Abstract
Short bowel syndrome occurs following the loss of a large portion of functional intestine and is associated with high morbidity and mortality. The intestine exhibits pronounced diurnal rhythms in glucose absorption and mounts a profound proliferative response following massive small bowel resection. Understanding the molecular pathways that underpin this could yield novel treatment options.
Two in vivo models were employed using the nocturnally active Sprague Dawley® rat, namely daytime feeding and massive small bowel resection. Glucose absorption exhibited a 24-hour periodicity in the gut and peaked during maximal nutrient delivery, mediated by rhythms in the glucose transporter sodium glucose co-transporter 1 (SGLT1). Feeding during the day shifted the peak in the circadian clock gene PER1 and SGLT1. RNA interference and luciferase assays demonstrated that PER1 transcriptionally regulates SGLT1, linking for the first time clock genes and intestinal glucose absorption.
Intestinal proliferation also exhibited diurnal rhythmicity, with peak absorptive surface area occurring during maximal nutrient availability. mir-16 is diurnally expressed in intestinal crypts, exhibiting minimal expression during maximal nutritional availability. mir-16 overexpression increased apoptosis and arrested proliferation in vitro. mir-125a was upregulated in intestinal crypts following 80% small bowel resection, and induced apoptosis and growth arrest upon overexpression in vitro.
This work provides novel insights into the role of circadian clock genes, intestinal transporters and microRNAs in regulating intestinal absorption and proliferation and is the first demonstration of a role for microRNAs in these adaptive phenomena. Modulation of these pathways may represent a new therapeutic option for the management of short bowel syndrome.
Keywords: Clock genes, Sodium glucose cotransporter 1, SGLT1, Diurnal, Intestinal adaptation, MicroRNA
Short bowel syndrome and the unmet clinical need
Despite many decades of research, short bowel syndrome remains a condition associated with high morbidity and mortality.1 This can occur following massive intestinal resection (often due to acute ischaemia in adults and necrotising enterocolitis in infants) or due to loss of functional intestinal length from stricturing or fistulation in pathologies such as Crohn’s disease. While the intestine is able to adapt to some extent to compensate for this, the degree of adaptation is often insufficient and the resulting malabsorption of water and nutrients by the remnant bowel necessitates dependence on total parenteral nutrition to meet nutritional requirements.2 Total parenteral nutrition is itself associated with adverse effects such as line sepsis and impairment of liver function,3 while other alternatives such as intestinal transplantation are associated with high morbidity and mortality,4 making current therapeutic options suboptimal. This highlights the need for novel approaches and new research strategies in the quest to improve the intestinal adaptive response. This article highlights our contribution to this search and outlines our findings pursuing the investigation of the role of molecular pathways that could be modulated to augment intestinal adaptation.
Diurnal rhythmicity – a unique model of intestinal adaptation
