Abstract
Functional meningeal lymphatic system plays a crucial role in outflow of cerebrospinal fluid. Metabolites and neurotoxins in the cerebrospinal fluid may be excreted via this system and accumulate in the cervical lymph nodes. In this letter, we highlighted the role of functional meningeal lymphatics and cerebrospinal fluid outflow.
Keywords: Human immunodeficiency virus -1 (HIV-1), Simian immunodeficiency virus (SIV), Follicular Dendritic Cells (FDCs), T Follicular Helper Cells (TFH), Cervical Lymph Nodes (CLNs), Glymphatics, Meningeal Lymphatics
Recent evidence of follicular dendritic cells (FDCs) within cervical lymph node (CLN) trapping the simian immunodeficiency virus (SIV) egressing from the brain (Dave et al., 2017) has continued the dialogue regarding the functional role of glymphatics and meningeal lymphatic system in communication pathways between the central nervous system (CNS) and the immune system via the CLN. In addition, virus trapped within the CLN FDC network remained infectious and transmissible to T follicular helper (TFH) cells. Together, these observations have implications for viral egress from the CNS to the CLNs. More importantly, these observations further strengthen the potential role of the glymphatic and the meningeal lymphatic system in cerebrospinal fluid (CSF) drainage and clearance of metabolites and virus through the cribiform plate and other passageways.
Of late, the functional meningeal lymphatic vessel system has garnered much attention (Andres et al., 1987; Yang et al., 2013; Mohammad et al., 2014; Aspelund et al., 2015; Iliff et al., 2015; Louveau et al., 2015a; Louveau et al., 2015b; Engelhardt et al., 2016; Ma et al., 2017). This system was first identified in the dura mater of rats (Andres et al., 1987). However, functional meningeal lymphatic vessels were identified recently in a study utilizing a mice model system and human dura tissues (Louveau et al., 2015b). This study altered the preexisting paradigm for the route of CSF drainage to dural venous sinuses through the arachnoid projections from the subarachnoid space. While it was known that there was a lymphatic component of CSF drainage; however, the study by Louveau and coworkers demonstrated that meningeal lymphatic system is a major pathway for clearance of molecular entities present in the CNS (Louveau et al., 2015b). In that study, authors inferred that the meningeal lymphatics were part of the CNS. Not surprisingly, many researchers have challenged their inference based on the premise that lymphatic vessels are a component of the surrounding connective tissue and not included within the CNS. In a similar study, Aspelund and coworkers identified lymphatic vessels adjacent to the superior sagittal sinus (Aspelund et al., 2015). They concluded that the lymphatic vessels might absorb CSF from the adjacent subarachnoid space and brain interstitial fluid via the glymphatics (Aspelund et al., 2015). Regardless of the discussion whether the functional meningeal lymphatic system is part of the CNS or not, in juxtaposition with the glymphatics, both systems appear to contribute to CSF drainage and clearance of molecular entities and CNS generated neurotoxic wastes (Figure 1).
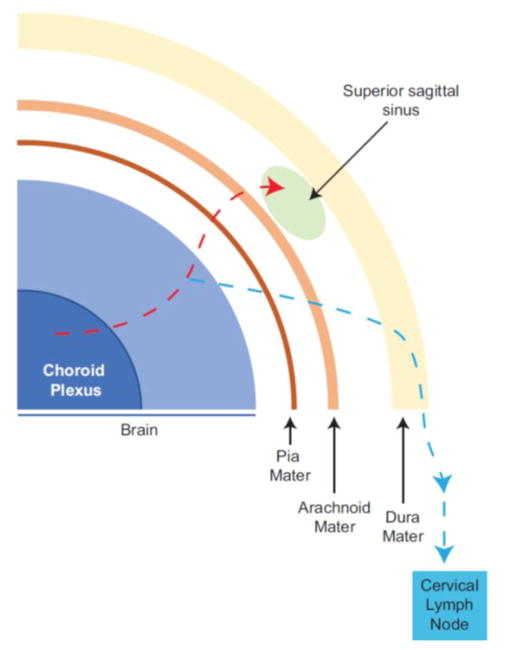
Figure 1. Schematic representation of cerebrospinal fluid pathway via the meningeal lymphatics.
