Abstract
Introduction
Conservative management of patients with a stable vestibular schwannoma (VS) places a significant burden on National Health Service (NHS) resources and yet patients’ surveillance management is often inconsistent. Our unit has developed a standardised pathway to guide surveillance imaging of patients with stable VS. In this article, we provide the basis for our imaging protocol by reviewing the measurement, natural history and growth patterns of VS, and we present a cost analysis of implementing the pathway both regionally and nationally.
Methods
Patients with an extrameatal VS measuring ≤20mm in maximal diameter receive magnetic resonance imaging (MRI) six months after their index imaging, followed by three annual MRI scans, two two-year interval MRI scans, a single three-year interval MRI scan and then five-yearly MRI scans to be continued lifelong. Patients with purely intrameatal tumours follow the same protocol but the initial six-month imaging is omitted. A cost analysis of the new pathway was modelled on our unit’s retrospective data for 2015 and extrapolated to reflect the cost of VS surveillance nationally.
Results
Based on an estimation that imaging surveillance would last approximately 25 years (+/- 10 years), the cost of implementing our regional surveillance programme would be £151,011 per year (for 99 new referrals per year) and it would cost the NHS £1,982,968 per year if implemented nationally.
Conclusions
A standardised surveillance pathway promotes safe practice in the conservative management of VS. The estimated cost of a national surveillance programme compares favourably with other tumour surveillance initiatives, and would enable the NHS to provide a safe and economical service to patients with VS.
Keywords: Vestibular schwannoma, Cost analysis, Magnetic resonance imaging
Vestibular schwannoma (VS) is a benign tumour arising from the nerve sheath of one of the vestibular nerves. The incidence of VS has risen significantly, and is now estimated to be between 14 and 20 cases per million per year in the UK,1,2 mirroring the increased incidence observed in other countries.3,4 This is at least partly due to the technical advances in medical imaging and the widespread availability of diagnostic magnetic resonance imaging (MRI), which have resulted in a greater number of patients with no audiovestibular symptoms being diagnosed with VS.5 As a result, conservative management with interval imaging is becoming increasingly common, and represents a significant burden to the multidisciplinary skull base and neuroimaging services in the National Health Service (NHS).
Conservative management of VS at our unit was often inconsistent so a standardised pathway was developed to guide the surveillance of these patients. The cost of implementing the pathway in our region was then analysed and the results were extrapolated to reflect the total cost of VS surveillance in the UK.
Methods
The published literature concerning the measurement, natural history and growth patterns of VS was reviewed, and other units’ practice of tumour surveillance was considered before developing our own regional guidelines.
Measurement of VS
At the last consensus meeting on systems for reporting results in VS, held in 2003,6 it was agreed that a VS should be defined as either purely intrameatal (intracanalicular) or intrameatal with extrameatal extension (simply referred to as extrameatal tumours in this paper). The size of both an intra and extrameatal component should be measured separately but the present criterion for growth of a purely intrameatal tumour is growth to extrameatal extension. For intrameatal tumours with an extrameatal extension and all other extrameatal tumours, it has been suggested that ‘absolute growth’ is defined as an increase of ≥3mm in maximal extrameatal diameter to rule out interindividual measuring variability and error due to unaligned scanning images.7
In 2012 a Norwegian group demonstrated that a tumour’s volume doubling time and the cm3/year-based model provided the most accurate methods for detecting subtle growth when compared with the standard mm/year-based model.8 Nevertheless, measuring a tumour’s largest extrameatal diameter is still deemed sensitive enough to determine clinically significant tumour growth and remains the accepted international standard.
Growth patterns
The natural history of VS growth is enigmatic and the percentage of growing tumours has been reported to vary widely from 30% to 90%5 depending (at least in part) on the length of the observation period. A systematic review from 2016 analysed the effect of various factors on tumour growth but neither patient age, nor sex, nor tumour size at presentation, nor location were demonstrated to influence VS growth.9 A patient’s presenting symptoms (including hearing loss and vertigo) are also unlikely to predict tumour growth but the predictive significance of tinnitus and balance disturbance on tumour growth remains unclear.9
A mean annual growth rate of 0.99–1.11mm has been reported9 but importantly, tumour growth within the first year has been found to be a statistically significant predictor of further tumour growth.10,11 Cystic and haemorrhagic tumours and tumours in patients with neurofibromatosis type 2 (NF2) are more unpredictable, and consequently have a higher mean annual growth.9,12,13
Surveillance imaging: current international practice
There is very little evidence to guide the frequency between surveillance imaging in patients with a stable VS (Table 1). In 2006 a Danish unit published a retrospective review of 30 years of experience of VS management.7 The authors recommended treatment for all tumours in excess of 15mm extrameatal diameter and provided a conservative surveillance algorithm for smaller tumours. They suggested yearly MRI for five years, followed by MRI every other year for four years, followed by a final MRI scan after a further five years. Observation of the patient is then terminated.