One robust and hitherto under-researched model of adaptation was the phenomenon of diurnal rhythmicity. This refers to the profound rhythms noted in mammalian intestinal glucose absorption coinciding with the arrival of food. Diurnal rhythms in glucose absorption are particularly prominent in the nocturnal-feeding rat, peaking during the dark.5 The exact mechanisms to cue, generate and modulate these rhythms were unclear. To investigate this further, we focused our efforts on the sodium glucose co-transporter 1 (SGLT1). This protein is expressed on the apical surface of villi in the small intestine and is responsible for more than 90% of glucose absorption in the bowel.6 Glucose absorption via SGLT1 is coupled with the absorption of water, which represents the principle behind the use of glucose in oral rehydration salts.6 By examining glucose uptake in rats harvested at 6-hourly intervals over a 24-hour period, we demonstrated peak glucose absorption during the nocturnal feeding period. This uptake was suppressed by the SGLT1-specific inhibitor phloridzin, confirming that the rhythmicity in glucose uptake was mediated entirely by SGLT1.7 Rhythmicity in SGLT1 protein levels was transcriptionally mediated, with a corresponding peak in Sglt1 mRNA expression 6 hours before the peak in protein levels.7
Circadian clock genes regulate intestinal glucose absorption
Circadian rhythms are controlled by an interlinked molecular feedback network of genes collectively known as ‘clock genes’ – transcriptional regulators that maintain 24-hour rhythmicity via two interacting transcription loops.8 These genes are Clock, Bmal1, Reverb A, Reverb B, Per1, Per2, Cry1 and Cry2. Cells in most tissues express circadian clock genes. The central clock in the suprachiasmatic nucleus responds specifically to light, while other local environmental triggers may also cue subsidiary clocks in peripheral tissues.9 Clock genes themselves can act as transcription factors to control rhythmic expression of other genes.8
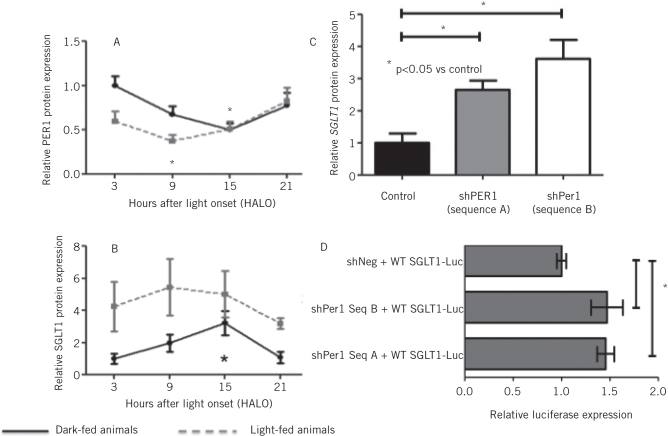
We examined the profile of intestinal clock gene rhythmicity in rats fed ad libitum and harvested at 3-hourly intervals, demonstrating a 24-hour periodicity in the expression of all eight clock genes.10 The peak in expression was phase-shifted when nutrients were delivered during the day only, in contrast to the usual nocturnal feeding pattern of rats. This phase shift was also identified in the expression of SGLT1 mRNA and protein levels. Of particular note, SGLT1 expression peaked during the trough in expression of the clock gene and known transcription factor PER1 (Fig 1a,b). Analysis of the promoter region for SGLT1 revealed the presence of four E-boxes, putative binding sites for PER1 and therefore a means for the regulation of SGLT1 expression. Knockdown of PER1 in vitro with short-hairpin RNA constructs increased SGLT1 expression by up to 3.5-fold compared with the scrambled control vector with no effect on the expression of other clock genes (Fig 1c). The exact mechanism of PER1-mediated regulation of SGLT1 was interrogated further using reporter assays by cloning the SGLT1 promoter into a luciferase reporter vector. Knockdown of PER1 increased SGLT1 promoter activity in vitro (Fig 1d). This was further increased by mutation of individual E-boxes, indicating that PER1 regulated SGLT1 transcription via activity on the E-boxes on the SGLT1 promoter.11
Figure 1.
