Abstract
The Src family kinase (SFK) is a subfamily of non-receptor tyrosine kinases. SFK members, Src and especially Fyn, are expressed in the striatum. These SFK members are involved in the regulation of neuronal and synaptic activities and are linked to the pathogenesis of a variety of neuropsychiatric and neurodegenerative disorders. Given the fact that muscarinic acetylcholine (mACh) receptors are highly expressed in striatal neurons and are critical for the regulation of striatal function, we investigated the role of mACh receptors in the regulation of SFKs in the adult rat striatum in vivo. We found that pharmacological blockade of mACh receptors by systemic administration of the mACh antagonist scopolamine induced a marked increase in phosphorylation of SFKs in the striatum of male and female rats. This scopolamine-induced increase in SFK phosphorylation occurred in the two subdivisions of the striatum (caudate putamen and nucleus accumbens) and was time-dependent and reversible. Another mACh antagonist atropine was also effective in stimulating SFK phosphorylation. Coadministration of subthreshold doses of scopolamine and a dopamine D1 receptor agonist SKF81297 enhanced striatal SFK phosphorylation. Between Fyn and Src proteins immunoprecipitated from striatal tissue, scopolamine selectively increased phosphorylation of Fyn. The increase in Fyn phosphorylation was accompanied by an increase in Fyn kinase activity in response to scopolamine. These results reveal a significant role of mACh receptors in the regulation of SFKs (mainly Fyn) in striatal neurons. Under normal conditions, endogenous mACh receptors appear to exert an inhibitory effect on Fyn activity.
Keywords: dopamine D1 receptor, Src, tyrosine kinase, caudate putamen, nucleus accumbens, scopolamine, atropine, SKF81297
Introduction
Non-receptor tyrosine kinases are divided into several subfamilies and are involved in the regulation of a large number of intracellular protein substrates via a tyrosine phosphorylation-dependent mechanism (Neet and Hunter, 1996). One of typical subfamilies of these kinases is the Src family kinase (SFK). In this family, Src and Fyn (also known as FynB) are key members and have been most extensively investigated. Src and Fyn are expressed in the mammalian brain and are present in cytoplasmic and synaptic locations (Ohnishi et al., 2011). Thus, Src and Fyn target a set of discrete substrates in these subcellular compartments. By directly phosphorylating specific tyrosine sites on these substrates, Src and Fyn regulate a variety of cellular and synaptic activities (Kalia et al., 2004; Ohnishi et al., 2011; Schenone et al., 2011). Of note, Src and Fyn are sensitive to changing cellular and synaptic input. Their activity could be upregulated by an increase in phosphorylation at a specific site. That is, phosphorylation of Src and Fyn at a conserved residue, tyrosine 416 (Y416), in the activation loop by an autophosphorylation mechanism leads to activation of these enzymes (Roskoski, 2005; Okada, 2012).
The transmitter acetylcholine (ACh) in the brain interacts with two types of receptors: nicotinic and muscarinic receptors. While the former is the ionotropic receptor, the latter is the G protein-coupled receptor. Muscarinic ACh (mACh) receptors have five subtypes (M1–M5) (Caulfield et al., 1998). M1, M3, and M5 receptors are Gq-coupled receptors, whereas M2 and M4 are Gi/o-coupled receptors (Wess, 1996). Based on the type of G proteins that mACh receptors are coupled to, activation of M1, M3, and M5 receptors enhances phospholipase Cβ1 activity, leading to yield of two signaling molecules, diacylglycerol and inositol-1,4,5-triphosphate. Activation of M2 and M4 receptors, on the other hand, inhibits adenylyl cyclase and thereby reduces formation of a downstream signaling molecule, cAMP. mACh receptors are among the receptors widely expressed in the brain. Thus, they are actively involved in the regulation of a variety of neural activities.
One brain area where mACh receptors (mainly M1 and M4 receptors) are highly expressed is the striatum (Levey et al., 1991). The striatum, as a key structure in the mesolimbic system, is essential for normal brain functions in mood, cognition and movement and is linked to the pathogenesis of various neurological and neuropsychiatric disorders. Within this region, ACh and dopamine notably form a dynamic balance controlling homeostasis of striatal medium spiny projection neurons. Dopamine has been well demonstrated to regulate SFKs in the brain. While the dopamine D1 receptor agonist increased SFK Y416 phosphorylation in the striatum, dopamine D2 receptors exerted an inhibitory effect on Y416 phosphorylation (Hattori et al., 2006; Mao and Wang, 2015; 2016; 2017). However, whether ACh through mACh receptors regulates SFKs in striatal neurons has been incompletely studied.
In this study, a series of neuropharmacological and neurochemical experiments were conducted in adult male and female rats to define the role of mACh receptors in the regulation of SFKs in vivo. Two non-selective mACh antagonists, scopolamine and atropine, were used to block mACh receptors. The effect of these antagonists after a systemic injection on phosphorylation of SFKs at Y416 and expression of total Fyn and Src proteins was investigated in the two subdivisions of striatum, the caudate putamen (CPu) and nucleus accumbens (NAc). The effect of coadministration of a D1 agonist SKF81297 and scopolamine on Y416 phosphorylation was also examined in the striatum. Finally, the effect of scopolamine on Y416 phosphorylation of immunopurified Fyn and Src and on Fyn kinase activity was tested.
Materials and Methods
Animals
Male and female Wistar rats were used. These animals (270–360 g; Charles River, New York, NY) were kept in our animal facility at 23°C and humidity of 50 ± 10%. Food and water were available ad libitum in animal rooms which were maintained on a 12/12 h light/dark cycle with lights on at 0700. The Guide for the Care and Use of Laboratory Animals of the NIH was used and followed throughout the course of experiment. Animal care and use was approved by the Institutional Animal Care and Use Committee.
