Abstract
Object
The purpose of the current study was to quantify the reproducibility, temporal stability and functional correlation of diffusion MR characteristics in the spinal cord in cervical stenosis patients with or without myelopathy. The association between longitudinal DTI measurements and serial neurological function assessment was explored at both the group and individual level.
Methods
Sixty-six nonoperatively treated patients with cervical stenosis were prospectively followed (3 months to 5 years) using synchronous serial MRI and functional outcome assessment. A total of 183 separate MRI examinations were performed, separated by at least 3 months, and each patient had a minimum of two MRI scans (range 2–5 scans). Anatomic and diffusion tensor imaging were performed within the spinal cord at the C1–2 region as well as area of highest compression. Coefficient of variance (COV) were compared across measurements in both reference tissue and areas of compression for anatomic measurements, fractional anisotropy (FA), and mean diffusivity (MD). The correlation between diffusion MR measures at the site of compression and evaluations of neurological function assessed using the modified Japanese orthopedic scale (mJOA) at multiple time points were evaluated.
Results
COV for anatomic measurements (Torg ratio and canal diameter) were between 7–10%. Median COV for FA measurements at the site of compression was 9% and reference tissue at C1–2 was 6%. Median COV for MD at the site of compression was approximately 12% and reference tissue at C1–2 was 10%. FA and MD measurements of C1–2 averaged 0.62 and 0.91 um2/ms, respectively, whereas FA and MD measurements at the site of compression averaged 0.51 and 1.26 um2/ms, respectively. Both FA (Slope = 0.037; R2 = 0.3281, P < 0.0001) and MD (Slope = −0.074; R2 = 0.1101, P = 0.0084) were significantly correlated with mJOA score. FA decreased by approximately 0.032 units per mJOA unit decrease (R2 = 0.1345, P < 0.0001), while MD was increased by approximately 0.084 um2/ms for every mJOA unit decrease (R2 = 0.1345, P < 0.0001).
Conclusion
Quantitative DTI measurements of the spinal cord in cervical stenosis patients with or without myelopathy have a median COV of 5–10%, similar to anatomic measurements. The reproducibility of these measurements and significant correlation with functional outcome status suggest a potential role in the evaluation and longitudinal surveillance of nonoperatively treated patients. With respect to the specific DTI measurements, FA within the spinal cord appears slightly more sensitive to neurological function and more stable than measures of MD. Therefore, DTI of the spinal cord may be a clinically feasible imaging technique for longitudinally monitoring patients with CSM.
Keywords: Diffusion tensor imaging, DTI, spinal cord, cervical spondylotic myelopathy, biomarker, CSM, stenosis
INTRODUCTION
Cervical spondylosis results from degeneration of intervertebral discs and supporting structures, which collapse and dehydrate during normal aging 2, increasing mechanical stress at cartilaginous end plates at the edge of the vertebral bodies 3,15,25,32. Over time, this repeated stress results in subperiosteal bone formation and/or osteophyte formation. Symptoms then arise during repeated spinal cord or nerve root compression 2,5,8 and manifest as neck pain syndromes, myelopathy, or radiculopathy 1,5,11.
Cervical spondylosis is diagnosed using anatomic MRI and is ubiquitous in the elderly. Myelopathy resulting from spondylosis is the most common cause of spinal cord dysfunction in the elderly 47; however, accurate estimation of spinal cord dysfunction using standard MRI remains a significant challenge, as common anatomic features 4,17 including MR signal change 16,28–31,33,34,37 and the degree of spinal cord compression 36,42,48 have not demonstrated a reliably strong and/or consistent association with neurological function. Decompression surgery is commonly performed in patients with moderate and severe myleopathy, whereas patients with asymptomatic stenosis or mild symptomatology may be treated nonoperatively and observed without continual progression 8,26,27,35 in many cases. Due to the aforementioned limitations of conventional MRI, there is significant interest in the development of non-invasive imaging biomarkers to quantify neurological function of the spinal cord in both operatively and non-operatively treated patients with advanced cervical spondylosis.
Diffusion tensor imaging (DTI), an advanced MR technique sensitive to the underlying microstructural organization of tissues 41, has previously shown to be useful for predicting neurological function in patients with cervical spondylosis 6,10,21,23,24,44,45 In particular, studies have shown that lowered diffusion fractional anisotropy (FA) at the site of compression is associated with increased neurological dysfunction, suggesting disruption in the directional coherence of nerve fibers in the spinal cord, and higher mean water diffusivity (MD) at the site of compression is associated with increasing neurological impairment.
While most DTI studies in patients with spondylosis have been cross-sectional, examining the relationship between DTI and functional status at a single time point, the variability and longitudinal stability of DTI measurements in the spinal cord in advanced cervical spondylosis has not been previously reported. The current study quantified the variation in DTI measurements by examining repeated MRI investigations in a cohort of asymptomatic cervical stenosis and CSM patients that were treated nonoperatively and prospectively monitored. The association between longitudinal DTI measurements and serial neurological function assessment was explored at both the group and individual level.