PER1 protein expression (A) reached a nadir at HALO15, coinciding with a peak in expression of SGLT1 protein expression (B). Knockdown of PER1 in vitro with two distinct shRNA sequences increased SGLT1 mRNA expression by almost 4-fold(C). Luciferase expression, as measured by promoter-reporter assays, increased with PER1 knockdown (D).
MicroRNAs act as regulators of intestinal proliferation
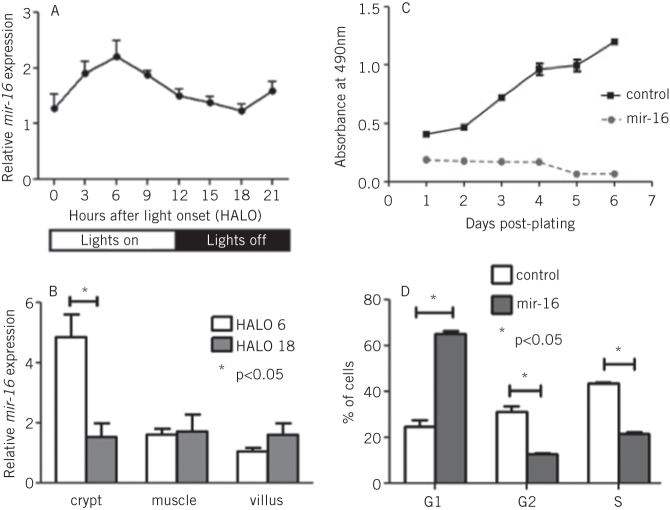
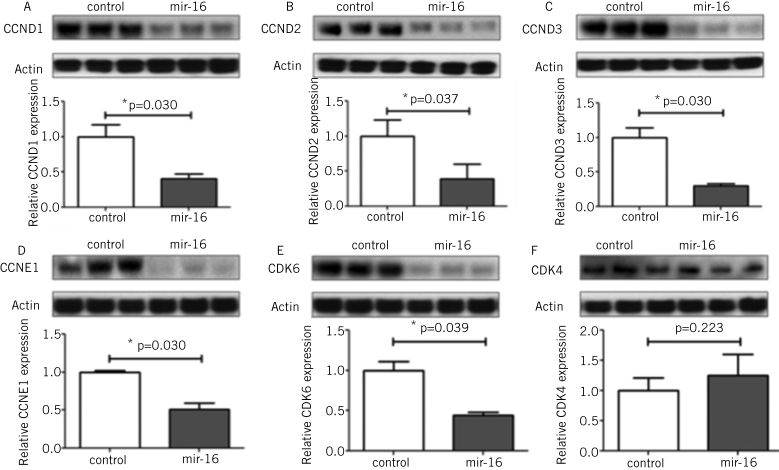
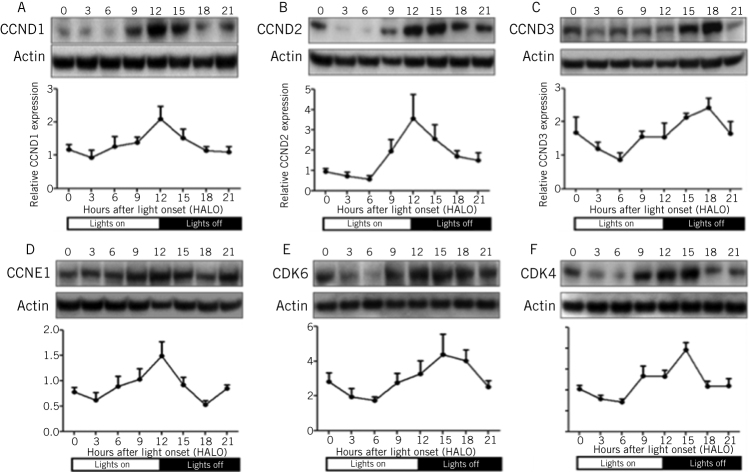
The studies described above have shown that one key element of adaptation, glucose absorption, could be regulated via the effect of clock genes on the rhythmicity of an individual transporter, SGLT1. We next sought to identify any other molecular pathways that could be responsible for another important aspect of adaptation, intestinal proliferation. MicroRNAs are short, non-coding RNAs that are able to downregulate the expression of multiple genes simultaneously and by binding to complementary sequences on the 3' untranslated regions of the transcripts of these genes.12–14 We examined the expression and rhythmicity of microRNAs in the intestine via microRNA microarrays carried out on the RNA extracted from the jejunum of rats harvested at 3-hourly intervals over a 24-hour period. Our study identified rhythmicity in the expression of three microRNAs, specifically mir-141, mir-20a and mir-16 (Fig 2a).15 We focused our further studies on mir-16, as this microRNA had previously been shown to affect proliferation in other organ systems.16 Indeed, mir-16 expression exhibited the greatest diurnal changes in the rapidly proliferating crypt fraction of the intestine, as determined via laser capture microdissection (Fig 2b). The effect of mir-16 on cell proliferation in vitro was assessed by cloning mir-16 into an overexpression vector and subsequent downstream analyses performed to measure cell viability, apoptosis and cell cycle progression. Overexpression of mir-16 suppressed cell growth and reduced cell numbers compared with the control vector, with no effect on apoptosis (Fig 2c).15 Cell-cycle analysis showed that mir-16 induced G1 arrest in enterocytes in vitro (Fig 2d). Bioinformatic microRNA target prediction algorithms identified five potential target genes that had well-described roles in the regulation of the G1/S transition, namely cyclinD1, cyclinD2 and cyclinD3, cyclin E and cyclin-dependent kinase 6.17,18 All five genes were suppressed when mir-16 was overexpressed in vitro (Fig 3) and exhibited diurnal rhythmicity in rat jejunum, with peak expression at the time of nocturnal feeding (Fig 4). Expression of cyclin-dependent kinase 4, a G1/S regulator not predicted to be a target of mir-16, did not change on mir-16 overexpression but did exhibit rhythms in expression on a diurnal basis, suggesting that other regulatory mechanisms may play a role in the regulation of expression of cell cycle besides microRNAs (Figs 3 and 4). Given the implications for enterocyte proliferation, we examined rhythmicity of morphological parameters of paraffin-embedded sections of jejunal villi. The dimensions of crypt depth and villus height both demonstrated 24-hour rhythms, peaking during the nocturnal feeding period when nutrient absorption would be at a maximum.7,15 We thus demonstrate that rhythmicity in mir-16 regulates enterocyte proliferation via G1 arrest and thereby maximal enterocyte villus surface area to peak nocturnally and temporally match absorptive capacity to nutrient delivery.
