Abstract
Introduction
The morbidity and significant health economic impact associated with the chondral lesion has led to a large number of strategies for therapeutic neochondrogenesis. The challenge has been to develop techniques that are cost effective single-stage procedures with minimal surgical trauma that have undergone rigorous preclinical scrutiny and robust reproducible assessment of effectiveness. A biological repair requires the generation of a cellular and matrix composite with appropriate signalling for chondrogenic differentiation.
Methods and results
A technique was developed that allowed chondrogenic primary (uncultured) cells from bone marrow aspirate concentrate, combined with a composite hydrophilic and fibrillar matrix to be applied arthroscopically to a site of a chondral lesion. The construct was tested in vitro and in animal experiments before clinical trials.
Clinical trials involved 60 patients in a prospective study. Symptomatic International Cartilage Repair Society grade 3 and 4a lesions were mapped and treated. Pre- and postoperative clinical assessments showed statistically significant improved outcomes; Lysholm Knee Scoring Scale (mean 52.8 to > 76.4; P < 0.05) International Knee Documentation Committee (mean 39 to > 79 P < 0.05) and Knee injury and Osteoarthritis Outcome Score (64.5 to >89.2 P < 0.05). Postoperative magnetic resonance imaging was evaluated morphologically (magnetic resonance observation of cartilage repair tissue, average MOCART score 72) and qualitatively; the regenerate was comparable to native cartilage.
Conclusions
This technique is effective, affordable, requires no complex tools and delivers a single-stage treatment that is potentially accessible to any centre capable of performing arthroscopic surgery. Good clinical results were found to be sustained at five years of follow-up with a regenerate that appears hyaline like using multiple magnetic resonance measures.
Keywords: Primary hyperparathyroidism, Adenoma, Hyperplasia
Introduction
The hallmark of tissue engineered therapeutic chondrogenesis is the delivery of chondrogenic cells to the articular surface, with a matrix that is either induced by these cells or delivered alongside them. Despite published successes of a number of such techniques,1–3 they have not been adopted widely and, to date, microfracture remains the most practiced method of biological resurfacing of the joint.4,5 Barriers to the adoption of advanced regenerative techniques include accessibility of the technique, weakness of evidence, any additional morbidity associated with the procedure and the relative cost compared with conventional treatments, palliation and arthroplasty. It is probable that for new therapeutic options to escape the confines of specialist centres, a robustly evidenced low-cost technique minimising the surgical insult that is accessible to the mainstream orthopaedic surgeon should be developed. Such a development should be evidence based from concept to application.
Background
Cartilage injuries are common and are naturally progressive with limited potential for intrinsic repair.6,7 Cartilage has evolved of into a tissue with properties that allow efficient force transfer and a frictionless surface necessary to prevent thermal and mechanical destruction of the tissue when subjected to physiological forces.8,9 The property of the cartilage matrix that makes this possible is a highly hydrophilic matrix, constrained within fibrillar collagen II arcades.8,10 This, combined with multiple methods of lubrication, yields a specialised tissue,8 which is produced and maintained by differentiated cells able to function in the absence of a vascular supply; such vasculature would not tolerate the forces that the tissue experiences. Damage to this structure triggers two vicious circles that lead to progressive disease. First, the loss of structural integrity causes the tissue to be less mechanically efficient, with consequence that the tissue becomes subject to further damage.9,11 Second, the tissue damage leads to an inflammatory response whose catabolic cascade through the release of metalloproteinases disrupts the fibrillar architecture, with loss of constraint of the hydrophilic hyaluronic acid and glycoconjugates and further structural damage.12 The development of subchondral sclerosis and cysts may further complicate the biology and the mechanics of the overlying damaged cartilage.13
The implication, evidenced by even the earliest observations of articular cartilage lesions, is that the biological equilibrium that exists in articular cartilage is very vulnerable to any mechanical or biochemical disruption and, once exceeding the functional capacity of the chondrogenic cells, leads to progressive failure of the tissue. Three things are required for effective chondrogenesis: cells with the potential to produce and maintain appropriate matrix, a scaffold that supports the cells and a subchondral plate with suitable mechanical and vascular properties to sustain the growing construct.