MATERIALS AND METHODS
Patient Population
A prospective observational study was performed in 66 nonoperatively treated cervical stenosis patients with or without myelopathy that were initially referred for surgical evaluation. Each patient had two or more DTI and conventional MRI scans, and a total of 183 separate MRI scans were performed across the entire cohort (mean 2.7 scans per patient, range 2 to 5 separate scans). The total surveillance time ranged from 3 months to 5½ years (average follow-up duration per patient = 520 days). The patients underwent synchronous MRI and neurological assessment at the same uniform time points: baseline, 3 months, 6 months, 1 year, 2 year, 3 year, and 5 years following their initial evaluation. Thirty-seven of the patients were male and 29 were female, and the average age was 64 years old (range 24 to 94).
The degree of neurological impairment was measured by the modified Japanese Orthopaedic Association (mJOA) 46 scale at the same time as each MRI scan. Forty-eight patients had some evidence of neurological symptomatology, as indicated by mJOA < 18, and eighteen patients had no neurological symptomatology but complaints of neck pain. The average mJOA score was 16.6 with a range from 11 to 18. All participants gave informed written consent to be part of this study. Surgical management was discussed with any patient whose mJOA score declined by two points or greater during the observation period. However, none of these patients opted for surgical intervention. All procedures complied with the principles of the Declaration of Helsinki and were approved by the Institutional Review Board within the Office for the Protection of Research Subjects at the University of California.
Conventional Magnetic Resonance Imaging
Standard MRI was obtained on a 3T MR scanner (3T Trio or Prisma; Siemens Healthcare, Erlangen, Germany) using a standard spine coil array for radiofrequency reception. Routine clinical MRI scans consisted of T1-weighted and T2-weighted sequences in the sagittal plane and T2-weighted images in the axial orientation. All patients had radiographic evidence of at least moderate cervical stenosis, including spinal canal narrowing related to advanced cervical spondylosis manifested by a combination of facet arthropathy, ligamentum flavum hypertrophy, and varying degrees of ventral disc-osteophyte compression. An MRI version of the Torg-Pavlov ratio36 (ratio of anterior-posterior diameter to thickness of the vertebral body) was documented and used for subsequent comparisons with diffusion measurements.
Diffusion Tensor Imaging (DTI)
Axial diffusion-weighted images were collected through the level of most significant canal narrowing. Excitation consisted of a custom two-dimensional, spatially selective radiofrequency excitation pulse (2D-RF) and a reduced FOV EPI readout with ramp sampling (Zoomed-EPI). The echo time (TE)/repetition time (TR) was set to 73–100ms/3000–10000ms, matrix size = 48×128; field-of-view (FOV) = 53 mm × 140 mm, slice thickness = 4–5mm with no gap, number of averages = 4–10, and 12–20 diffusion sensitizing directions with b=500 s/mm2 and 1–2 b = 0 s/mm2 images. After acquisition of DWIs, eddy-current and motion correction was performed using a 12-degree of freedom affine transformation using FSL (FMRIB; Oxford, UK; http://www.fmrib.ox.ac.uk/fsl/). Mean diffusivity (MD), or average apparent diffusion coefficient relating to the mean water motility, as well as fractional anisotropy (FA), the degree of diffusion anisotropy, were calculated from the resulting diffusion MR data.
Regions of Interest
Manual segmentation of the spinal cord was performed for the whole cord at each axial image slice location using the T2-weighted anatomical images. DTI measurements at the site of highest compression, including voxels in areas of T2 hyperintensity, were used for comparison. DTI measurements of the cord at the C1–2 spinal levels were also made for a reference.
Statistical Analysis
Coefficient of variance (COV) was calculated by estimating the ratio of the standard deviation of mean FA and MD in the spinal cord over time divided by the average of the mean FA and MD measurements over the same time period (COV = σ/μ). Temporal trends in both average and percentage change in FA and MD were displayed for each individual patient. Lastly, the linear relationship between DTI measurements and mJOA were determined using Pearson’s correlation coefficient and multiple measurements per patient. For these associations, statistical significance was determined by testing whether the slope of the linear trend line was significantly different from zero using an F-test. All statistical analyses were performed using GraphPad Prism v6.0d (GraphPad Software, Inc., La Jolla, CA) and Matlab (R2011b; Mathworks, Inc., Natick, MA).
