Abstract
Introduction
One of the most feared complications of colorectal surgery is anastomotic leak. Numerous techniques have been studied in the hope of decreasing leakage. This study was designed to assess the handling characteristics of a novel adhesive tissue patch (TissuePatch™; Tissuemed, Leeds, UK) applied to colorectal anastomoses in a pilot study. This was with a view to assessing its potential role in aiding anastomotic healing in subsequent trials.
Methods
A patch was applied to colorectal anastomoses after the surgeon had completed the anastomosis and prior to abdominal closure. Handling characteristics and patient outcomes were recorded prospectively.
Results
Nine patients were recruited before the study was prematurely terminated. In one patient, the patch fell off and in another patient, the surgeon omitted to apply it. Six patients had significant postoperative problems (1 confirmed leak necessitating return to theatre and excision anastomosis, 3 suspicious of leak on computed tomography delaying discharge, 2 perianastomotic collections). One patient had an uneventful recovery.
Conclusions
Although the handling characteristics of this novel tissue patch were deemed satisfactory, it appears that wrapping a colorectal anastomosis with an adhesive hydrophilic patch has significant deleterious effects on anastomotic healing. This could be a consequence of the creation of a microenvironment between the patch and the anastomosis that impairs healing. Further research is required to better understand the mechanisms involved. At present, the use of such patches on colorectal anastomoses should be discouraged outside the confines of a well monitored trial.
Keywords: Colon, Surgery, Abdomen, Rectum, Large intestine, Small intestine
Anastomotic leak remains one of the most feared complications in colorectal surgery, with a prevalence of approximately 5% and considerable associated mortality.1 Not surprisingly, much has been written on the possible causes and prevention of anastomotic leakage but to date, no single intervention has been shown to be linked to significant and reliable benefit. It is disappointing that advances in surgical techniques have not led to a commensurate reduction in colorectal anastomotic leak rates.
In recent years, adhesive patches have been introduced that claim to convey clinical benefit when applied to varied surgical sites by buttressing these areas of surgical trauma. One such device is TissuePatch™ (Tissuemed, Leeds, UK). This is a hydrophilic self-adhesive tissue patch that has been used extensively in thoracic, head and neck, and neurosurgery in the UK and Europe2,3 with varied stated benefits including reduction of dural seepage and air leaks. To our knowledge, use of this patch has not yet been described in association with gastrointestinal anastomoses. Additionally, at the time of setting up this study, it was not known whether the sound deployment of such hydrophilic patches during potentially moister abdominal and pelvic procedures would even be technically feasible on the bowel.
Our study was designed to assess the safety and handling characteristics of the patch in wrapping anastomoses during abdominal surgery. This small scale pilot study was conceived as essential preliminary groundwork prior to the ultimate intention of setting up a larger randomised clinical trial aspiring to investigate the efficacy of this particular patch on minimising gastrointestinal anastomotic leaks in the eventuality that TissuePatch™ application on the bowel was deemed both technically achievable and clinically safe.
Methods
In this observational study, data were collected prospectively. The study was granted ethical approval by the South Yorkshire Research Ethics Committee, and sponsored by the research and development panel at York Teaching Hospital NHS Foundation Trust. The primary endpoint was the handling characteristics of the TissuePatch™ on application to a gastrointestinal anastomosis. Secondary endpoints comprised parameters of safety including clinical outcomes and complications. All potential adverse events and postoperative length of hospital stay were recorded.
All patients undergoing a colorectal procedure (open or laparoscopic) involving creation of an intestinal anastomosis were eligible for inclusion in the study. Those under 18 years of age and pregnant females were excluded. Subject to these criteria, this pilot study was set up with the intention to recruit 40 consecutive patients.