Systemic administration of drugs
Drugs were administered at a volume of 1 ml/kg via an intraperitoneal (i.p.) injection. Rats were sacrificed at a time point indicated after drug administration. The dose of scopolamine, atropine, and SKF81297 was calculated as the salt. Scopolamine was administered at a dose of 5 mg/kg. Selection of this dose was due to the fact that scopolamine injected at this dose increased immediate early gene expression and phosphorylation of extracellular signal-regulated kinases in the rat striatum in vivo (Wang and McGinty, 1996a; Mao et al., 2017). Atropine was injected at 5 mg/kg (i.p.). At this dose, atropine was effective in inducing immediate early gene expression (Bernard et al., 1993) and reducing the dopamine D2 receptor antagonist raclopride-induced catalepsy (Alcock et al., 2001). An acute injection of saline (1 ml/kg) was made in age-matched rats and served as controls.
Western blot analysis
Rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.). Animals were then sacrificed by decapitation. Brains were removed and cut into coronal slices. The two subdivisions of the striatum, the CPu and NAc, were dissected from the slices. Brain tissue was homogenized in a radioimmunoprecipitation assay (RIPA) buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin (Cell Signaling Technology, Danvers, MA). Homogenates were centrifuged at 800 g for 10 min (4°C). Supernatants were collected and protein concentrations were determined. Samples were stored at −80°C until use.
Western blots were performed to analyze changes in protein levels as described previously (Jin et al., 2013). Briefly, proteins were separated on SDS NuPAGE Novex 4–12% gels (Invitrogen, Carlsbad, CA). Separated proteins were transferred to polyvinylidene fluoride membranes. Membranes were then blocked, washed, and incubated in a buffer solution containing a primary antibody (see below) overnight at 4°C. Membranes were incubated in a buffer solution containing a secondary antibody. Proteins on membranes were visualized by an enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Piscataway, NJ) and were measured using ImageJ analysis software. Values of optical density were normalized to a loading control protein (actin).
Immunoprecipitation
This was carried out as described previously (Mao and Wang, 2016). Dissected brain tissue was homogenized in a RIPA lysis buffer. Homogenates were centrifuged at 800 g for 10 min (4°C). Supernatants were collected and used for immunoprecipitation. Proteins in supernatants (300–500 μg) were incubated in a buffer solution containing a mouse antibody against Src (2 μg) or Fyn (3 μg). Protein complexes were precipitated with 50% protein A and G agarose/sepharose bead slurry (GE Healthcare). Precipitated proteins were resolved on SDS-PAGE with a rabbit antibody against Src, Fyn, or phosphorylated Y416 (pY416) (see below).
Fyn kinase activity assay
Fyn kinase activity was assayed using the Takara Universal Tyrosine Kinase Assay Kit (Clontech Laboratory, Inc., Mountain View, CA) according to the manufacturer’s instructions as described previously (Liu et al., 2014). After anesthesia with pentobarbital and decapitation, the rat striatum was dissected and was homogenized in an equal volume of the extraction buffer provided with the kit. Fyn proteins from lysates were immunoprecipitated with a mouse anti-Fyn antibody. Immunopurified Fyn proteins were eluted in a kinase reaction buffer and added with ATP to wells of microplates covered with an immobilized tyrosine kinase substrate poly(Glu-Tyr) (37°C, 30 min). Samples were washed three times, blocked with a blocking solution, and incubated with an anti-phosphotyrosine antibody conjugated to horseradish peroxidase. The absorbance of phosphorylated substrates was measured at 450 nm.
Antibodies and pharmacological agents
Rabbit polyclonal antibodies used in this study include antibodies against Src (Cell Signaling), Fyn (Santa Cruz Biotechnology, Santa Cruz, CA), or β-actin (Sigma-Aldrich, St. Louis, MO). Mouse antibodies include those against Src (Cell Signaling) or Fyn (Santa Cruz). A rabbit polyclonal antibody against pY416 (Cell Signaling) was used. This antibody reacts with the Src family members when phosphorylated at the conserved activation residue: Y416 (chicken Src), Y419 (rat Src), and Y420 (rat Fyn). Pharmacological agents include (−)-scopolamine hydrobromide, SKF81297 [(±)-6-chloro-PB hydrobromide], and atropine sulfate. These agents were purchased from Sigma-Aldrich (St. Louis, MO). All agents were freshly prepared at the day of experiments. They were dissolved in physiological saline.
Statistics
Our data were evaluated statistically and these data are expressed as means ± SEM. One statistical method we used was a one- or two-way analysis of variance followed by a Bonferroni (Dunn) comparison. Another method was a two-tailed unpaired Student’s t-test.