Table 1.
Surveillance imaging protocols for the conservative management of vestibular schwannoma
| CPH | BHM | NHNN | KCH | |||
| Intrameatal | Extrameatal | Intrameatal | Extrameatal | |||
| Total scans in 15-year period | 8 | 6 | 8 | 9 | 7 | 8 |
| Duration of surveillance | 14 years | Lifelong | Lifelong | Lifelong | Lifelong | Lifelong |
| Index scan | ||||||
| 6 months | MRI | MRI | MRI | |||
| 1 year | MRI | MRI | MRI | MRI | MRI | MRI |
| 2 years | MRI | MRI | MRI | MRI | MRI | MRI |
| 3 years | MRI | MRI | MRI | MRI | MRI | |
| 4 years | MRI | MRI | ||||
| 5 years | MRI | MRI | MRI | MRI | MRI | |
| 6 years | ||||||
| 7 years | MRI | MRI | MRI | MRI | MRI | |
| 8 years | ||||||
| 9 years | MRI | MRI | MRI | MRI | ||
| 10 years | MRI | MRI | ||||
| 11 years | ||||||
| 12 years | MRI | MRI | ||||
| 13 years | ||||||
| 14 years | MRI | MRI | ||||
| 15 years | MRI | MRI | MRI | MRI | ||
| Ongoing | N/A | Every 5 years | Every 5 years | Every 5 years | Every 5 years | Every 5 years |
CPH = Copenhagen; BHM = Birmingham; NHNN = National Hospital for Neurology and Neurosurgery, London; KCH = King’s College Hospital, London; MRI = magnetic resonance imaging
The only other protocol published in the medical literature comes from Birmingham, UK.13 In their paper, the authors retrospectively reviewed the records of all patients treated in a 20-year period and subsequently devised a protocol for the conservative management of VS measuring up to 20mm in diameter. Their data indicated that growth usually manifests itself in the first three years after presentation and they recommended an initial MRI scan at six months following diagnosis, followed by imaging at annual intervals for two years and another scan two years later to identify any patient with an indolent tumour. They suggested that patients should undergo lifelong surveillance with subsequent follow-up imaging performed every five years.
A third protocol, from the National Hospital for Neurology and Neurosurgery (NHNN) in London, was obtained via personal correspondence with Chandra Pierre-Davis (data manager for the brain tumour unit at NHNN). This was the only protocol where tumour location determines the frequency of the surveillance imaging, differentiating between intrameatal and extrameatal tumours. Patients with a purely intrameatal tumour measuring <5mm in diameter receive three 1-year interval scans, three 2-year interval scans, two 3-year interval scans followed by lifelong surveillance with MRI every five years. Those with an extrameatal tumour receive additional MRI six months following diagnosis before commencing the aforementioned imaging protocol.
The King’s surveillance pathway for patients with stable VS
In line with current UK practice, our unit applies continued conservative management for all stable VSs measuring <20mm in maximal extrameatal diameter.5,14 Decisions regarding the timing of surveillance imaging in patients with larger tumours or in those with a cystic, haemorrhagic or NF2 related tumour are made on an individual basis.
Patients with a purely intrameatal tumour undergo three annual MRI scans, two 2-year interval scans, one 3-year interval scan and then 5-yearly imaging to be continued lifelong. Those with extrameatal tumours receive MRI six months after their index scan, followed by three annual MRI scans, two 2-year interval MRI scans, a single 3-year interval MRI scan and then 5-yearly MRI to be continued lifelong (Fig 1).
Figure 1.