The participants were identified preoperatively and approached in a preassessment clinic, where they were given written and verbal information about the study. All participants provided written informed consent. All anastomoses were fashioned using standard techniques as deemed appropriate by one of three operating surgeons. No effort was made to standardise anastomotic techniques or influence the surgical teams’ decisions in any other respect. A single film of TissuePatch™ (measuring 50 mm × 100 mm × 0.04 mm) was placed around the completed anastomosis immediately prior to abdominal closure. Abdominal drains were not used in accordance with our standard practice. All patients were managed using a validated perioperative enhanced recovery protocol.4
The operating surgeon completed a data sheet on the handling characteristics of the patch immediately after surgery. The characteristics assessed comprised ease of handling and application, adherence to the anastomosis, memory, laparoscopic use and convenience for nursing staff. The surgeon was asked to grade these characteristics on a scale of 1–10, with a grade of 1–3 equating to ‘easy’, a grade of 4–6 equating to ‘moderately easy’, a grade of 7–9 equating to ‘difficult’ and a grade of 10 equating to ‘very difficult’. Investigations were requested by the overseeing clinical teams as deemed clinically appropriate and with no input from the researchers. Outcomes from these investigations were recorded prospectively.
Throughout their hospital stay, each patient was reviewed at least once daily by the research team until hospital discharge or transfer to another institution. Patients were followed up to the point of discharge/transfer or for 30 days postoperatively (whichever was the longer) so as to capture early readmissions. Any complications during this time period (including death) were recorded prospectively.
Results
A total of nine patients (Table 1) were recruited before the study was terminated because of perceived adverse clinical outcomes associated with patch placement. Eight patients had TissuePatch™ applied and seven had the patch left in situ. Stapled side-to-side anastomoses were performed following right hemicolectomy and reversal of stoma procedures while end-to-end anastomosis anastomoses were fashioned for all left-sided procedures. All left-sided and anterior resections were performed via open surgery and where right-sided resections were undertaken laparoscopically, the anastomosis was created in an extracorporeal fashion following removal of the specimen.
Table 1.
Patient characteristics and operative details
| Patient | TissuePatch™ applied | Age | Pathology | Location of anastomosis | Operation | Postoperative tumour staging | TissuePatch™ left in situ |
| 1 | Yes | 76 years | Cancer | Ascending colon | RH | pT2 pN0 | Yes |
| 2 | Yes | 59 years | Cancer | Caecum | RH | pT3 pN0 | Yes |
| 3 | No | 80 years | Post AR | Terminal ileum | IC | N/A | No |
| 4 | No | 47 years | Diverticulosis | Sigmoid colon | AR | N/A | No |
| 5 | Yes | 73 years | Cancer | Ascending colon | RH | pT2 pN1 | Yes |
| 6 | Yes | 75 years | Cancer | Ascending colon | RH | pT4 pN1 | Yes |
| 7 | Yes | 65 years | Cancer | Ascending colon | RH | pT3 pN1 | Yes |
| 8 | Yes | 70 years | Cancer | Descending colon | AR | pT4 pN2 | Yes |
| 9 | Yes | 67 years | Diverticulosis | Sigmoid colon | AR | N/A | Yes |
AR = anterior resection; RH = right hemicolectomy; IC = ileostomy closure
In one patient, patch placement was inadvertently omitted after placement was interrupted to deal with anastomotic bleeding. In the remaining eight patients, the handling characteristics of the patch were largely satisfactory (Table 2). TissuePatch™ was considered an easy product to handle and apply, displayed good adherence, was easy to trim when necessary and allowed complete coverage of the anastomosis.
Table 2.