Results
Scopolamine time-dependently increases SFK phosphorylation
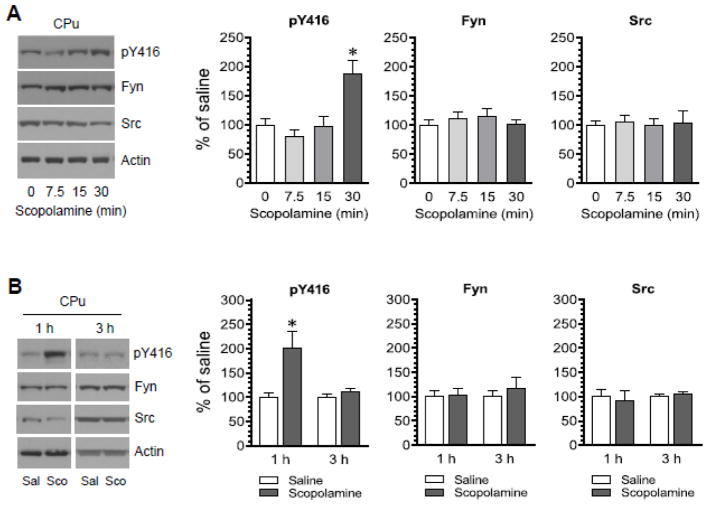
To determine the effect of pharmacological blockade of mACh receptors on SFK phosphorylation, the mACh antagonist scopolamine was injected at a single dose (5 mg/kg, i.p.). Rats were sacrificed at a different time point (7.5, 15, or 30 min) after scopolamine injection. Changes in phosphorylation of SFKs at a pan autophosphorylation site within the activation loop (Y416) were analyzed in the CPu by immunoblots. We found that pY416 levels were not altered at two early time points surveyed (7.5 and 15 min) in scopolamine-treated rats as compared to saline-treated rats (Fig. 1A). Saline-treated rats were sacrificed immediately (0 min) after saline injection and served as a control. Remarkably, pY416 levels were significantly elevated at a late time point (30 min) following scopolamine injection. No significant change in the total amount of Fyn and Src proteins was observed at all time points surveyed. These results indicate that blockade of mACh receptors with scopolamine increases SFK Y416 phosphorylation in the CPu. To determine the effect of scopolamine at further later time points, we injected the antagonist at the same dose (5 mg/kg, i.p.) and sacrificed rats at 1 or 3 h after scopolamine injection. Scopolamine persisted to increase pY416 signals in the CPu at 1 h (Fig. 1B). However, at 3 h, the pY416 level in scopolamine-treated rats was not significantly different from that in saline-treated rats. At 1 and 3 h, scopolamine did not alter total Fyn and Src protein levels. Thus, a systemic administration of scopolamine induces a time-dependent increase in SFK Y416 phosphorylation in the CPu in vivo.
Figure 1. Time-dependent effects of scopolamine on SFK Y416 phosphorylation in the rat CPu.
(A) Effects of scopolamine on phosphorylation of SFK Y416 and expression of Fyn and Src. Rats were administered with scopolamine (5 mg/kg, i.p.) and were sacrificed at a different time point (7.5, 15, or 30 min) after scopolamine injection. (B) Effects of saline and scopolamine on phosphorylation of SFK Y416 and expression of Fyn and Src. Rats were administered with saline (Sal) or scopolamine (Sco, 5 mg/kg, i.p.) and were sacrificed 1 or 3 h after saline or scopolamine injection. Representative immunoblots are shown to the left of the quantified data. Note that scopolamine induced a time-dependent increase in Y416 phosphorylation, while the antagonist did not alter expression of total Fyn and Src proteins. Data are presented as means ± SEM (n = 3–5 per group). *P < 0.05 versus saline (0 min) (A) or saline at the same time point (B).
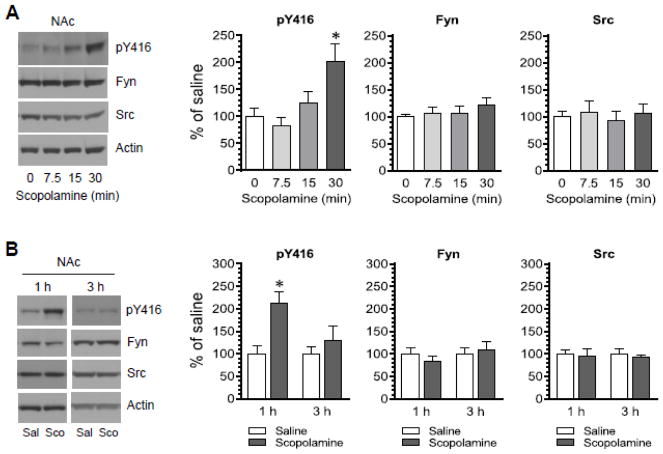
The effect of scopolamine on SFK Y416 phosphorylation was also examined in the NAc of the same rats. Similar to the effect of scopolamine seen in the CPu, scopolamine time-dependently elevated Y416 phosphorylation in the NAc. As shown in Fig. 2A, scopolamine induced a minimal change in Y416 phosphorylation at 7.5 and 15 min after scopolamine injection. At 30 min, scopolamine induced a significant increase in pY416 levels. An increase in pY416 levels was still seen at 1 but not 3 h in scopolamine-treated rats relative to saline-treated rats (Fig. 2B). No significant change in total Fyn and Src protein levels was seen at all time points surveyed. These results indicate that scopolamine can upregulate SFK Y416 phosphorylation in the NAc.
Figure 2. Time-dependent effects of scopolamine on SFK Y416 phosphorylation in the rat NAc.
(A) Effects of scopolamine on phosphorylation of SFK Y416 and expression of Fyn and Src. Rats were administered with scopolamine (5 mg/kg, i.p.) and were sacrificed at a different time point (7.5, 15, or 30 min) after scopolamine injection. (B) Effects of saline and scopolamine on phosphorylation of SFK Y416 and expression of Fyn and Src. Rats were administered with saline (Sal) or scopolamine (Sco, 5 mg/kg, i.p.) and were sacrificed 1 or 3 h after saline or scopolamine injection. Representative immunoblots are shown to the left of the quantified data. Data are presented as means ± SEM (n = 3–5 per group). *P < 0.05 versus saline (0 min) (A) or saline at the same time point (B).