Figure 2.
mir-16 expression exhibited a diurnal rhythm in the jejunum of rats harvested at 3-hourly intervals, as measured by quantitative PCR (A). Laser capture microdissection identified the greatest difference between peak and trough levels to occur in the crypt fraction of the jejunum (B). Cells bearing overexpression vectors for mir-16 had significant impairment of proliferative capacity compared with the scrambled control vector (measured by the cell viability assays, C). Cell-cycle analysis via flow cytometry showed that cells overexpressing mir-16 were arrested in the G1 phase of the cell cycle compared with controls (D).
Figure 3.
Protein expression of cell cycle genes cyclin D1 (A), cyclin D2 (B), cyclin D3 (C), cyclin E1 (D), cyclin-dependent kinase 6 (E) and cyclin-dependent kinase 4 (F) was measured in cells bearing overexpression vectors for mir-125a compared to cells bearing a scrambled control vector. Levels were measured by Western blotting and normalised to the housekeeping protein Actin. Expression of cyclin D1, cyclin D2, cyclin D3, cyclin E1 and cyclin-dependent kinase 6 was lower in mir-16 overexpression compared to the control. Cyclin-dependent kinase 4, a cell cycle regulator not predicted to be a target of mir-16 showed no change in protein expression following mir-16 overexpression.
Figure 4.
Protein expression of cell cycle genes cyclin D1 (A), cyclin D2 (B), cyclin D3 (C), cyclin E1 (D), cyclin-dependent kinase 6 (E) and cyclin-dependent kinase 4 (F) was measured in the jejunum of rats harvested at 3-hourly intervals by Western blotting. Protein levels were normalised to the housekeeping protein Actin. Diurnal rhythmicity of expression was demonstrated for all six proteins with peak expression falling between 12 to 15 hours after light onset.
MicroRNA regulation of the adaptive response to resection
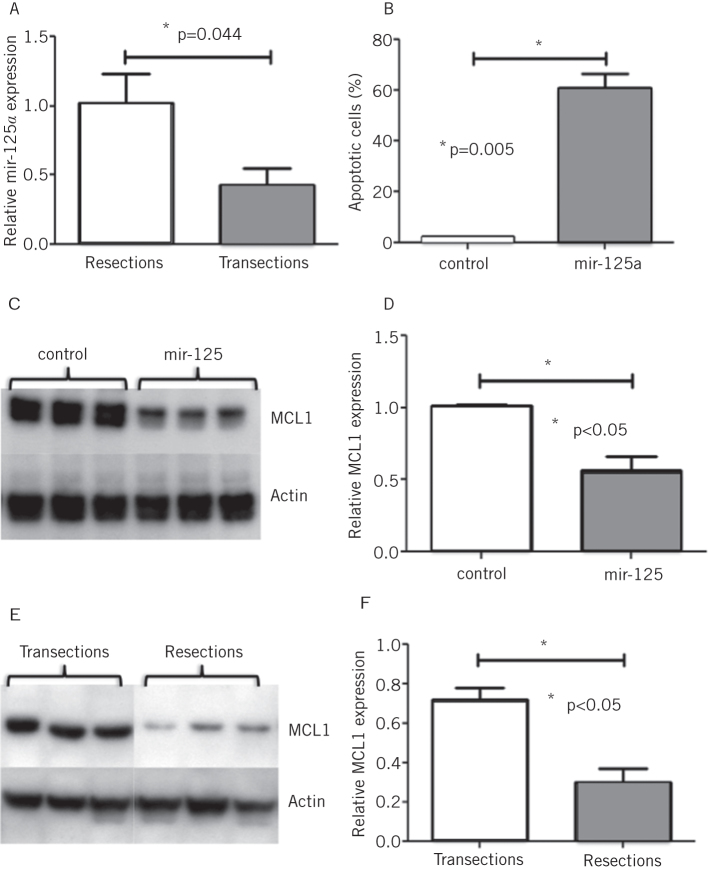
We next sought to determine whether microRNAs, shown in the studies above to mediate the physiological adaptive process of diurnal rhythmicity, could similarly regulate an adaptive response to a pathological process such as intestinal resection.19,20 We assessed this with a model of massive small bowel resection, involving removal of 80% intestinal length from one group of rats, compared with simply transecting and reanastomosing the intestine, with no loss of length, in a control group of rats. MicroRNA expression in the jejunum of both groups of rats was analysed with microRNA microarrays, 48 hours after the surgical procedure; mir-125a was identified to be elevated following intestinal resection, particularly in the crypt fraction of the intestine (Fig 5a).21 Overexpression of mir-125a in an enterocyte