EM, Bjorck IM. Endosperm and whole grain rye breads are characterized by low post-prandial insulin response and a beneficial blood glucose profile. Nutrition journal. 2009;8:42. doi: 10.1186/1475-2891-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilsson AC, Ostman EM, Knudsen KE, Holst JJ, Bjorck ME. A cereal-based evening meal rich in indigestible carbohydrates increases plasma butyrate the next morning. J Nutr. 2010;140(11):1932–6. doi: 10.3945/jn.110.123604. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh GH, Noakes M, Royle PJ, Foster PR. Whole-grain rye and wheat foods and markers of bowel health in overweight middle-aged men. Am J Clin Nutr. 2003;77(4):967–74. doi: 10.1093/ajcn/77.4.967. [DOI] [PubMed] [Google Scholar]
- 28.Brownlee IA, Moore C, Chatfield M, et al. Markers of cardiovascular risk are not changed by increased whole-grain intake: the WHOLEheart study, a randomised, controlled dietary intervention. Br J Nutr. 2010;104(1):125–34. doi: 10.1017/S0007114510000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorburn A, Muir J, Proietto J. Carbohydrate fermentation decreases hepatic glucose output in healthy subjects. Metabolism. 1993;42(6):780–5. doi: 10.1016/0026-0495(93)90249-n. [DOI] [PubMed] [Google Scholar]
- 30.Wirstrom T, Hilding A, Gu HF, Ostenson CG, Bjorklund A. Consumption of whole grain reduces risk of deteriorating glucose tolerance, including progression to prediabetes. Am J Clin Nutr. 2013;97(1):179–87. doi: 10.3945/ajcn.112.045583. [DOI] [PubMed] [Google Scholar]
- 31.Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, Kris-Etherton PM. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr. 2008;87:79–90. doi: 10.1093/ajcn/87.1.79. [DOI] [PubMed] [Google Scholar]
- 32.Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, Bügel S Tetens I, Astrup A. Whole grain compared to refined wheat decreases the percentage body fat following a 12-week energy restricted dietary intervention in postmenopausal women. J Nutr. 2012;142:710–6. doi: 10.3945/jn.111.142315. [DOI] [PubMed] [Google Scholar]
- 33.Kirk E, Reeds DN, Finck BN, et al. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136(5):1552–60. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49(5):677–83. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 35.Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82(3):559–67. doi: 10.1093/ajcn.82.3.559. [DOI] [PubMed] [Google Scholar]
- 36.Eelderink C, Schepers M, Preston T, et al. Slowly and rapidly digestible starchy foods can elicit a similar glycemic response because of differential tissue glucose uptake in healthy men. Am J Clin Nutr. 2012;96(5):1017–24. doi: 10.3945/ajcn.112.041947. [DOI] [PubMed] [Google Scholar]
- 37.Oishi K, Yamamoto S, Itoh N, et al. Wheat alkylresorcinols suppress high-fat, high-sucrose diet-induced obesity and glucose intolerance by increasing insulin sensitivity and cholesterol excretion in male mice. J Nutr. 2015;145(2):199–206. doi: 10.3945/jn.114.202754. [DOI] [PubMed] [Google Scholar]
- 38.Faerch K, Borch Johnsen K, Vaag A, et al. Sex differences in glucose levels: a consequence of physiology or methodological convenience? The Inter99 study. Diabetologia. 2010;53(5):858–65. doi: 10.1007/s00125-010-1673-4. [DOI] [PubMed] [Google Scholar]