Coadministration of scopolamine and SKF81297 increases SFK phosphorylation
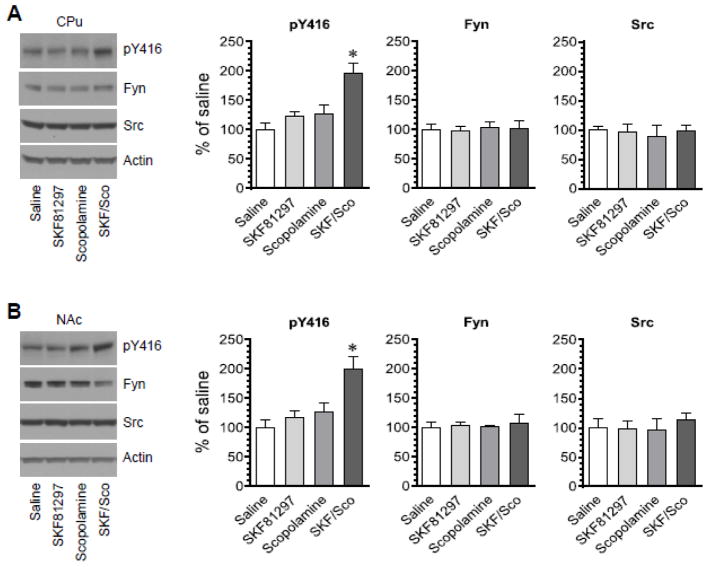
D1 receptors are positively linked to SFK Y416 phosphorylation based on the fact that a D1 agonist SFK81297 (3 mg/kg, i.p.) induced a small but significant increase in Y416 phosphorylation in the rat striatum (Mao and Wang, 2015). To determine the effect of D1 receptor activation together with mACh blockade on Y416 phosphorylation, we coadministered SFK81297 and scopolamine at a low dose (1 mg/kg for both drugs, i.p.) and sacrificed rats 45 min after drug administration. Changes in pY416 levels were investigated in the CPu and NAc using Western blots. We found that administration of either SKF81297 or scopolamine alone at the low dose did not significantly affect Y416 phosphorylation in the CPu (Fig. 3A) and NAc (Fig. 3B). Interestingly, coadministration of the two agents induced a significant increase in pY416 levels in the two regions. SKF81297 and scopolamine when administered alone or together had no effect on total Fyn and Src protein expression. These data suggest that coadministration of subthreshold doses of the D1 agonist and the mACh antagonist is able to enhance SFK Y416 phosphorylation in the CPu and NAc.
Figure 3. Effects of coadministration of SKF81297 and scopolamine on SFK Y416 phosphorylation in the rat striatum.
(A) Effects of coadministration of SKF81297 and scopolamine on phosphorylation of SFK Y416 and expression of Fyn and Src in the CPu. (B) Effects of coadministration of SKF81297 and scopolamine on phosphorylation of SFK Y416 and expression of Fyn and Src in the NAc. Representative immunoblots are shown to the left of the quantified data. Note that SKF81297 and scopolamine when coadministered induced a marked increase in pY416 levels in both the CPu and NAc, while the two agents injected alone had no effect. Rats were administered with SKF81297 (SKF, 1 mg/kg, i.p.) or scopolamine (Sco, 1 mg/kg, i.p.) or the two agents together and were sacrificed 45 min after drug injection for Western blot analysis. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus saline.
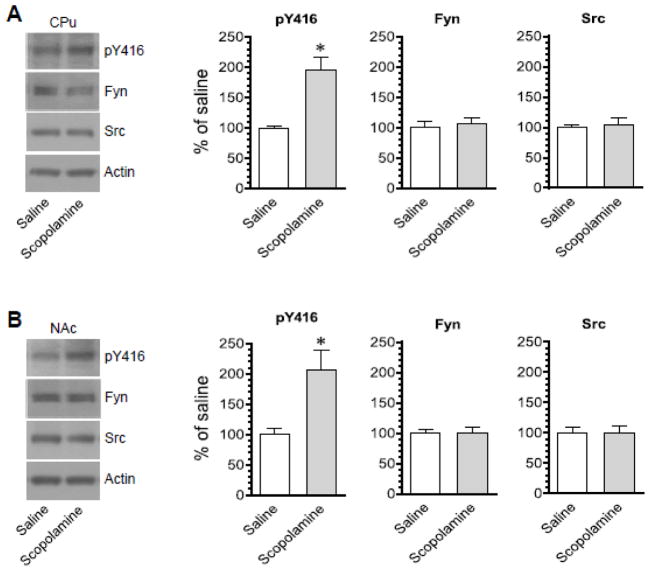
Scopolamine increases SFK phosphorylation in female rats
In this study, female rats were used, while male rats were used in all other studies of the present work. This was set to explore whether there exists a sex influence over the SFK response to scopolamine. An i.p. injection of scopolamine (5 mg/kg, 45 min prior to brain tissue collection) induced a marked increase in pY416 levels in the CPu of female rats (Fig. 4A). Similar results were observed in the NAc as the pY416 level was higher in scopolamine-treated rats than saline-treated rats (Fig. 4B). No significant change in Fyn and Src protein levels after scopolamine injection was seen in both regions. Thus, like male rats, female rats exhibited a positive response of SFK Y416 phosphorylation to scopolamine.
Figure 4. Effects of scopolamine on SFK Y416 phosphorylation in the striatum of female rats.
(A) Effects of scopolamine on phosphorylation of SFK Y416 and expression of Fyn and Src in the CPu. (B) Effects of scopolamine on phosphorylation of SFK Y416 and expression of Fyn and Src in the NAc. Representative immunoblots are shown to the left of the quantified data. Note that scopolamine induced a marked increase in pY416 levels in both the CPu and NAc. Rats were administered with scopolamine (5 mg/kg, i.p.) and were sacrificed 45 min after scopolamine injection for Western blot analysis. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus saline.