RESULTS
Coefficient of Variance (COV) in Repeated Anatomic and DTI Measures of the Spine
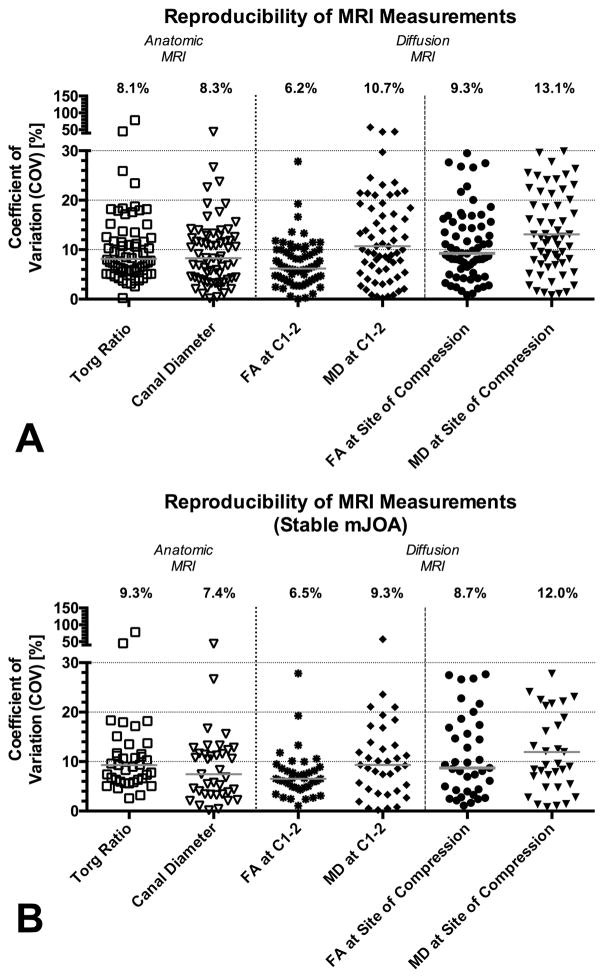
Fig 1 illustrates example images from a neurologically stable patient with cervical stenosis, demonstrating the relative consistency in DTI measurements over time. The median mJOA score for the patient cohort was 17, the median FA at the lesion site was 0.50, the median MD at the lesion site was 1.25 um2/ms, and the median MRI-equivalent Torg ratio was 0.36. Median COV was significantly different across anatomic and DTI measurements (Friedman, P < 0.0001), indicating COV in repeated DTI measurements were slightly higher than anatomical measurements of the spinal column and there was substantial variability when examining repeated MD measurements at the site of compression. More precisely, the median COV for anatomical measurements of MRI-equivalent Torg ratio was 8.1% and the median COV for measurements of canal diameter was 8.3% (Fig 2A; Table 1), whereas the median COV of FA and MD measurements at the site of compression were 9.3% and 13.1%, respectively. Dunn’s test for multiple comparisons indicated that both canal diameter (Adjusted P = 0.0012) and Torg ratio (Adjusted P = 0.0073) had a significantly lower median COV compared with median COV of MD at the site of compression, but no difference in COV was detected between canal diameter or Torg ratio when compared with FA (Adjusted P > 0.05). For reference, median COV of FA and MD measurements in reference tissue at spinal level C1–2 were 6.2% and 10.7%, respectively, and median COV for FA was significantly lower than MD in these reference tissues (Adjusted P = 0.0121). Additionally, there was a significant difference in median FOV between FA measurements at C1–2 (lowest COV) and MD at the site of compression (highest COV) (Adjusted P < 0.0001). When examining COV in patients with no change in mJOA, trends were similar (Fig 2B; Table 2; P = 0.5855 comparing median COV for all patients vs. median COV for subset with constant mJOA).
Fig. 1. Serial longitudinal anatomic and DTI measurements in a 77-year-old female patient with spinal cord compression at C3–4 and subtle T2 signal change, but no neurological impairment (mJOA = 18).
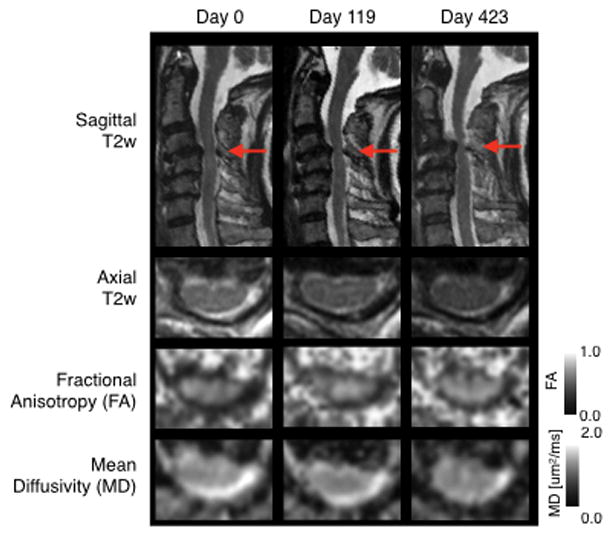
Red arrows show site of most significant cervical stenosis and area of evaluation.
Fig. 2. Coefficient of variance (COV) in repeated anatomic and DTI measurements of the spinal cord in patients with cervical spondylosis.
A) COV in all patients (N = 66). B) COV in only patients with no change in mJOA (N = 37).
Table 1.
Coefficient of Variance for Anatomic and Diffusion MRI Measurements in All patients (N = 66).
| Canal Diameter | Torg Ratio | FA at C1–2 [%] | MD at C1–2 [%] | FA at Site of Compression [%] | MD at Site of Compression [%] | |
|---|---|---|---|---|---|---|
| Minimum | 0.1 | 0.2 | 0.0 | 0.2 | 0.8 | 0.8 |
| 25% Percentile | 4.2 | 5.6 | 4.0 | 5.6 | 5.9 | 7.8 |
| Median | 8.3 | 8.1 | 6.2 | 10.7 | 9.3 | 13.1 |
| 75% Percentile | 12.8 | 12.8 | 10.0 | 19.8 | 15.6 | 23.4 |
| Maximum | 44.0 | 78.4 | 27.8 | 57.1 | 29.5 | 34.6 |
| Mean | 9.6 | 11.1 | 7.1 | 13.8 | 11.0 | 15.3 |
| Std. Deviation | 7.2 | 10.9 | 4.6 | 11.4 | 7.1 | 9.7 |
| Std. Error of Mean | 0.9 | 1.3 | 0.6 | 1.4 | 0.9 | 1.2 |
| Lower 95% CI of mean | 7.8 | 8.4 | 6.0 | 11.0 | 9.3 | 12.9 |
| Upper 95% CI of mean | 11.4 | 13.8 | 8.3 | 16.6 | 12.8 | 17.7 |
Table 2.