cell line suppressed cell viability by increasing the rate of apoptosis in these cells (Fig 5b). No effect was noted on cell cycle progression. It is likely that the increase in apoptosis following intestinal resection is a reflection of the balance between proliferation and apoptosis that maintains tight regulation of the adaptive response.22 Bioinformatic algorithms for prediction of microRNA targets were interrogated for specific targets implicated in apoptosis. MCL-1, a ‘pro-survival’ protein that inhibits apoptosis was found to be a potential candidate of mir-125a.23,24 MCL1 protein levels were suppressed by mir-125a overexpression in vitro (Figs 5c and 5d). Correspondingly protein expression of MCL1 was low in animals that had undergone massive small bowel resection with high levels of mir-125a (Figs 5e and 5f).21
Figure 5.
mir-125a was shown to be elevated following massive small bowel resection compared with transected controls (identified by microRNA microarrays and confirmed by quantitative PCR; A). mir-125a overexpressing cells had significantly higher rates of apoptosis (measured via cleaved caspase 3 levels) compared with cells transfected with the control vector (B). Western blotting showed lower levels of MCL1 protein in cells overexpressing mir-125a compared with transfection with the control vector (C and D) and correspondingly lower levels of MCL1 following resection compared with transection (E and F). Protein levels of MCL1 were normalised to the housekeeping protein Actin.
Conclusions
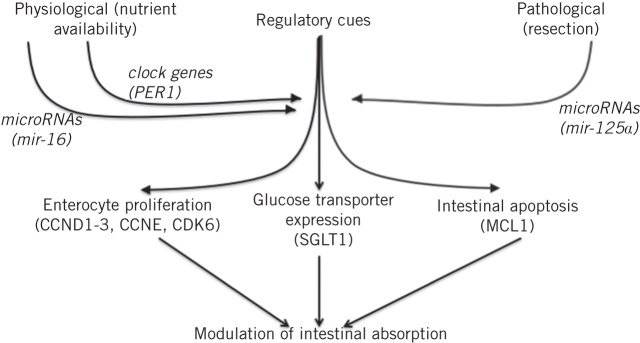
These studies collaboratively identify molecular pathways that regulate the intestinal adaptive response to both physiological and pathological stimuli. Nutrient availability acts as a regulatory cue on a physiological basis via clock genes and microRNAs, specifically mir-16, while mir-125a regulates the adaptation to small bowel resection (Fig 6). These cues are able to individually and cooperatively regulate key components of the adaptive response, namely enterocyte proliferation and apoptosis and transporter expression. Modulation of these pathways may facilitate the development of new therapies to boost absorptive and other functions in malabsorptive conditions, thereby improving the management of intestinal failure and short bowel syndrome.
Figure 6.
Modulation of intestinal absorption is mediated by both physiological and pathological triggers such as nutrient availability and resection respectively. These cues, acting via regulatory pathways including microRNAs and clock genes, are able to mediate key features of the adaptive process such as enterocyte proliferation, glucose transporter expression and intestinal apoptosis.