Atropine increases SFK phosphorylation
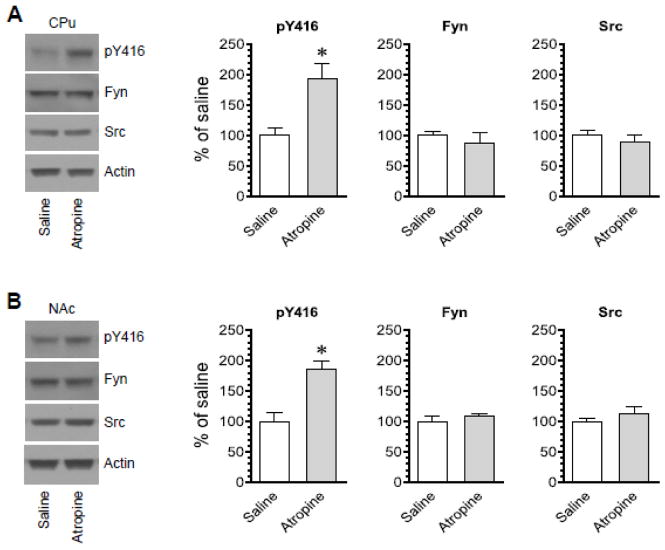
Another non-selective mACh antagonist atropine was used to test its effects on SFK Y416 phosphorylation. A single injection of atropine at a dose of 5 mg/kg (i.p., 45 min prior to brain tissue harvest) increased pY416 levels in the CPu (Fig. 5A). Similarly, atropine increased pY416 levels in the NAc (Fig. 5B). In both regions, atropine did not significantly alter Fyn and Src protein levels. Thus, like scopolamine, atropine possesses the ability to enhance SFK Y416 phosphorylation in the CPu and NAc.
Figure 5. Effects of atropine on SFK Y416 phosphorylation in the rat striatum.
(A) Effects of atropine on phosphorylation of SFK Y416 and expression of Fyn and Src in the CPu. (B) Effects of atropine on phosphorylation of SFK Y416 and expression of Fyn and Src in the NAc. Representative immunoblots are shown to the left of the quantified data. Note that atropine induced a marked increase in pY416 levels in both the CPu and NAc. Rats were administered with atropine (5 mg/kg, i.p.) and were sacrificed 45 min after scopolamine injection for Western blot analysis. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus saline.
Scopolamine selectively increases Fyn phosphorylation
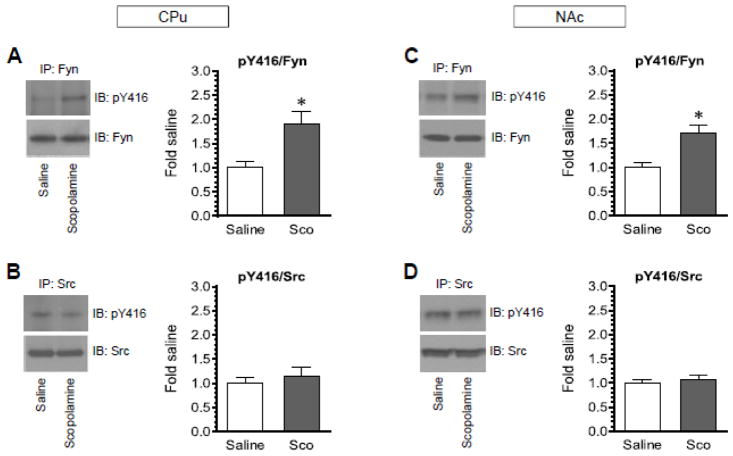
Both Fyn and Src can be phosphorylated at the conserved autophosphorylation site Y416 (Roskoski, 2005; Okada, 2012). To determine whether scopolamine increases Y416 phosphorylation in either Fyn or Src or both, Fyn or Src proteins were immunoprecipitated by an antibody against Fyn or Src, respectively, from the CPu and NAc. The level of pY416 in these immunoprecipitated Fyn and Src proteins was then analyzed by Western blot. Interestingly, following a single injection of scopolamine (5 mg/kg, i.p.), pY416 levels were significantly elevated in immunoprecipitated Fyn proteins from the CPu (Fig. 6A). In contrast, pY416 levels in immunoprecipitated Src proteins remained unchanged in scopolamine-treated rats relative to saline-treated rats (Fig. 6B). Similar results were observed in the NAc (Fig. 6C and 6D). A significant increase in pY416 signals was seen in immunoprecipitated Fyn but not Src. Thus, scopolamine selectively upregulates Y416 phosphorylation in Fyn rather than Src in the two subregions of the striatum.
Figure 6. Effects of scopolamine on Y416 phosphorylation in immunoprecipitated Fyn and Src proteins from the rat striatum.
(A and B) Effects of scopolamine on Y416 phosphorylation in immunoprecipitated Fyn (A) and Src (B) in the CPu. (C and D) Effects of scopolamine on Y416 phosphorylation in immunoprecipitated Fyn (C) and Src (D) in the NAc. Note that scopolamine selectively increased Fyn but not Src phosphorylation in the two regions. Representative immunoblots are shown at left of quantified data. Rats were injected with a single dose of scopolamine (Sco, 5 mg/kg, i.p.) and were sacrificed 45 min after scopolamine injection for immunoprecipitation (IP) of Fyn and Src, followed by immunoblot (IB) analysis of Y416 phosphorylation in immunoprecipitated Fyn and Src proteins. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus saline.