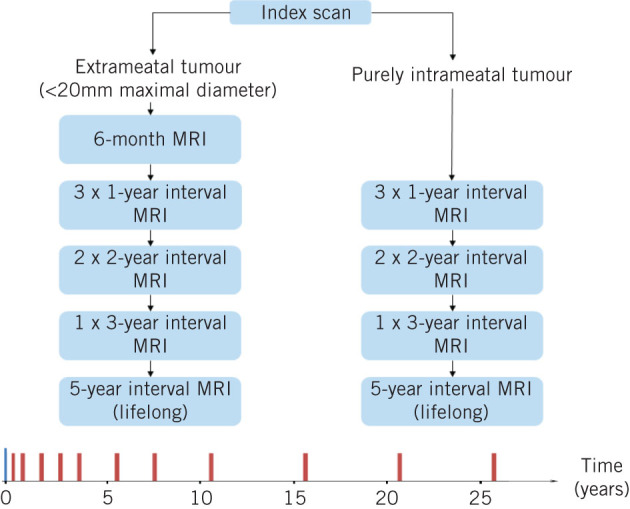
The King’s College Hospital surveillance pathway for the conservative management of patients with stable vestibular schwannoma
The patient’s index imaging is considered to be the first MRI scan that the patient receives and on which the diagnosis of VS is made or the first scan that is referred to the skull base multidisciplinary team (MDT) for review (whichever applies to the patient in question). Patients who have undergone surgery receive baseline imaging at three months and this is considered their new index scan. Subsequent surveillance imaging is the same as for the extrameatal tumour protocol described above.
A measurement of maximal tumour diameter is compared with the corresponding measurement on the patient’s index imaging. Tumours demonstrating an increase of ≥3mm are defined as ‘growing’; this applies to both intrameatal and extrameatal tumours. Patients who demonstrate absolute growth no longer follow the surveillance pathway and any decisions concerning their continued surveillance with imaging are made on an individual basis. Any patient under surveillance who develops new or changing symptoms is reviewed in clinic and the timing of ongoing surveillance imaging is also made on an individual basis.
Cost analysis
Retrospective analysis was conducted on a prospectively collected database of all patients with a VS referred to the skull base unit at King’s College Hospital in a 12-month period (1 January – 31 December 2015). Data collected included patient demographic features, tumour size at presentation and the MDT’s initial management decision. Our local clinical commissioning group (Southwark) was contacted to obtain the tariff cost of a brain MRI scan with gadolinium performed and reported at King’s College Hospital. The cost of lifelong surveillance in our patient population was calculated using the UK’s average life expectancy and this was extrapolated to the UK population as a whole.
Results
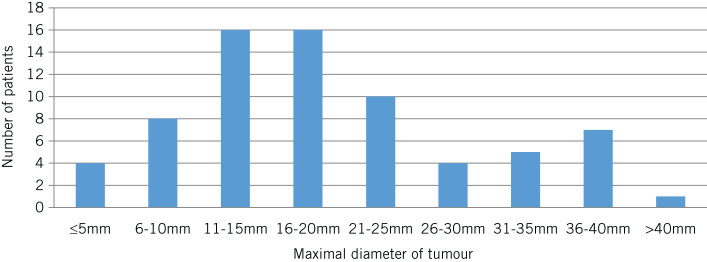
In 2015, 309 patients with VS were reviewed by the MDT, of whom 99 were new referrals (58 male, 41 female; median age: 57 years, interquartile range: 46–69 years). Seventy-two patients had an extrameatal tumour and twenty-seven had a purely intrameatal tumour. Data on the original tumour size were available in 96 patients. The median maximal extrameatal tumour diameter was 17mm (range: 1.5–42mm) (Fig 2) and 62% of patients had a tumour measuring <20mm at presentation. The median diameter for intrameatal tumours was 8mm (range: 2–12mm). All patients with a purely intrameatal tumour were managed conservatively with interval imaging surveillance. Of those patients with an extrameatal tumour, 95% (42/44) with tumours measuring ≤20mm were managed conservatively whereas 44% (12/28) with tumours measuring >20mm continued with conservative surveillance.
Figure 2.