Coefficient of Variance for Anatomic and Diffusion MRI Measurements in Patients with No Change in mJOA (N = 37).
| Canal Diameter | Torg Ratio | FA at C1–2 | MD at C1–2 | FA at Site of Compression | MD at Site of Compression | |
|---|---|---|---|---|---|---|
| Minimum | 0.1 | 2.6 | 1.1 | 1.1 | 0.8 | 0.2 |
| 25% Percentile | 3.4 | 6.1 | 4.1 | 4.7 | 6.2 | 4.2 |
| Median | 7.4 | 9.3 | 8.7 | 6.5 | 12.0 | 9.3 |
| 75% Percentile | 12.0 | 12.6 | 17.1 | 8.8 | 22.3 | 15.4 |
| Maximum | 44.0 | 78.4 | 27.7 | 27.8 | 34.1 | 57.1 |
| Mean | 9.0 | 12.2 | 11.2 | 7.4 | 14.2 | 11.4 |
| Std. Deviation | 8.1 | 13.3 | 8.2 | 4.9 | 10.2 | 10.6 |
| Std. Error of Mean | 1.3 | 2.2 | 1.4 | 0.8 | 1.7 | 1.7 |
| Lower 95% CI of mean | 6.3 | 7.8 | 8.4 | 5.8 | 10.8 | 7.9 |
| Upper 95% CI of mean | 11.7 | 16.7 | 13.9 | 9.0 | 17.6 | 15.0 |
Temporal Patterns in DTI Measurements of the Spinal Cord in Cervical Spondylosis During Long-Term Surveillance
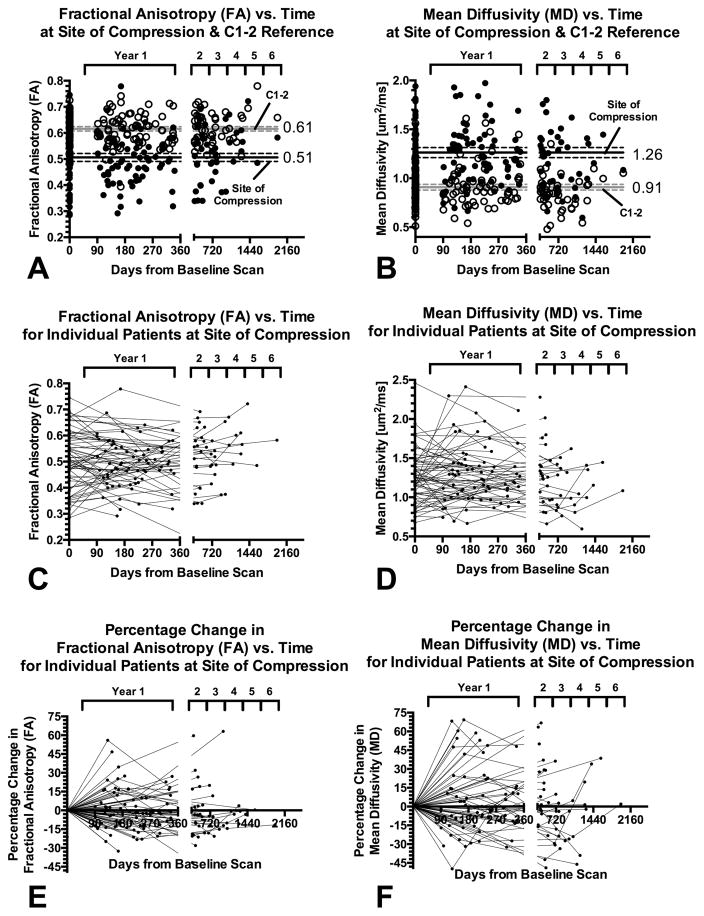
Multiple DTI measurements of reference spinal cord tissue at C1–2 for all patients taken independently resulted in an average FA measurement of 0.61 ± 0.06 (standard deviation, s.d.) and MD measurements of 0.91 ± 0.20 s.d. um2/ms (Fig 3A–B). Grouping all cervical spondylosis patients together and assuming each DTI scan represents an independent measurement, the average FA at the site of compression was 0.50 ± 0.10 s.d. and average MD was 1.26 ± 0.35 s.d. um2/ms, which was significantly lower than the reference tissues (Paired t-test, P < 0.0001 for both FA and MD). Both measurements at C1–2 and measurements at the site of compression did not show linear or polynomial trends (P > 0.1), suggesting stability of these measurements over time.
Fig. 3. Long-term temporal DTI measurements and trends in patients with cervical spondylosis.
A) Fractional anisotropy (FA) evaluated over time at the site of compression (solid circles) and C1–2 reference tissue (open circles). B) Mean diffusivity (MD) evaluated over time at the site of compression (solid circles) and C1–2 reference tissue (open circles). C) FA values at the site of compression for individual patients with cervical spondylosis (connected by lines). D) MD values at the site of compression for individual patients with cervical spondylosis (connected by lines). E) Percentage change in FA measurements at the site of compression over the surveillance period, with respect to the first imaging examination time point. F) Percentage change in MD measurements at the site of compression over the surveillance period, with respect to the first imaging examination time point.