Scopolamine increases Fyn kinase activity
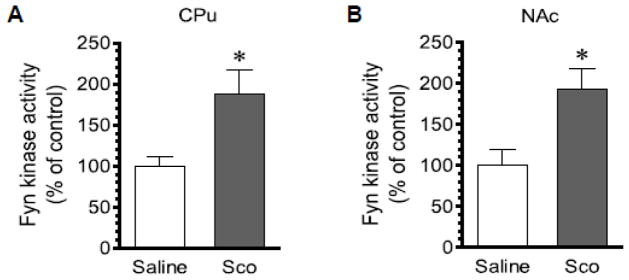
An increase in Y416 phosphorylation is expected to enhance SFK kinase activity. To determine if this is the case for Fyn responses to scopolamine, we analyzed changes in Fyn kinase activity following scopolamine administration. To this end, we administered scopolamine at 5 mg/kg (i.p.). We then sacrificed rats 45 min after scopolamine injection. CPu and NAc lysates were prepared and Fyn proteins were immunoprecipitated. Kinase activity levels of immunopurified Fyn were then assayed. We found that scopolamine showed the ability to elevate Fyn kinase activity. As can be seen from Fig. 7, the activity level of Fyn was significantly elevated in the CPu (Fig. 7A) and NAc (Fig. 7B) of scopolamine-treated rats compared to saline-treated rats.
Figure 7. Effects of scopolamine on Fyn kinase activity in the rat striatum.
(A) Effects of scopolamine on Fyn kinase activity in the CPu. (B) Effects of scopolamine on Fyn kinase activity in the NAc. Note that a single injection of scopolamine (Sco) induced an increase in Fyn kinase activity in the CPu (A) and NAc (B). Rats were administered with saline or scopolamine (5 mg/kg, i.p.) and were sacrificed 45 min after scopolamine injection for Fyn kinase activity assays. Data are presented as means ± SEM (n = 5 per group). *P < 0.05 versus saline.
Discussion
We investigated whether mACh receptors regulate SFKs by testing the effect of the mACh antagonists on SFK Y416 phosphorylation in the striatum of male and female rats. We found that a single i.p. injection of scopolamine induced an increase in Y416 phosphorylation in the CPu and NAc. Another mACh antagonist atropine also increased Y416 phosphorylation. The effect of scopolamine was evidently time-dependent. Scopolamine seems to selectively target Fyn since scopolamine elevated Y416 phosphorylation of immunopurified Fyn but not Src and enhanced Fyn kinase activity in the striatum. Of note, scopolamine and a D1 agonist when coadministered at subthreshold doses induced an increase in Y416 phosphorylation. These results indicate that there exists a tonic mACh tone in inhibiting Fyn activity in striatal neurons under normal conditions.
The effect of scopolamine on striatal SFK phosphorylation has several characteristics. First, the effect of scopolamine was time-dependent. A significant increase in SFK Y416 phosphorylation in the striatum was detected at 30 min after scopolamine injection. The increase remained at 1 h and declined to an insignificant level at 3 h. Thus, the increase in SFK phosphorylation induced by scopolamine is a reversible event. Second, the effect of scopolamine was not drug-specific. Another commonly used mACh antagonist atropine produced the same effect. Third, we tested the sex influence over the SFK response to scopolamine. We found that scopolamine produced a comparable level of phosphorylation of SFK Y416 in the striatum of both male and female rats. Thus, there is no significant sex influence over the SFK response to scopolamine. Finally, our results showed a different response of Fyn and Src to scopolamine. Fyn and Src, due to their homology in amino acid sequence and structures, share a common step of activation, i.e., Y416 autophosphorylation (Thomas and Brugge, 1997). However, Fyn exhibits a much higher level of expression than Src in the striatum (Pascoli et al., 2011). Moreover, Fyn is a preferred SFK member responsive to D1 signaling and linking D1 receptors to N-methyl-D-aspartate (NMDA) receptors in striatal neurons (Dunah and Standaert, 2001; Dunah et al., 2004; Pascoli et al., 2011; Mao and Wang, 2015). Like D1 receptors, mACh receptors in the striatum appear to preferentially regulate Fyn. This notion is supported by our finding in the present study that blockade of mACh receptors elevated phosphorylation of immunopurified Fyn but not Src.
The study on the regulation of SFKs by acetylcholine receptors is limited. An early study found a regulatory role of acetylcholine receptors in controlling SFK activity. Application of carbachol, an agonist stimulating both muscarinic and nicotinic receptors, elevated Src Y416 phosphorylation in a human-derived cell line (Watcharasit et al., 2001), indicating a role of acetylcholine receptors in upregulating Src activity. However, in contrast to this finding in a cell line, we found that the mACh antagonists, both scopolamine and atropine, consistently increased Y416 phosphorylation in the striatum in vivo. Apparently, in striatal neurons, mACh receptors exert an overall inhibitory regulation of SFKs under normal conditions. With regard to the mACh subtype possibly responsible for mediating the effect of scopolamine and atropine, the striatum is knowingly enriched with M1 and M4 subtypes (Levey et al., 1991). M1 receptors activate a Gq-coupled pathway to yield diacylglycerol and inositol-1,4,5-trisphosphate, whereas M4 receptors inhibit Gi/o-coupled cAMP formation and thus suppress protein kinase A (PKA) (Wess, 1996). Gq-coupled receptors are positively linked to non-receptor tyrosine kinases (Dikic et al., 1996; Della Rocca et al., 1997). Activation of M1 receptors activated SFKs in COS-7 cells via a mechanism involving β and γ subunits of G proteins (Igishi and Gutkind, 1998). In hippocampal CA1 neurons that express the M1 subtype of mACh receptors (Levey, 1996), muscarine sequentially activated PKC and Src to potentiate NMDA receptors (Lu et al., 1999). Thus, M1 receptors generally upregulate SFKs and therefore less likely mediate the effect of scopolamine. In contrast to the M1 subtype, the M4 receptor might be a site where scopolamine interacts with to alter SFKs. cAMP and PKA have been shown to act as a signaling system effective in regulating SFKs. For instance, PKA upregulated Fyn phosphorylation at serine 21 and Src phosphorylation at serine 17, which promoted Y416 autophosphorylation of these SFKs (Schmitt and Stork, 2002; Yeo et al., 2011). Similarly, activation of PKA with forskolin positively regulates Fyn although not Src in spinal neurons (Yang et al., 2011). Thus, it is possible that M4 receptors inhibit the cAMP-PKA pathway to inhibit SFK phosphorylation. Blockade of M4 receptors by scopolamine or atropine could therefore result in an increase in SFK phosphorylation (this study).