Maximal extrameatal diameter of vestibular schwannomas referred to the King’s College Hospital skull base multidisciplinary team meeting in 2015
Since the UK has an average life expectancy of 81 years,15 it can be estimated that imaging surveillance will last approximately 25 years (+/- 10 years) in our population. The cost of obtaining a MRI scan at our unit is £223. The cost for lifetime surveillance (∼10 scans) in an individual patient would therefore amount to £2,230. The cost of implementing our regional surveillance programme was calculated to be £151,011 per year (for 99 new referrals per year). Consequently, the annual cost of introducing this pathway across the entire NHS is estimated to be £1,982,968 (based on a UK population of 65 million,16 a VS incidence of 20 cases per million population per year2 and a cost of £223 per MRI scan).
Discussion
It is clear that the incidence of VS has increased significantly over the last 30 years.1,3,4 Several reasons are likely to have contributed to this, including improved diagnostic imaging and the widespread availability of MRI, a heightened awareness of symptoms among the general population, increasing life expectancy, and easier access to audiological testing among general practitioners and otolaryngologists. As the incidence of VS has increased,2 the average tumour size has decreased and up to 20% of patients are likely to have no audiovestibular symptoms at the time of diagnosis.4
Most centres would now recommend conservative treatment with surveillance MRI for incidentally diagnosed asymptomatic small tumours; however, there is no consensus regarding the optimal timing of interval imaging. As a result, there is significant variability in the treatment of these patients and even the management delivered at a single unit is often inconsistent, with several patients continuing with annual surveillance imaging for many years. Unnecessarily frequent imaging places an additional strain on the limited clinical and financial resources available in the NHS.
We have developed a standardised pathway to guide the surveillance imaging of patients with a stable VS in order to achieve three key objectives: 1) to maintain patient safety, 2) to eliminate unnecessary imaging requests, and 3) to reduce the cost of imaging surveillance. The literature concerning the measurement, natural history and growth patterns of VS was reviewed, and the practice of VS tumour surveillance at other units was considered before developing our own regional guidelines.
Our pathway is designed to be used in patients with tumours measuring <20mm in maximal extrameatal diameter. It is known that larger tumours are at risk of rapid and unpredictable growth so it was felt that an upper limit of 20mm would ensure that patients with larger tumours are initially followed up more closely. Nevertheless, if a patient who harbours a large tumour remains stable and has limited symptoms (eg deafness alone), then intervention may not be indicated and it may be appropriate to consider following the same surveillance pathway in these patients too.
Our unit adheres to the current international standard of measuring a tumour by its maximal extrameatal diameter6 and absolute growth is defined as growth of ≥3mm. It is accepted that other measurements of growth (such as volume doubling time) may be superior in detecting subtle growth.8 However, we did not feel that detecting such small changes in growth (<3mm) would alter our management strategy so we continued with a simpler measurement of tumour size. Previous authors have defined absolute growth differently, depending on the degree of the intra or extrameatal extension.6 We believe that this is confusing and that intrameatal growth of ≥3mm should still be considered significant, prompting careful consideration of the available treatment options (including ongoing surveillance) in all tumours.
There is a wide variation in the reported rate of growing tumours. No clear predictive factors of growth behaviour have been identified except that tumour growth within the first year is the best predictor of future growth.10,11 Martin et al demonstrated that 90% of growing tumours are detected within the first three years of surveillance.13 In order to capture early tumour growth at our unit, all extrameatal tumours have initial six-month imaging, followed by annual imaging for the first three years. Thereafter, the interval of imaging surveillance gradually increases over ten years.
Contrary to some other authors, we believe that surveillance should continue lifelong. Lifelong five-year interval imaging will not significantly alter the cost of surveillance but ensures that those patients who exhibit late tumour growth are not missed. Certain types of tumour such as cystic and haemorrhagic tumours, and those associated with NF2 are known for their unpredictable growth patterns. For this reason these cases do not follow the standardised pathway and surveillance imaging is tailored to the individual patient.
In order to perform a cost analysis, all cases of VS discussed at our MDT meeting were reviewed retrospectively. Ninety-nine new cases of VS were referred to our unit in the twelve-month study period. The median patient age was 58 years so our cost analysis was modelled on a 25-year surveillance programme receiving 99 new referrals every year with all patients following our imaging protocol. It was also assumed that all tumours remained stable for the entire duration of surveillance. Consequently, this should be a very conservative cost estimate as 30–90% of these tumours will demonstrate some growth,5 which will inevitably prompt closer interval imaging (either as part of increased surveillance or following treatment). Furthermore, our analysis does not factor in time for discussion at the MDT meeting, clinic time or the logistical costs of transporting patients for their imaging.