Longitudinal DTI measurements for both FA and MD at the site of compression for individual patients during the period of radiographic surveillance are illustrated in Fig 3C–D. The percentage change in DTI measurements for FA and MD from the first evaluation time point are illustrated in Fig 3E–F. Despite some fluctuations in DTI acquisition protocols over the period of evaluation, the majority of the patients (47 of 66) showed relatively steady measurements over time, consistent with their stable neurological status. However, a few patients (13 of 66) did demonstrate fluctuations (greater than 10% COV) in DTI measurements over time. In the some of these cases (7 of 13), significant issues with DTI image quality including severe susceptibility artifacts were observed only at specific time points. In 2 of 13 patients, these artifacts were present in >2 evaluation time points. In the remaining 4 patients, spinal cord motion or other issues may have contributed to the high fluctuations in measurements.
Functional Correlation of DTI Measurements
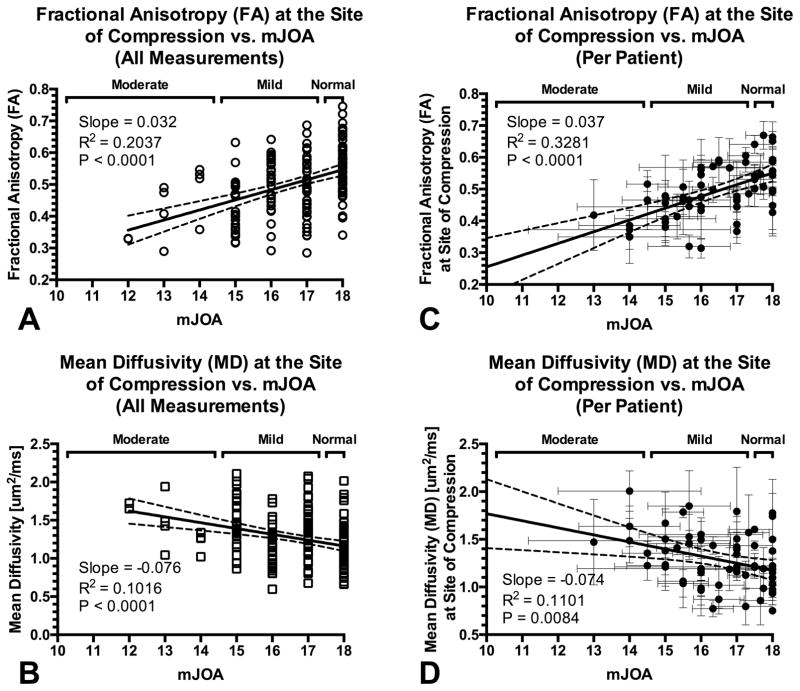
Next, we examined the relationship between DTI measurements and mJOA in order to determine whether FA or MD were valuable predictors of neurological status. Pooling all DTI measurements from all patients and all time points, both FA and MD at the site of compression showed a significant correlation with neurological status (Fig 4A–B). Specifically, FA was shown to decrease approximately 0.032 units per mJOA unit decrease (R2 = 0.2037, P < 0.0001), while MD was shown to increase approximately 0.084 um2/ms for every mJOA unit decrease (R2 = 0.1016, P < 0.0001). When evaluating each patient individually by averaging both their DTI and mJOA over their respective surveillance period, FA (R2 = 0.3281, P < 0.0001) and MD (R2 = 0.1101, P = 0.0084) were correlated with approximately the same sensitivity to mJOA (Fig 4C–D). Together, these results further support the hypothesis that DTI measurements at the site of compression, particularly FA and MD, may be valuable predictors of neurological status.
Fig. 4. Correlation between DTI measurements of the spinal cord at the site of compression and functional impairment assessed with mJOA.
A) Fractional anisotropy (FA) measurements at the site of compression versus mJOA using all available measurements from all patients (R2 = 0.2037, P < 0.0001). B) Mean diffusivity (MD) measurements at the site of compression versus mJOA using all available measurements (R2 = 0.1016, P < 0.0001). C) Association between FA measurements at the site of compression and mJOA evaluated per patient, averaging multiple DTI and neurological assessment exams (R2 = 0.3281, P < 0.0001). D) Association between MD at the site of compression and mJOA evaluated per patient by averaging multiple DTI and neurological exams (R2 = 0.1101, P = 0.0084).
DISCUSSION
Degenerative changes within the cervical spine are a normal part of aging and are commonly encountered in middle aged and older patients. Because of this ubiquity among older patients, one of the greatest challenges associated with the clinical management of patients with advanced cervical spondylosis is determining when to intervene, either surgically or therapeutically, as many changes within the spinal cord may be relatively benign and not result in permanent neurological damage. Additionally, patients may have similar appearing conventional MRI features such as degree of stenosis and spinal cord signal change, yet have distinctly different degrees of functional impairment. Moreover, since asymptomatic and mildly symptomatic cervical myelopathy patients are frequently treated nonoperatively, a tool for obtaining objective measurements of spinal cord integrity would be highly beneficial for long-term surveillance and clinical management of this patient in population.