In addition to ACh, dopamine is another principal transmitter in the striatum. It is generally agreed that acetylcholine and dopamine form an intrinsic dynamic balance to cooperatively control striatal neuronal activity (Chou et al., 1992; Bernard et al., 1993; Morelli et al., 1993; Wang and McGinty, 1996a; 1996b; 1997). At the cellular level, D1 and D2 receptors are expressed in medium spiny projection neurons in the striatum in a segregated manner: D1 receptors in striatonigral neurons and D2 receptors in striatopallidal neurons (Gerfen et al., 1990; Aubert et al., 2000; Bertran-Gonzalez et al., 2010). Stimulation of D1 receptors with agonists has been shown to increase Fyn rather than Src phosphorylation in mouse cultured striatal neurons (Pascoli et al., 2011), rat hippocampal slices (Yang et al., 2012), and the adult rat striatum (Mao and Wang, 2015). In contrast, the D2 receptor antagonists elevated Fyn but not Src phosphorylation in the striatum (Hattori et al., 2006; Mao and Wang, 2015; 2016). Thus, D1 receptors stimulate Fyn phosphorylation, while D2 receptors inhibit it. Of note, M4 receptors and D1 receptors are coexpressed in striatonigral neurons (Ince et al., 1997; Santiago and Potter, 2001). Given the fact that Gi/o-coupled M4 receptors and Gs-coupled D1 receptors antagonistically converge to the cAMP-PKA pathway, it is reasoned that the two receptors regulate PKA and subsequently Fyn in an opposed manner in the same neurons. Consistent with this notion, a positive allosteric modulator VU0152100 selective for M4 receptors suppressed SFK Y416 phosphorylation induced by the D1 agonist SKF81297 in the rat striatum (Mao and Wang, 2015). Moreover, coadministration of SKF81297 and scopolamine at subthreshold doses induced an increase in rat striatal Y416 phosphorylation (this study).
Acknowledgments
This work was supported by NIH grants DA10355 (J.Q.W.) and MH61469 (J.Q.W.). Authors want to thank Dr. Nan He for his technical assistance. We also thank the help from the UMKC student programs (ADVANCER and Sarah Morrison).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Alcock SJ, Hemsley KM, Crocker AD. Atropine acts in the ventral striatum to reduce raclopride-induced catalepsy. Eur J Pharmacol. 2001;424:179–187. doi: 10.1016/s0014-2999(01)01146-3. [DOI] [PubMed] [Google Scholar]
- Aubert I, Ghorayeb I, Normand E, Bloch B. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. J Comp Neurol. 2000;418:22–32. [PubMed] [Google Scholar]
- Bernard V, Dumartin B, Lamy E, Bloch B. Fos immunoreactivity after stimulation or inhibition of muscarinic receptors indicates anatomical specificity for cholinergic control of striatal efferent neurons and cortical neurons in the rat. Eur J Neurosci. 1993;5:1218–1225. doi: 10.1111/j.1460-9568.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanat. 2010;4:136. doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acethycholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- Chou H, Ogawa N, Asanuma M, Hirata H, Mori A. Muscarinic cholinergic receptor-mediated modulation on striatal c-fos mRNA expression induced by levodopa in rat brain. J Neural Transm. 1992;90:171–181. doi: 10.1007/BF01250959. [DOI] [PubMed] [Google Scholar]
- Della Rocca GJ, van Biesen T, Daaka Y, Luttrell DK, Luttrell LM, Lefkowitz RJ. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dopamine D1-dependent trafficking of striatal N-methyl-D-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Mol Pharmacol. 2004;65:121–129. doi: 10.1124/mol.65.1.121. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Hattori K, Uchino S, Isosaka T, Maekawa M, Iyo M, Sato T, Kohsaka S, Yagi T, Yuasa S. Fyn is required for haloperiodol-induced catalepsy in mice. J Biol Chem. 2006;281:7129–7135. doi: 10.1074/jbc.M511608200. [DOI] [PubMed] [Google Scholar]
- Igishi T, Gutkind JS. Tyrosine kinases of the Src family participate in signaling to MAP kinase from both Gq and Gi-coupled receptors. Biochem Biophys Res Commun. 1998;244:5–10. doi: 10.1006/bbrc.1998.8208. [DOI] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Jin DZ, Guo ML, Xue B, Fibuch EE, Choe ES, Mao LM, Wang JQ. Phosphorylation and feedback regulation of metabotropic glutamate receptor 1 by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2013;33:3402–3412. doi: 10.1523/JNEUROSCI.3192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene. 2004;23:8007–8016. doi: 10.1038/sj.onc.1208158. [DOI] [PubMed] [Google Scholar]