Many attempts at reversing damage to articular cartilage have been developed. They may be classified by the component of the tissue that is involved in initiating the regenerative. Chondrostimulation may introduce cells to the surface that may form articular cartilage, usually fibrocartilage, lacking in any hydrophilic matrix.14 This can be improved if the matrix produced by the chondrogenic cell can be contained within a ‘bioactive chamber’.15 An acellular fibrillar scaffold using collagen (almost always type I) has also been found to be effective.16 Collagen type I, while readily available and widely used, may not be the optimal scaffold.17
Delivery of chondrogenic cells transforms the repair to an active process. The now classical autologous chondrocyte implantation,2 intensifies the chondrotypical cellular component by the use of cultured chondrocytes. The use of periosteal patches over the repair site have been used but the additional cellular component of these patches appears to provide no benefit.18 Cultured chondrocytes may dedifferentiate in monolayer culture. Characterisation techniques selecting cells for their phenotypic behaviour to counter this dedifferentiation has been shown to improve results but the mortality of differentiated cells is a limiting factor.19 Bone marrow-derived stems cells offer the potential for delivering a source of chondrogenic cells after preconditioning.20
Three-dimensional scaffolds further enhance the matrix composition of the regenerated tissue. Techniques combining matrix and cells represent a progression in the evolution of chondroregenerative therapies.21,22 Experiments with nonfibrillar–protein hydrogel matrices also suggest that a hydrophilic component may be important.23,24
Despite such a proliferation of techniques, few have achieved the sustained acceptance required to become a mainstream therapeutic offering for patients with chondral lesions to replace microfracture.25 Many factors may account for this situation: the surgical trauma of a procedure requiring arthrotomy, the need for a two-stage procedure, the expense of the culture process despite longer-term economic benefits,26,27 relatively small numbers in trials and the availability of an alternative definitive therapeutic options in the form of replacement arthroplasty.
Aims
The goal has been to develop a technique that is robust, single stage, minimally invasive, cost effective and acceptable to both patient and clinician. Robustness relies on having a vertical translation of research starting a logically derived concept, tested in in-vitro studies, applied in in-vivo standardised animal models and delivered to clinical practice after a sound ethical review. The expense of culture processing should be avoided. The approach should be minimally invasive and the surgical trauma should not exceed conventional microfracture. The repair construct, as stated earlier, should combine chondrogenic cells, hydrophilic matrix and fibrillar protein constraint. The composite should be adherent and should have mechanical integrity.
Mesenchymal cell-induced chondrogenesis (MCIC) is a technique that has been developed with these objectives. Three factors enabled the development of this technique. Concentrating chondrogenic cells using fractionation centrifugation enables intensification without culture.28 The use of fibrin glue allows the formation of an adhesive gel that can act as a carrier for both cells and adjuvant matrix components.16 Arthroscopic application of this gel is enabled by the use of carbon dioxide insufflation and dry arthroscopy. While the use dry arthroscopy has been found to be effective,29 a modification using CO2 insufflation allowed improved delivery of the composite construct even against gravity.
Assessment of repair may be symptomatic, functional or morphological. While it is expected that symptomatic relief is what is being targeted with treatments, some assessment of the regenerate itself is important. While an invasive histological assessment may cause some ethical concerns, high resolution magnetic resonance imaging (MRI) techniques can non-invasively evaluate the repair tissue.30
In vitro tests
Primary cells with chondrogenic differentiation potential can be derived from bone marrow aspirate. These cells can be selectively concentrated by centrifugal fractionation. The bone marrow aspirate concentrate (BMAC) yields an increase of nucleated cell density by a factor of 5.8 (± 2.74 standard error; n = 10) and an increase in the number of colony-forming units by 5.23 times (Fig 1). The levels of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) AB, stromal cell-derived factor 1 alpha (SDF1α) and transforming growth factor beta 1 (TGFβ1) in the supernatants were measured by enzyme-linked immunosorbent assay. In the BMAC group, the VEGF, PDGF AB, SDF1α and TGFβ1 was significantly increased compared with bone marrow. The mean concentration of VEGF was 318.33 ± 249.93 pg/ml in bone marrow and 2203.33 ± 855.91 pg/ml in BMAC. The mean concentration of PDGF AB was 658.33 ± 456.53 pg/ml in bone marrow and 4438.33 ± 2695.18 pg/ml in BMAC; the mean concentration of SDF1α was 1650.00 ± 1866.28 pg/ml in bone marrow and 24581.67 ± 18198.13 pg/ml in BMAC, the mean concentration of TGFβ1 was 13.67 ± 22.70 pg/ml in bone marrow and 190.00 ± 119.33 pg/ml in BMAC. Surface markers were tested using fluorescence-activated cell sorting analysis demonstrating an expression pattern consistent with mesenchymal stem cells (Fig 2).