While conventional MRI is the current standard for radiographic assessment of the spinal cord in patients with cervical spondylosis and myelopathy, measures including spinal cord compression36,42,48, spinal canal diameter, and even presence of spinal cord signal change 16,28–31,33,34,37 have failed to show a consistent significant association with neurological impairment in these patients. To overcome these limitations, studies have explored the use of advanced imaging techniques such as DTI 6,10,21,23,28,44,9,19,20,38 to inspect microstructural characteristics and evaluate the degree of spinal cord injury and functional impairment. Despite promising initial studies, questions remain regarding the reproducibility and reliability of using DTI as a tool for longitudinal surveillance of the spinal cord in patients with spinal cord disease.
To this concern, the current study sought to investigate the reproducibility, temporal trends, and functional correlates of DTI measurements within the cervical spinal cord in a large cohort of patients with asymptomatic cervical stenosis or cervical myelopathy over a surveillance period that ranged from 3 months to more than 5 years. We report a COV for repeated measurements in patients with cervical spondylosis of between 5–10%, similar to variability in simple anatomic measurements and similar to variability in DTI parameters reported in the literature. A previous study by our team showed COV of multiple DTI measurements of MD and FA within reference tissues in 15 patients evaluated over approximately 200 days of approximately 8.7% and 4.6%, respectively 12, which is slightly lower than the 10.7% and 6.2% COV reported in in the current study.
The average FA and MD measurements reported in reference C1–2 tissues within the current study are consistent with those previously reported in the normal cervical spinal cord. For example, we report average FA and MD measurements of 0.61 and 0.91 um2/ms, respectively, whereas Mamata et al. 28 reported values of 0.70 and 0.81 um2/ms, Facon et al. 14 reported values of approximately 0.75 and 1.0 um2/ms, and Budzik et al. reported values of 0.54 and 0.78 um2/ms. Additionally, Jones et al. 23 reported FA values of the spinal cord at C2–3 of approximately 0.64, which is also consistent with our reported values at C1–2.
In general, we observed an increased MD and decreased FA at the site of compression compared with reference normal values. This is consistent with previous observations in patients with cervical spondylosis 6,10,12,14,22,23,28. Additionally, we observed a significant correlation between degree of neurological impairment assessed using the mJOA and DTI measurements including FA and MD, which is also consistent with previous clinical studies 13,23. Together, these results support the hypothesis that DTI measurements at the site of compression may be valuable for estimating the degree of neurological impairment in patients with cervical spondylosis.
The present investigation is novel and clinically significant for several reasons. First, having performed 183 separate MRI scans in a cohort of 66 patients, this is the first and largest study evaluating temporal stability and reproducibility of DTI in patients with advanced cervical spondylosis. This helps to address a gap in our knowledge base regarding the stability and efficacy of repeated DTI measurements over time, as the vast majority of studies have been focused on single time-point assessments. Second, we were able to determine the expected change in mJOA based on changes in FA and MD. FA was shown to decrease by approximately 0.032 units and MD was demonstrated to increase approximately 0.084 um2/ms for every point decrease in mJOA score. Lastly, when examining each individual patient by averaging their DTI and mJOA over their respective surveillance period, FA and MD were strongly correlated with sensitivity to mJOA. Taken together, these three key findings suggest that DTI could potentially be utilized as a method to longitudinally assay spinal cord integrity in patients with asymptomatic cervical stenosis and CSM that are being treated nonoperatively. Additional future study with a larger cohort of patients and longer follow-up time will be needed.
Study Limitations
Although DTI can provide new information regarding the health of the spinal cord there remains limitations to widespread use due to technical challenges and issues with consistency in slice prescriptions between evaluation time points. Many of the technical challenges relate to the small size and movement of the cord, namely inaccuracies arising from partial volume and susceptibility-related artifacts. Additionally, general issues with consistency and accuracy of slice prescription for even the anatomic evaluation of stenosis is well-documented 18,40 and thus adequate attention should be given to align slices across exams in the same patient.
It is also important to note that technological advances in MR system hardware and software evolved over the surveillance period in the current study, resulting in slight variability in the acquisition protocols over time. Thus, our measurements of variability and temporal stability documented in the present study reflect the realistic variations in DTI measurements expected during routine clinical use of DTI for evaluation of the spinal cord using clinically available, state-of-the-art acquisition protocols. The current version of our acquisition protocol used for the last 3 years of examinations, consisting of 30 diffusion sensitizing directions and 4 averages for b=0 s/mm2 images, has tolerable accuracy in DTI parameter estimation 7,43 and is consistent with current consensus recommendations from the scientific community 39.
CONCLUSION
Quantitative DTI measurements of the spinal cord in patients with advanced cervical spondylosis have a median COV of 5–10%, which is similar to anatomic measurements at the site of compression. Mean measurements of FA and MD in normal reference tissues as well as the increase in MD and decrease in FA in patients with increasing neurological impairment are consistent with previous studies. Results also suggest FA within the cord is slightly more sensitive to neurological function and more stable than measures of MD. The reproducibility of these measurements and significant correlation with functional outcome status suggest a potential role in the evaluation and longitudinal surveillance of nonoperatively treated patients.