- Levey AI. Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proc Natl Acad Sci USA. 1996;93:1351–13546. doi: 10.1073/pnas.93.24.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DZ, Sharp FR, Van KC, Ander BP, Ghiasvand R, Zhan X, Stamova B, Jickling GC, Lyeth BG. Inhibition of Src family kinases protects hippocampal neurons and improves cognitive function after traumatic brain injury. J Neurotrauma. 2014;31:1268–1276. doi: 10.1089/neu.2013.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Mao L, Wang HH, Wang JQ. Antagonism of muscarinic acetylcholine receptors alters synaptic ERK phosphorylation in the rat forebrain. Neurochem Res. 2017;42:1202–1210. doi: 10.1007/s11064-016-2157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Dopaminergic and cholinergic regulation of Fyn tyrosine kinase phosphorylation in the rat striatum in vivo. Neuropharmacology. 2015;99:491–499. doi: 10.1016/j.neuropharm.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Dopamine D2 receptors are involved in the regulation of Fyn and metabotropic glutamate receptor 5 phosphorylation in the rat striatum in vivo. J Neurosci Res. 2016;94:329–338. doi: 10.1002/jnr.23713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Wang JQ. Antagonism of dopamine D2 receptors alters phosphorylation of Fyn in the rat medial prefrontal cortex. J Mol Neurosci. 2017;61:524–530. doi: 10.1007/s12031-017-0894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M, Fenu S, Cozzolino A, Pinna A, Carta A, Di Chiara G. Blockade of muscarinic receptors potentiates D1 dependent turning behavior and c-fos expression in 6-hydroxydopamine-lesioned rats but does not influence D2 mediated response. Neuroscience. 1993;53:673–678. doi: 10.1016/0306-4522(93)90615-m. [DOI] [PubMed] [Google Scholar]
- Neet K, Hunter T. Vertebrate non-receptor protein-tyrosine kinase families. Genes Cell. 1996;1:147–169. doi: 10.1046/j.1365-2443.1996.d01-234.x. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Murata Y, Okazawa H, Matozaki T. Src family kinases: modulators of neurotransmitter receptor function and behavior. Trends Neurosci. 2011;34:629–637. doi: 10.1016/j.tins.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Okada M. Regulation of the Src family kinase by Csk. Int J Biol Sci. 2012;8:1385–1397. doi: 10.7150/ijbs.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Besnard A, Herve D, Pages C, Heck N, Girault JA, Caboche J, Vanhoutte P. Cyclic adenosine monophosphate-independent tyrosine phosphorylation of NR2B mediates cocaine-induced extracellular signal-regulated kinase activation. Biol Psychiatry. 2011;69:218–227. doi: 10.1016/j.biopsych.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Santiago MP, Potter LT. Biotinylated m4-toxin demonstrates more M4 muscarinic receptor protein on direct than indirect striatal projection neurons. Brain Res. 2001;894:12–20. doi: 10.1016/s0006-8993(00)03170-x. [DOI] [PubMed] [Google Scholar]
- Schenone S, Brullo C, Musumeci F, Biava M, Falchi F, Botta M. Fyn kinase in brain diseases and cancer: the search for inhibitors. Curr Med Chem. 2011;18:2921–2942. doi: 10.2174/092986711796150531. [DOI] [PubMed] [Google Scholar]
- Schmitt JM, Stork PJ. PKA phosphorylation of Src mediates cAMP’s inhibition of cell growth via Rap1. Mol Cell. 2002;9:85–94. doi: 10.1016/s1097-2765(01)00432-4. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Scopolamine augments c-fos and zif/268 messenger RNA expression induced by the full D1 dopamine receptor agonist SKF-82958 in the intact rat striatum. Neuroscience. 1996a;72:601–616. doi: 10.1016/0306-4522(95)00597-8. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Muscarinic receptors regulate striatal neuropeptide gene expression in normal and amphetamine-treated rats. Neuroscience. 1996b;75:43–56. doi: 10.1016/0306-4522(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Intrastriatal injection of a muscarinic receptor agonist and antagonist regulates striatal neuropeptide mRNA expression in normal and amphetamine-treated rats. Brain Res. 1997;748:62–70. doi: 10.1016/s0006-8993(96)01244-9. [DOI] [PubMed] [Google Scholar]
- Watcharasit P, Tucholski J, Jope RS. Src family kinase involvement in muscarinic receptor-induced tyrosine phosphorylation in differentiated SH-SY5Y cells. Neurochem Res. 2001;26:809–816. doi: 10.1023/a:1011612118779. [DOI] [PubMed] [Google Scholar]
- Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- Yang HB, Yang X, Cao J, Li S, Liu YN, Suo ZW, Cui HB, Guo Z, Hu XD. cAMP-dependent protein kinase activated Fyn in spinal dorsal horn to regulate NMDA receptor function during inflammatory pain. J Neurochem. 2011;116:93–104. doi: 10.1111/j.1471-4159.2010.07088.x. [DOI] [PubMed] [Google Scholar]
- Yang K, Trepanier C, Sidhu B, Xie YF, Li H, Lei G, Salter MW, Orser BA, Nakazawa T, Yamamoto T, Jackson MF, MacDonald JF. Metaplasticity gated through differential regulation of GluN2A versus GluN2B receptors by Src family kinases. EMBO J. 2012;31:805–816. doi: 10.1038/emboj.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo MG, Oh HJ, Cho HS, Chun JS, Marcantonio EE, Song WK. Phosphorylation of Ser 21 in Fyn regulates its kinase activity, focal adhesion targeting, and is required for cell migration. J Cell Physiol. 2011;226:236–247. doi: 10.1002/jcp.22335. [DOI] [PubMed] [Google Scholar]