Figure 1.
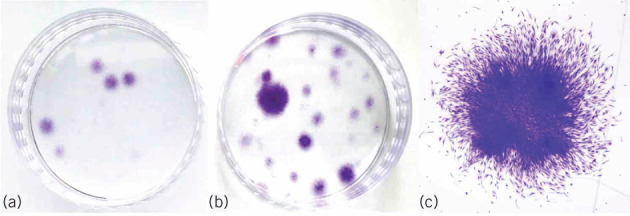
Crystal violet-stained colony forming units-fibroblast. Representative samples are shown. a) The bone marrow sample was a small number of variable sized colonies. b) The bone marrow aspirate concentrate (BMAC) sample was many colonies more than bone marrow, which varies widely in size, cell density and cell number. c) A BMAC sample of a single-medium sized colony (x 12.5 magnification image of a)
Figure 2.
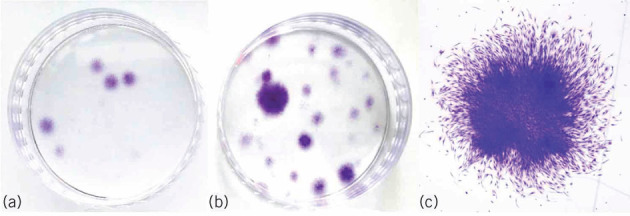
Flow cytometry analysis of bone marrow aspirate concentrate (BMAC) and bone marrow (BM). Nucleated cells of isolation labelled with antibodies against the indicated antigens and analyzed by flow cytometry. Representative histograms are displayed. On the y axis is the cell count (the cells were gated mononuclear cells) and on the x axis is the fluorescence intensity in a log (100–105) scale. The isotype control is shown as a red line histogram
Two commercially available matrix agents with approval for human use have been chosen for the composite microscaffold. Hyaluronic acid provides a hydrophilic matrix and fibrinogen forms the fibrillar adhesive component that undergoes phase change from liquid to hydrogel under controllable physiological conditions. The cell matrix composite has good adhesion and structural integrity, while fluorescent dye staining demonstrates viability of nucleated cells of over 90% (Fig 3). Phenotypical behaviour was evaluated using cell morphology and in vitro production of collagen type II. Adhesion of the construct required an irregular surface.
Figure 3.
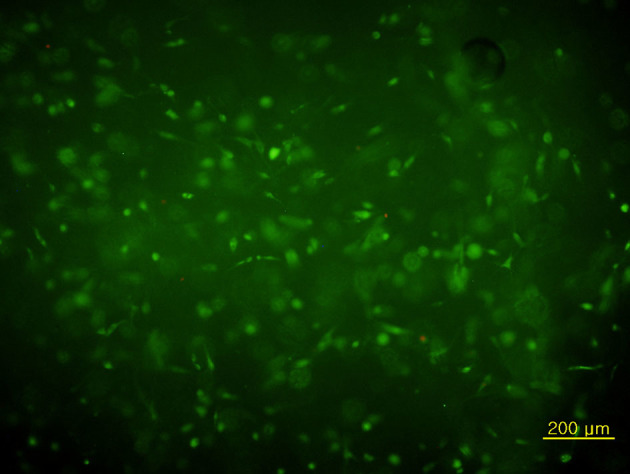
Biochemical staining of the sections obtained from bone marrow aspirate concentrate gel beads. Fluorescent dye staining of the sections taken from beads incubated for four days (original magnification × 40). Viable cells appeared green and dead cells were red, when they were stained with calcein acetoxymethyl ester and ethidium homodimer, respectively
Preclinical tests
We used a standard animal model for cartilage repair. Adult New Zealand white rabbits with a trochlear lesion were treated with microfracture (M group, control), microfracture with acellular matrix (MH group), microfracture with matrix plus BMAC (MBH group). After retrieval of these lesions at six weeks, the repair tissue was investigated with Masson trichrome staining and immunohistochemistry for collagen types I and II. The results with BMAC and the composite microscaffold exceed controls and microscaffold without cells, and are comparable to those found in other studies (Fig 4).