Cerebrospinal fluid outflow represents an excretory system for the central nervous system. The pathway of CSF drainage and resorption involved transmission through the pia mater, into the sub-arachnoid spaces and eventual drainage via arachnoid projections into the dural sinuses (Red dash line). The identification of meningeal lymphatics (Blue dash line) in the dura mater demonstrated that these vessels are not part of the blood vasculature and associate with superior sagittal and transverse sinuses. These lymphatic vessels transport solutes to the deep cervical lymph nodes (Blue dash line).
The underlying processes and pathways of CSF drainage and metabolite clearance require further elucidation. Indeed, new insights into the molecular transport in the CSF along the glymphatics and meningeal lymphatics pathways to the CLNs were recently described using a lymphatic reporter mouse model (Ma et al., 2017). In that model, the authors utilized near infrared (NIR) tracers with high-resolution stereomicroscopy. The NIR tracers were PEGylated and ideally suited for characterizing the lymphatic system as they are not directly taken up by blood vessels nor do they adhere to tissue components or get phagocytosed by macrophages. One such tracer P40D680 accumulated within the CLNs 10 minutes after the infusion (Ma et al., 2017). The P40D680 tracer continued to accumulate with peak accumulation at 60 minutes post infusion. In addition, there was slower accumulation in the mandibular lymph nodes. Furthermore, they observed reduced CSF drainage in aging mice, which has potential implications for neurodegenerative diseases such as multiple sclerosis and Alzheimer’s disease. These studies offer support for trafficking of small molecular entities such as PD40D680 (molecular weight 40KDa) along the meningeal lymphatics to the CLNs. The accumulation of SIV in the CLNs suggests that much larger molecular entities may also be trafficked along the meningeal lymphatics (Dave et al., 2017).
Increased understanding of the functional meningeal lymphatics system has necessitated addressing the issue of immune privilege within the CNS. In the pre-existing paradigm of CNS immune privilege, key immune system components are excluded from the CNS. Contrary to this belief, identification of CD3e T cells and MHC II expressing immune cells within the meningeal lymphatic vessels suggests that there is constant immune surveillance within the meningeal compartment (Mohammad et al., 2014; Louveau et al., 2015a; Engelhardt et al., 2016). The impact of CSF drainage and immune surveillance by the functional meningeal lymphatic system on neurodegenerative diseases and viral infections in the CNS needs to be further investigated. More importantly, it is crucial to carefully extend these studies to humans and non-human primates. Our observations of SIV accumulation in FDCs of CLNs and transmission of the infected virus to TFH cells suggest that there is an additional level of complexity that needs to be unraveled to understand HIV/SIV infection of the CNS and the unique CLN viral reservoir associated with it. Expanding these investigations to other neurodegenerative diseases besides HIV/SIV infection will provide greater clarity in this developing area of research.
Acknowledgments
We thank Dr. Gendelman for critical reading and Robin Taylor for editorial assistance. This work is supported in part by National Institutes of Health grants R21MH11355 and P30MH062261 to SNB.
Footnotes
Conflict of Interest: Authors declared no conflicting of interests exist
References
- Andres KH, von During M, Muszynski K, Schmidt RF. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl) 1987;175:289–301. doi: 10.1007/BF00309843. [DOI] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave RS, Sharma RK, Muir RR, Haddad E, Gumber S, Villinger F, Nehra AP, Khan ZK, Wigdahl B, Ansari AA, Byrareddy SN, Jain P. FDC:TFH Interactions within Cervical Lymph Nodes of SIV-Infected Rhesus Macaques. J Neuroimmune Pharmacol. 2017 doi: 10.1007/s11481-017-9775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Carare RO, Bechmann I, Flugel A, Laman JD, Weller RO. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 2016;132:317–338. doi: 10.1007/s00401-016-1606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Goldman SA, Nedergaard M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015;14:977–979. doi: 10.1016/S1474-4422(15)00221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Harris TH, Kipnis J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015a;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015b;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Ineichen BV, Detmar M, Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun. 2017;8:1434. doi: 10.1038/s41467-017-01484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad MG, Tsai VW, Ruitenberg MJ, Hassanpour M, Li H, Hart PH, Breit SN, Sawchenko PE, Brown DA. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clin Invest. 2014;124:1228–1241. doi: 10.1172/JCI71544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, Benveniste H, Iliff JJ, Nedergaard M. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med. 2013;11:107. doi: 10.1186/1479-5876-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]