References
- 1.Arnold JG., Jr The clinical manifestations of spondylochondrosis (spondylosis) of the cervical spine. Ann Surg. 1955;141:872–889. doi: 10.1097/00000658-195514160-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey P, Casa Major L. Osteoarthritis of the spine as a cause of compression of the spinal cord and its roots. J Nerv Ment Dis. 1911;38:588–609. [Google Scholar]
- 3.Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6:190S–197S. doi: 10.1016/j.spinee.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:1178–1184. [PubMed] [Google Scholar]
- 5.Brain WR, Northfield D, Wilkinson M. The neurological manifestations of cervical spondylosis. Brain. 1952;75:187–225. doi: 10.1093/brain/75.2.187. [DOI] [PubMed] [Google Scholar]
- 6.Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol. 2011;21:426–433. doi: 10.1007/s00330-010-1927-z. [DOI] [PubMed] [Google Scholar]
- 7.By S, Smith AK, Dethrage LM, Lyttle BD, Landman BA, Creasy JL, et al. Quantifying the impact of underlying measurement error on cervical spinal cord diffusion tensor imaging at 3T. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke E, Robinson PK. Cervical myelopathy: a complication of cervical spondylosis. Brain. 1956;79:483–510. doi: 10.1093/brain/79.3.483. [DOI] [PubMed] [Google Scholar]
- 9.Cooke FJ, Blamire AM, Manners DN, Styles P, Rajagopalan B. Quantitative proton magnetic resonance spectroscopy of the cervical spinal cord. Magn Reson Med. 2004;51:1122–1128. doi: 10.1002/mrm.20084. [DOI] [PubMed] [Google Scholar]
- 10.Demir A, Ries M, Moonen CT, Vital JM, Dehais J, Arne P, et al. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology. 2003;229:37–43. doi: 10.1148/radiol.2291020658. [DOI] [PubMed] [Google Scholar]
- 11.Ellenberg MR, Honet JC, Treanor WJ. Cervical radiculopathy. Arch Phys Med Rehabil. 1994;75:342–352. doi: 10.1016/0003-9993(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 12.Ellingson BM, Salamon N, Grinstead JW, Holly LT. Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy. Spine J. 2014;14:2589–2597. doi: 10.1016/j.spinee.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellingson BM, Salamon N, Hardy AJ, Holly LT. Prediction of Neurological Impairment in Cervical Spondylotic Myelopathy using a Combination of Diffusion MRI and Proton MR Spectroscopy. PLoS One. 2015;10:e0139451. doi: 10.1371/journal.pone.0139451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005;26:1587–1594. [PMC free article] [PubMed] [Google Scholar]
- 15.Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine (Phila Pa 1976) 1998;23:2730–2737. doi: 10.1097/00007632-199812150-00012. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez de Rota JJ, Meschian S, Fernandez de Rota A, Urbano V, Baron M. Cervical spondylotic myelopathy due to chronic compression: the role of signal intensity changes in magnetic resonance images. J Neurosurg Spine. 2007;6:17–22. doi: 10.3171/spi.2007.6.1.4. [DOI] [PubMed] [Google Scholar]
- 17.Friedenberg ZB, Miller WT. Degenerative Disc Disease of the Cervical Spine. J Bone Joint Surg Am. 1963;45:1171–1178. [PubMed] [Google Scholar]
- 18.Henderson L, Kulik G, Richarme D, Theumann N, Schizas C. Is spinal stenosis assessment dependent on slice orientation? A magnetic resonance imaging study. Eur Spine J. 2012;21(Suppl 6):S760–764. doi: 10.1007/s00586-011-1857-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holly LT, Ellingson BM, Salamon N. Metabolic imaging using proton magnetic spectroscopy as a predictor of outcome following surgery for cervical spondylotic myelopathy. J Spinal Disord Tech. 2015 doi: 10.1097/BSD.0000000000000248. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holly LT, Freitas B, McArthur DL, Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;10:194–200. doi: 10.3171/2008.12.SPINE08367. [DOI] [PubMed] [Google Scholar]
- 21.Hori M, Fukunaga I, Masutani Y, Nakanishi A, Shimoji K, Kamagata K, et al. New diffusion metrics for spondylotic myelopathy at an early clinical stage. Eur Radiol. 2012;22:1797–1802. doi: 10.1007/s00330-012-2410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hori M, Okubo T, Aoki S, Kumagai H, Araki T. Line scan diffusion tensor MRI at low magnetic field strength: feasibility study of cervical spondylotic myelopathy in an early clinical stage. J Magn Reson Imaging. 2006;23:183–188. doi: 10.1002/jmri.20488. [DOI] [PubMed] [Google Scholar]
- 23.Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M. Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol. 2013;34:471–478. doi: 10.3174/ajnr.A3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kara B, Celik A, Karadereler S, Ulusoy L, Ganiyusufoglu K, Onat L, et al. The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology. 2011;53:609–616. doi: 10.1007/s00234-011-0844-4. [DOI] [PubMed] [Google Scholar]
- 25.Karadimas SK, Erwin WM, Ely CG, Dettori JR, Fehlings MG. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2013;38:S21–36. doi: 10.1097/BRS.0b013e3182a7f2c3. [DOI] [PubMed] [Google Scholar]