Acknowledgements
The work to decipher these pathways formed the basis of the research for my PhD, performed in the laboratory of Professor Stanley Ashley at Harvard Medical School, Boston, Massachusetts. This article summarises the work performed and the findings that resulted from this work, and was a Hunterian Lecture delivered on 4 May 2017 at the Association of Surgeons of Great Britain and Ireland.
References
- 1.Pironi L. Definitions of intestinal failure and the short bowel syndrome. 2016; (2): 173–185. [DOI] [PubMed] [Google Scholar]
- 2.Vanderhoof JA, Langnas AN. Short-bowel syndrome in children and adults. 1997; (5): 1,767–1,778.. [DOI] [PubMed] [Google Scholar]
- 3.Moukarzel AA, Haddad I, Ament ME et al. 230 patient years of experience with home long-term parenteral nutrition in childhood: natural history and life of central venous catheters. 1994; (10): 1,323–1,327.. [DOI] [PubMed] [Google Scholar]
- 4.Todo S, Reyes J, Furukawa H et al. Outcome analysis of 71 clinical intestinal transplantations. 1995; (3): 270–282.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavakkolizadeh A, Berger UV, Shen KR et al. Diurnal rhythmicity in intestinal SGLT-1 function, V(max), and mRNA expression topography. 2001; (2): G209–G215.. [DOI] [PubMed] [Google Scholar]
- 6.Turk E, Zabel B, Mundlos S et al. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. 1991; (6316): 354–356.. [DOI] [PubMed] [Google Scholar]
- 7.Balakrishnan A, Stearns AT, Rounds J et al. Diurnal rhythmicity in glucose uptake is mediated by temporal periodicity in the expression of the sodium-glucose cotransporter (SGLT1). 2008; (6): 813–818.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson I, Reddy AB. Molecular mechanisms of the circadian clockwork in mammals. 2014; (15): 2,477–2,483.. [DOI] [PubMed] [Google Scholar]
- 9.Panda S. Circadian physiology of metabolism. 2016; (6315): 1,008–1,015.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balakrishnan A, Stearns AT, Ashley SW et al. Restricted feeding phase shifts clock gene and sodium glucose cotransporter 1 (SGLT1) expression in rats. 2010; (5): 908–914.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balakrishnan A, Stearns AT, Ashley SW et al. PER1 modulates SGLT1 transcription in vitro independent of E-box status. 2012; (6): 1,525–1,536.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. 2005; (1): 15–20.. [DOI] [PubMed] [Google Scholar]
- 13.Lim LP, Lau NC, Garrett-Engele P et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. 2005; (7027): 769–773.. [DOI] [PubMed] [Google Scholar]
- 14.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. 2001; (5543): 858–862.. [DOI] [PubMed] [Google Scholar]
- 15.Balakrishnan A, Stearns AT, Park PJ et al. MicroRNA mir-16 is anti-proliferative in enterocytes and exhibits diurnal rhythmicity in intestinal crypts. 2010; (20): 3,512–3,521.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonci D, Coppola V, Musumeci M et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. 2008; (11): 1,271–1,277.. [DOI] [PubMed] [Google Scholar]
- 17.Sherr CJ. Cancer cell cycles. 1996; (5293): 1,672–1,677.. [DOI] [PubMed] [Google Scholar]
- 18.Sherr CJ. G1 phase progression: cycling on cue. 1994; (4): 551–555.. [DOI] [PubMed] [Google Scholar]
- 19.Stern LE, Huang F, Kemp CJ et al. Bax is required for increased enterocyte apoptosis after massive small bowel resection. 2000; (2): 165–170.. [DOI] [PubMed] [Google Scholar]
- 20.Dowling RH, Booth CC. Structural and functional changes following small intestinal resection in the rat. 1967; (1): 139–149.. [PubMed] [Google Scholar]
- 21.Balakrishnan A, Stearns AT, Park PJ et al. Upregulation of proapoptotic microRNA mir-125a after massive small bowel resection in rats. 2012; (4): 747–753.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin CE, Falcone RA, Kemp CJ et al. Intestinal adaptation and enterocyte apoptosis following small bowel resection is p53 independent. 1999; (3 Pt 1): G717–G724.. [DOI] [PubMed] [Google Scholar]
- 23.Craig RW. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. 2002; (4): 444–454.. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds JE, Yang T, Qian L et al. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. 1994; (24): 6,348–6,352.. [PubMed] [Google Scholar]