Figure 4.
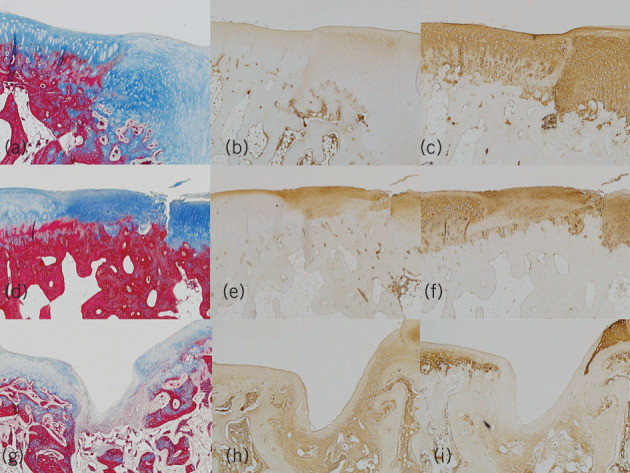
Photomicrograph of the section of regenerated cartilage of the microfracture with matrix plus bone marrow aspirate concentrate group with a) Masson trichrome staining; b) immunohistochemical staining with anti-collagen type I antibody; and c) immunohistochemical staining with anti-collagen type II antibody and the microfracture with acellular matrix group (d–f); and microfracture group (x 40) (g–i), respectively
Clinical protocol
Clinical trial was performed in UK and the comparative clinical data were supported from the South Korean cohort. Patient selection criteria were defined and thorough ethical review was undertaken, scrutinised by governance teams. Patients were recruited after counselling and a robust pathway for clinical support and follow-up was in place. Assessment was with standard symptom scores and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 mapping of chondral lesions. This was a prospective trial with no synchronous controls, comparing symptomatic and functional scoring for patients prior to surgery. The study included 60 patients (38 male and 22 female) (mean age 44.4 years, range 19–61 years) with an average chondral lesion size of 3.8 (Table 1). All patients were followed-up for five years.
Table 1.
Demographic information (N = 60).
| Variable | Mean ± SE (range) | n (%) |
| Age (year) | 44.4 ± 1.7 (19–61) | |
| Lesion size (cm2) | 3.8 ± 0.2 (2–9) | |
| Chondral lesions (n) | ||
| 1 | 38 (63.3) | |
| 2 | 14 (23.3) | |
| 3 or more | 8 (13.3) | |
| Location (multi-responses): | ||
| Medial femoral condyle | 39 (65) | |
| Trochlea | 14 (23.3) | |
| Lateral femoral condyle | 12 (20) | |
| Lateral tibial plateau | 10 (16.7) | |
| Patella | 9 (15) | |
| Medial tibial plateau | 6 (10) |
SE, standard error
One prime consideration was that the surgical trauma should not significantly exceed the trauma induced by the well-established conventional microfracture. This implied an arthroscopic procedure. Delivering a gel matrix to the articular surface with fluid irrigation would be naturally challenging, so the arthroscopic procedure is adapted to use the CO2 insufflation employed in laparoscopic surgery after the initial debridement and preparation of the lesion. The surgical workflow is initially similar to conventional microfracture, including:
bone marrow aspiration from the ipsilateral iliac crest
evaluation of the joint condition and mapping of lesions pre-debridement during initial joint survey
debridement of any degenerate menisci and the unstable portion of the articular cartilage lesions
reduction of subchondral sclerosis
microfracture and mapping of the post debridement lesion
washout of any debris induced
CO2 insufflation and drying of the lesions to be treated
application of the composite gel to the surface.
For the purposes of the study microfracture was deemed necessary to provide shear stability of the construct and this was tested by cycling the joint through a range of motion (Fig 5).
Figure 5.