Results of handling characteristics after attempted application in 8 patients
| Characteristics assessed | Options | Number of patients |
| How easy was TissuePatch™ to handle? | Easy | 6 |
| Moderately easy | 2 | |
| Difficult | 0 | |
| Very difficult | 0 | |
| N/A | 0 | |
| How easy was TissuePatch™ to apply around the anastomosis? | Easy | 7 |
| Moderately easy | 1 | |
| Difficult | 0 | |
| Very difficult | 0 | |
| N/A | 0 | |
| How well did TissuePatch™ adhere to the target tissue? | Very well | 5 |
| Well | 2 | |
| Poor | 0 | |
| Very poor | 1 | |
| N/A | 0 | |
| Did TissuePatch™ have a memory that affected application? | No memory | 8 |
| Minimal memory | 0 | |
| Moderate memory | 0 | |
| Significant memory | 0 | |
| N/A | 0 | |
| How easy was TissuePatch™ to use laparoscopically? | Easy | 0 |
| Moderately easy | 0 | |
| Difficult | 0 | |
| Very difficult | 0 | |
| N/A | 8 | |
| How easy was TissuePatch™ to handle with gloves/instruments? | Easy | 8 |
| Moderately easy | 0 | |
| Difficult | 0 | |
| Very difficult | 0 | |
| N/A | 0 | |
| How easy was TissuePatch™ to use for theatre staff from packaging to application? | Easy | 8 |
| Moderately easy | 0 | |
| Difficult | 0 | |
| Very difficult | 0 | |
| N/A | 0 | |
| How easy was TissuePatch™ to cut/trim to shape? | Easy | 4 |
| Moderately easy | 0 | |
| Difficult | 0 | |
| Very difficult | 0 | |
| N/A | 4 | |
| Overall opinion | Easy | 6 |
| Moderately easy | 2 | |
| Difficult | 0 | |
| Very difficult | 0 | |
| N/A | 0 |
The patch failed to adhere in one of these eight patients. In this case, the patch was removed prior to abdominal closure and was therefore not left in situ around the anastomosis. As a result, complication and length of stay data are only presented for the remaining seven patients where TissuePatch™ was left in situ after surgery. Of these, six (86%) developed significant complications and only one made an uneventful recovery. All patients with a complication underwent abdominal computed tomography in the postoperative period: one patient had a clinical leak necessitating a return to theatre, excision of the friable anastomosis (with an obvious defect in the staple line) and formation of a defunctioning stoma; three had imaging reported as a contained leak with extraluminal air that was managed conservatively with intravenous antimicrobial therapy; and two showed perianastomotic collections without extraluminal air and were managed successfully with antimicrobials.
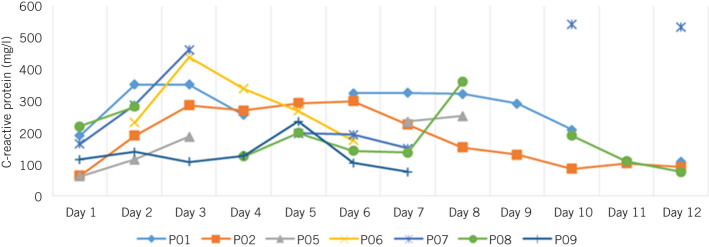
The median length of hospital stay for the seven patients with an indwelling TissuePatch™ was 9 days (interquartile range: 3–18 days). One patient was discharged after 3 days and readmitted shortly thereafter with a contained anastomotic leak. All seven patients demonstrated a rise in C-reactive protein of >150mg/l in their early postoperative course (Fig 1). There were no recorded deaths within 30 days of surgery.
Figure 1.
C-reactive protein values over time in all 7 patients with a patch in situ
Discussion
This pilot study was designed primarily to assess the handling characteristics and safety of a proprietary hydrophilic adhesive tissue patch during abdominal procedures involving a gastrointestinal anastomosis. This patch had been used extensively in other fields (particularly in cardiothoracic and neurosurgery) with apparently good results but its applicability and safety for gastrointestinal surgery was unknown. Based on the results of this small scale pilot study, TissuePatch™ appears to be easy to handle and deploy around a gastrointestinal anastomosis. However, the study was discontinued prematurely because of an alarming incidence of perianastomotic problems (86%). Only one patient had an uneventful recovery. All patients experienced a considerable rise in the inflammatory marker C-reactive protein following surgery.