- 26.LaRocca H. Cervical spondylotic myelopathy: natural history. Spine (Phila Pa 1976) 1988;13:854–855. doi: 10.1097/00007632-198807000-00028. [DOI] [PubMed] [Google Scholar]
- 27.Lees F, Turner JW. Natural History and Prognosis of Cervical Spondylosis. Br Med J. 1963;2:1607–1610. doi: 10.1136/bmj.2.5373.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging. 2005;22:38–43. doi: 10.1002/jmri.20357. [DOI] [PubMed] [Google Scholar]
- 29.Mastronardi L, Elsawaf A, Roperto R, Bozzao A, Caroli M, Ferrante M, et al. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J Neurosurg Spine. 2007;7:615–622. doi: 10.3171/SPI-07/12/615. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda Y, Miyazaki K, Tada K, Yasuda A, Nakayama T, Murakami H, et al. Increased MR signal intensity due to cervical myelopathy. Analysis of 29 surgical cases. J Neurosurg. 1991;74:887–892. doi: 10.3171/jns.1991.74.6.0887. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto M, Toyama Y, Ishikawa M, Chiba K, Suzuki N, Fujimura Y. Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy. Does it predict the outcome of conservative treatment? Spine (Phila Pa 1976) 2000;25:677–682. doi: 10.1097/00007632-200003150-00005. [DOI] [PubMed] [Google Scholar]
- 32.McCormick WE, Steinmetz MP, Benzel EC. Cervical spondylotic myelopathy: make the difficult diagnosis, then refer for surgery. Cleve Clin J Med. 2003;70:899–904. doi: 10.3949/ccjm.70.10.899. [DOI] [PubMed] [Google Scholar]
- 33.Mehalic TF, Pezzuti RT, Applebaum BI. Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery. 1990;26:217–226. doi: 10.1097/00006123-199002000-00006. discussion 226–217. [DOI] [PubMed] [Google Scholar]
- 34.Morio Y, Yamamoto K, Kuranobu K, Murata M, Tuda K. Does increased signal intensity of the spinal cord on MR images due to cervical myelopathy predict prognosis? Arch Orthop Trauma Surg. 1994;113:254–259. doi: 10.1007/BF00443813. [DOI] [PubMed] [Google Scholar]
- 35.Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101–108. doi: 10.1093/brain/95.1.101. [DOI] [PubMed] [Google Scholar]
- 36.Pavlov H, Torg JS, Robie B, Jahre C. Cervical spinal stenosis: determination with vertebral body ratio method. Radiology. 1987;164:771–775. doi: 10.1148/radiology.164.3.3615879. [DOI] [PubMed] [Google Scholar]
- 37.Puzzilli F, Mastronardi L, Ruggeri A, Lunardi P. Intramedullary increased MR signal intensity and its relation to clinical features in cervical myelopathy. J Neurosurg Sci. 1999;43:135–139. discussion 139. [PubMed] [Google Scholar]
- 38.Salamon N, Ellingson BM, Nagarajan R, Gebara N, Thomas A, Holly LT. Proton magnetic resonance spectroscopy of human cervical spondylosis at 3T. Spinal Cord. 2013;51:558–563. doi: 10.1038/sc.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samson RS, Levy S, Schneider T, Smith AK, Smith SA, Cohen-Adad J, et al. ZOOM or Non-ZOOM? Assessing Spinal Cord Diffusion Tensor Imaging Protocols for Multi-Centre Studies. PLoS One. 2016;11:e0155557. doi: 10.1371/journal.pone.0155557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schonstrom N. The significance of oblique cuts on CT scans of the spinal canal in terms of anatomic measurements. Spine (Phila Pa 1976) 1988;13:435–436. doi: 10.1097/00007632-198804000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz ED, Cooper ET, Fan Y, Jawad AF, Chin CL, Nissanov J, et al. MRI diffusion coefficients in spinal cord correlate with axon morphometry. Neuroreport. 2005;16:73–76. doi: 10.1097/00001756-200501190-00017. [DOI] [PubMed] [Google Scholar]
- 42.Suk KS, Kim KT, Lee JH, Lee SH, Kim JS, Kim JY. Reevaluation of the Pavlov ratio in patients with cervical myelopathy. Clin Orthop Surg. 2009;1:6–10. doi: 10.4055/cios.2009.1.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu TW, Kim JH, Wang J, Song SK. Full tensor diffusion imaging is not required to assess the white-matter integrity in mouse contusion spinal cord injury. J Neurotrauma. 2010;27:253–262. doi: 10.1089/neu.2009.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen CY, Cui JL, Liu HS, Mak KC, Cheung WY, Luk KD, et al. Is diffusion anisotropy a biomarker for disease severity and surgical prognosis of cervical spondylotic myelopathy? Radiology. 2014;270:197–204. doi: 10.1148/radiol.13121885. [DOI] [PubMed] [Google Scholar]
- 45.Wen CY, Cui JL, Mak KC, Luk KD, Hu Y. Diffusion tensor imaging of somatosensory tract in cervical spondylotic myelopathy and its link with electrophysiological evaluation. Spine J. 2014;14:1493–1500. doi: 10.1016/j.spinee.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 46.Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976) 2001;26:1890–1894. doi: 10.1097/00007632-200109010-00014. discussion 1895. [DOI] [PubMed] [Google Scholar]
- 47.Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am Fam Physician. 2000;62:1064–1070. 1073. [PubMed] [Google Scholar]
- 48.Yue WM, Tan SB, Tan MH, Koh DC, Tan CT. The Torg--Pavlov ratio in cervical spondylotic myelopathy: a comparative study between patients with cervical spondylotic myelopathy and a nonspondylotic, nonmyelopathic population. Spine (Phila Pa 1976) 2001;26:1760–1764. doi: 10.1097/00007632-200108150-00006. [DOI] [PubMed] [Google Scholar]