The cost of obtaining and reporting on enhanced MRI with gadolinium at our institution is £223 so imaging surveillance on patients with stable VS in our region is estimated to amount to at least £151,011 per year over a 25-year period. A similar active surveillance programme is recommended by the National Institute for Health and Care Excellence in cases of low risk prostate cancer,17 with a study from the US giving an estimated ten-year cost of $28,784 per patient.18 The estimated ten-year cost for implementing our VS surveillance pathway is £1,561 per patient ($1,944 – www.xe.com, March 2017), which compares very favourably with surveillance of low risk prostate cancer. National implementation of our VS surveillance pathway would cost the NHS approximately £1,982,968 per year.
Conclusions
We have developed a regional pathway for the conservative management of VS. A standardised surveillance pathway promotes safe practice while providing an economical service and we believe that this represents excellent value for money for the NHS. We now propose to establish a national multidisciplinary collaborative in conjunction with the British Skull Base Society to develop a national protocol for the management of this increasingly common patient group.
References
- 1.Evans DG, Moran A, King A et al. . Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. 2005; : 93–97. [DOI] [PubMed] [Google Scholar]
- 2.Moffat DA, Hardy DG, Irving RM et al. . Referral patterns in vestibular schwannomas. 1995; : 80–83. [DOI] [PubMed] [Google Scholar]
- 3.Stangerup SE, Tos M, Thomsen J, Caye-Thomasen P. True incidence of vestibular schwannoma?. 2010; : 1,335–1,340. [DOI] [PubMed] [Google Scholar]
- 4.Frohlich AM, Sutherland GR. Epidemiology and clinical features of vestibular schwannoma in Manitoba, Canada. 1993; : 126–130. [DOI] [PubMed] [Google Scholar]
- 5.Stangerup SE, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. 2012; : 257–268. [DOI] [PubMed] [Google Scholar]
- 6.Kanzaki J, Tos M, Sanna M et al. . New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. 2003; : 642–648. [DOI] [PubMed] [Google Scholar]
- 7.Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. 2006; : 547–552. [DOI] [PubMed] [Google Scholar]
- 8.Varughese JK, Breivik CN, Wentzel-Larsen T, Lund-Johansen M. Growth of untreated vestibular schwannoma: a prospective study. 2012; : 706–712. [DOI] [PubMed] [Google Scholar]
- 9.Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. 2016; : 1–8. [DOI] [PubMed] [Google Scholar]
- 10.van de Langenberg R, de Bondt BJ, Nelemans PJ et al. . Predictors of volumetric growth and auditory deterioration in vestibular schwannomas followed in a wait and scan policy. 2011; : 338–344. [DOI] [PubMed] [Google Scholar]
- 11.Tschudi DC, Linder TE, Fisch U. Conservative management of unilateral acoustic neuromas. 2000; : 722–728. [PubMed] [Google Scholar]
- 12.Suryanarayanan R, Ramsden RT, Saeed SR et al. . Vestibular schwannoma: role of conservative management. 2010; : 251–257. [DOI] [PubMed] [Google Scholar]
- 13.Martin TP, Senthil L, Chavda SV et al. . A protocol for the conservative management of vestibular schwannomas. 2009; : 381–385. [DOI] [PubMed] [Google Scholar]
- 14.Reddy CE, Lewis-Jones HG, Javadpour M et al. . Conservative management of vestibular schwannomas of 15 to 31 mm intracranial diameter. 2014; : 752–758. [DOI] [PubMed] [Google Scholar]
- 15.Office for National Statistics . Newport: ONS; 2017. [Google Scholar]
- 16.Office for National Statistics . Newport: ONS; 2017. [Google Scholar]
- 17.National Institute for Health and Care Excellence . London: NICE; 2014. [Google Scholar]
- 18.Keegan KA, Dall’Era MA, Durbin-Johnson B, Evans CP. Active surveillance for prostate cancer compared with immediate treatment: an economic analysis. 2012; : 3,512–3,518. [DOI] [PMC free article] [PubMed] [Google Scholar]