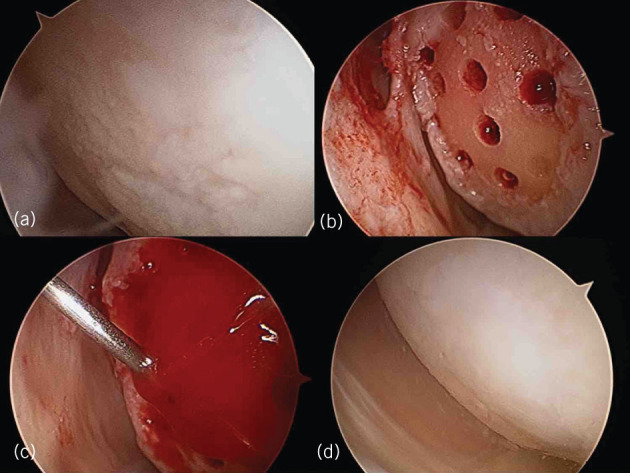
Mesenchymal cell-induced chondrogenesis procedure. a) Arthroscopic evaluation of knee joint and cartilage lesion; b) lesion preparation and microfracture; c) bone marrow aspirate concentrate and gel scaffold injection under CO2 inflation; d) second-look arthroscopic findings after two years
The postoperative protocol was initial continuous passive motion for four hours, followed by either partial weight bearing but allowing full range of motion for tibiofemoral lesions or mobilising in a brace when load bearing with full weight bearing allowed for patellofemoral lesions for six weeks.
Results of clinical trials
Follow-up included symptom and functional scoring tests using International Knee Documentation Committee, Knee injury and Osteoarthritis Outcome Score and Lysholm Knee Scoring Scale.3 Six-monthly MRI follow-up was required for evaluation using repair scores in the trial phase. Qualitative and quantitative MRI scans were analysed by an independent radiologist with a special interest in mapping of cartilage defects and repair tissue. The results are shown in Fig 6 and Table 2. Statistically significant improvement in symptom scores were seen in all scoring systems.
Figure 6.
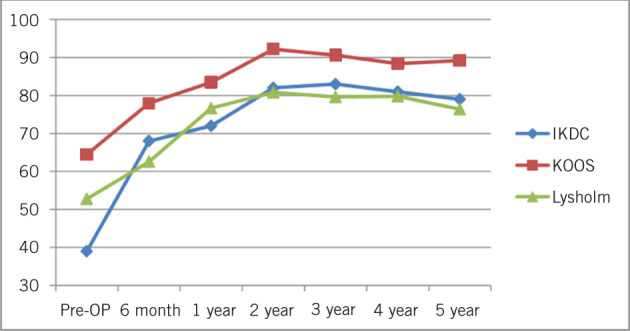
Summary of clinical outcome. All the scores showed significant improvement from preoperative evaluation to 60-month follow-up. KOOS, Knee injury and Osteoarthritis Outcome Score; IKDC, International Knee Documentation Committee
Table 2.
Summary of clinical and radiological outcome (N = 60).
| Variable | Preoperative (a) | 6-month (b) | 1-year (c) | 2-year (d) | 3-year (e) | 4-year (f) | 5-year (g) | P (post hoc) |
| Mean (SE) | ||||||||
| IKDC | 39.0 (0.6) | 68.0 (0.9) | 72.0 (0.9) | 82.0 (1.0) | 83.0 (1) | 81.0 (1.2) | 79.0 (1.1) | < .001i (a < b, c, d, e, f, g)ii |
| KOOS | 64.5 (1.3) | 78.0 (1.1) | 83.5 (0.9) | 92.2 (0.7) | 90.6 (0.7) | 88.4 (0.7) | 89.2 (0.7) | < .001i (a < b, c, d, e, f, g)ii |
| Lysholm | 52.8 (1.2) | 62.6 (0.9) | 76.7 (1.2) | 80.8 (1.3) | 79.6 (1.4) | 79.8 (1.3) | 76.4 (1.1) | < .001i (a < b, c, d, e, f, g)ii |
| MOCART | 60.0 (1.2) | 66.0 (1.3) | 72.0 (1.4) | < .001i (b < c < d)b | ||||
| T2* mapping | 29.0 (0.7) | 29.2 (0.7) | < .001iii | |||||
IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; MOCART, magnetic resonance observation of cartilage repair tissue; SE, standard error.
i Greenhouse–Geisser
ii Significant different were compared by Bonferroni test following one-way repeated-measures analysis of variance, with significant difference at P < 0.05.
iii Paired t test
Improvements were seen on MRI using both qualitative and quantitative (T2*-mapping and dGEMRIC) assessments. For the structured morphological assessment, the magnetic resonance observation of cartilage repair tissue (MOCART) score was used for postoperative magnetic resonance examinations. A MOCART score of 0 represents the worst possible result and 100 the best possible MRI outcome. A mean MOCART of 72 ± 21 (mean ± standard deviation, SD, standard error 1.4), T2*-mapping and dGEMRIC scores comparing regenerate with adjacent native articular cartilage (relaxation times 29.2 ± 8.5 and 29.9 ± 6.3 (mean ± SD) respectively, over the first two-year period of the follow-up showed the regenerate to be hyaline-like in signal (Table 2). The MOCART scores show improvement continues over the two-year period of magnetic resonance assessment.
The financial cost of a procedure is a factor that often determines acceptability of regenerative techniques to health service providers. Tables 3 and 4 show representative costs converted to US dollars for comparison. A significant contributor to the cost of MCIC had been the need for three MRI scans for the purposes of this study. The two centres for the trial have different methods of determining the cost of a procedure. For the UK cohort, costs were itemised and calculated by service provider for this particular procedure, and compared with standard tariffs for routine joint replacement and arthroscopy plus microfracture for NHS patients by the same provider. The costs in South Korea have many more confounding variables.
Table 3.
Comparison of costs according to treatment methods Costs South Korean Cohort
| Item | MCIC | TKR |
| Average hospital fee per patient | $4,713.6 | $6,754.0 |
| Corporate contribution | $3,100.4 | $4,546.4 |
| Personal contribution | $1,613.2 | $2,2076 |
| Special doctor fee | $553.0 | $658.4 |
The exchange rate for the hospital fees of Korean won for US dollars was 1,095.04 won.
Table 4.
Comparison of costs according to treatment methods (UK cohort).
| Procedure | Fixed price tariff for NHS patients | |
| (£) | US $ equivalenta | |
| Arthroscopy | 3131 | 3,757 |
| Mesenchymal cell induced chondrogenesis | 5817 | 6,980 |
| Total knee replacement | 9924 | 11,908 |
a Exchange rate of 1.2 was used to derive the US dollar rate.
The mean surgical time reduced from 55 minutes to 35 minutes over the five years of the initial trial. The serious complications rate is comparable to that of arthroscopic procedures; in our trials, there were no serious complications in these arthroscopic procedures.
Conclusions
MCIC has benefitted from the thorough review of existing methods of chondroregenerative therapeutic options and their limitations. A vertical translational methodology was followed from developing and testing the construct in culture, followed by representative standardised animal studies before proceeding to clinical studies. The procedure has a shallow learning curve being an evolutionary progression of other well-established techniques. Surgical trauma is minimal and this may lead to low complication rates compared with open procedures in other studies. The biggest hurdle limiting acceptability of chondroregenerative techniques remains the health economic justification of an expensive procedure which may, for many patients, merely delay implant arthroplasty rather than replacing it. This procedure is much more cost effective than other regenerative techniques (partly by using primary cells rather than relying on cell culture), to the extent that it may be a reasonable alternative to arthroplasty even in older patient with symptomatic single compartment lesions.
References
- 1.Bekkers JEJ, Inklaar M, Saris DBF. Treatment selection in articular cartilage lesions of the knee. 2009; (Suppl 1): 148S–155S. [DOI] [PubMed] [Google Scholar]
- 2.Brittberg M, Lindahl A, Nilsson A et al. . Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. 1994; (14): 889–895. [DOI] [PubMed] [Google Scholar]
- 3.Ananthram Shetty A, Kim S-J et al., . Mannheim: Springer Science; 2014. [Google Scholar]
- 4.Johnson LL. Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. 1986; (1): 54–69. [DOI] [PubMed] [Google Scholar]
- 5.Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. 2002; (3): 170–176. [PubMed] [Google Scholar]
- 6.Arøen A, Løken S, Heir S et al. . Articular cartilage lesions in 993 consecutive knee arthroscopies. 2004; (1): 211–215. [DOI] [PubMed] [Google Scholar]
- 7.Squires GR, Okouneff S, Ionescu M, Poole AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. 2003; (5): 1,261–1,270. [DOI] [PubMed] [Google Scholar]
- 8.Dowson D, Wright V. Lubrication and wear in joints. 1970; (2): 112–113. [Google Scholar]
- 9.Maquet P. The biomechanics of the knee and surgical possibilities of healing osteoarthritic knee joints. 1980; (146): 102–110. [PubMed] [Google Scholar]
- 10.Korhonen RK, Laasanen MS, Töyräs J et al. . Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. 2003; (9): 1,373–1,379. [DOI] [PubMed] [Google Scholar]
- 11.Chen C-T, Bhargava M, Lin PM, Torzilli PA. Time, stress, and location dependent chondrocyte death and collagen damage in cyclically loaded articular cartilage. 2003; (5): 888–898. [DOI] [PubMed] [Google Scholar]
- 12.Lin PM, Chen C-TC, Torzilli PA. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. 2004; (6): 485–496. [DOI] [PubMed] [Google Scholar]
- 13.Temple-Wong MM, Bae WC, Chen MQ et al. . Biomechanical, structural, and biochemical indices of degenerative and osteoarthritic deterioration of adult human articular cartilage of the femoral condyle. 2009; (11): 1,469–1,476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinwachs MR, Guggi T, Kreuz PC. Marrow stimulation techniques. 2008; (1): 26–31. [DOI] [PubMed] [Google Scholar]
- 15.Gille J, Anders S, Volpi P et al. . Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. 2013; (10): e49–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shetty AA, Kim SJ, Shetty V et al. . Autologous collagen induced chondrogenesis (ACIC: Shetty-Kim technique). A matrix based acellular single stage arthroscopic cartilage repair technique. 2016; (3): 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farjanel J, Schürmann G, Bruckner P. Contacts with fibrils containing collagen I, but not collagens II, IX, and XI, can destabilize the cartilage phenotype of chondrocytes. 2001; : S55–63. [DOI] [PubMed] [Google Scholar]
- 18.Gooding CR, Bartlett W, Bentley G et al. . A prospective, ranomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. 2006; (3): 203–210. [DOI] [PubMed] [Google Scholar]
- 19.Saris DB, Vanlauwe J, Victor J et al. . 29.4 Comparison of structural repair following characterized chondrocyte implantation or microfracture for the treatment of symptomatic cartilage defects of the knee. 2007; : B84. [Google Scholar]
- 20.Matsuda C, Takagi M, Hattori T et al. . Differentiation of human bone marrow mesenchymal stem cells to chondrocytes for construction of three-dimensional cartilage tissue. 2005; (1–3): 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gobbi A, Karnatzikos G, Scotti C et al. . One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. 2011; (3): 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klemt C. Tissue engineered cartilage with cultured chondrocytes and a collagen sponge matrix. : Stark G.B., Horch R., Táczos E. (eds) . Berlin: Springer; 1998. pp. 169–172. [Google Scholar]
- 23.Nishimoto S, Takagi M, Wakitani S et al. . Effect of chondroitin sulfate and hyaluronic acid on gene expression in a three-dimensional culture of chondrocytes. 2005; (1): 123–126. [DOI] [PubMed] [Google Scholar]
- 24.Saw K-Y, Anz A, Merican S et al. . Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. 2011; (4): 493–506. [DOI] [PubMed] [Google Scholar]
- 25.Heir S, Ulstein S, Årøen A et al. . Microfracture technique vs mosaic plasty: no difference in knee scores at 5-11 years follow-up in a prospective randomized clinical trial. 2013; (10): e136. [Google Scholar]
- 26.de Windt TS, Sorel JC, Vonk LA et al. . Early health economic modelling of single-stage cartilage repair. Guiding implementation of technologies in regenerative medicine. 2017; (10): 2,950–2,959. [DOI] [PubMed] [Google Scholar]
- 27.Lindahl A, Brittberg M, Peterson L. Health economics benefits following autologous chondrocyte transplantation for patients with focal chondral lesions of the knee. 2001; (6): 358–363. [DOI] [PubMed] [Google Scholar]
- 28.Benders KEM, Boot W, Cokelaere SM et al. . Multipotent stromal cells outperform chondrocytes on cartilage-derived matrix scaffolds. 2014; (4): 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piontek T, Ciemniewska-Gorzela K, Szulc A et al. . All-arthroscopic AMIC procedure for repair of cartilage defects of the knee. 2011; (5): 922–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trattnig S, Mamisch TC, Pinker K et al. . Differentiating normal hyaline cartilage from post-surgical repair tissue using fast gradient echo imaging in delayed gadolinium-enhanced MRI (dGEMRIC) at 3 Tesla. 2008; (6): 1,251–1,259. [DOI] [PubMed] [Google Scholar]