Many previous studies have reported on the use of adhesive sealants, barriers and patches (both in animal and in human studies) with conflicting results. The majority of these have not described significant morbidity related to the use of adhesives, and some have concluded that topically applied patches and adherent substances do not interfere with anastomotic healing.5–12
In contrast, some authors have reported morbidity when using sealants and adhesives in animal studies. Chmelnik et al evaluated the use of TachoSil® (a fibrin coated collagen patch; Takeda, Tokyo, Japan) in small diameter intestinal anastomoses in rats.13 This was a well designed prospective study where the authors found a significantly higher incidence of complications associated with the patch and microabscess formation in the sealed anastomoses. Giuratrabocchetta et al investigated the use of a synthetic glue or a fibrin sealant in colonic anastomoses in rabbits and compared the results with a control group.14 No differences were identified in terms of anastomotic healing but the use of either glue was linked to a more intense inflammatory response at the site of the anastomosis.
Van der Ham et al studied the effect of fibrin glue in a rat model of both complete and incomplete anastomoses in the colon.15 They concluded that fibrin glue inhibited wound healing and that it was associated in particular with reduced collagen content at the anastomosis. These results were mirrored by Byrne et al, who also investigated the use of fibrin glue in rats, finding that the glue impaired anastomotic healing.16 They postulated that it provided resistance to ingrowth of vascular granulation tissue.
Published information pertaining to human studies and the use of fibrin glue or sealants is even sparser. In 2007 Wang et al described the application of a fibrin sealant to anastomoses in 48 patients with existing abdominal sepsis, concluding that it might be useful in preventing leakage.17 More recently, Morks et al reported the results of a pilot study that investigated the use of a biodegradable sheath that forms a protective layer in the bowel lumen.18 Their preliminary results were satisfactory but a multicentre randomised clinical trial of this method has shown no statistical difference in the leak rate.19
The largest study relevant to this topic was reported by Beck et al, who described the use of an adhesion barrier in abdominal and pelvic surgery.20 This was a multicentre randomised controlled trial looking into the safety of Seprafilm® (Genzyme, Cambridge, MA, US) in 1,791 patients with benign disease. They found a higher incidence of anastomotic leak or of an adverse event related to a leak (eg abscess, peritonitis or fistula) in the subgroup where Seprafilm® was placed directly on the staple or suture line of a fresh bowel anastomosis than in those who did not receive the barrier (13.5% vs 6.2%). The authors concluded that Seprafilm® is safe to use in abdominopelvic surgery but that application around a fresh intestinal anastomosis should be avoided.19 To our knowledge, no published data exist on the use of hydrophilic patches including TissuePatch™ but the results from our small scale pilot study concord with those from similar literature.
The mechanism by which application of an adhesive patch may be deleterious to healing is unclear. One possible explanation is that application of a patch around a fresh anastomosis precludes local adhesion formation and isolates the anastomosis from intraperitoneal defence mechanisms. This accords with the observations of Beck et al, who suggested that the development of adhesions prevents adverse consequences from minor leaks.20 Another possibility is that the patch itself stimulates an intense inflammatory response, which deters healing by inhibiting fibroblast function and deposition of collagen. Finally, it is possible that application of a ‘wrap’ leads to the creation of a microenvironment that adversely affects healing. This hypothesis is supported by recent evidence implicating certain intestinal bacteria (eg Enterococcus and Escherichia) in the aetiology of anastomotic breakdown.21,22
Study limitations
Significant limitations are recognised with this study. Most importantly, the numbers were small as a consequence of early termination of the study. It could be argued that attributing complications and longer hospital stays to the use of the patch is unjustified given the small numbers. We consider this highly unlikely; our published results of outcomes after colorectal surgery have consistently reported a median duration of stay of ≤5 days with few major complications.4 A rolling departmental audit of surgical outcomes (data not published) persistently demonstrated a gastrointestinal anastomotic leak rate below 5%. Immediately after pausing this trial, complication rates returned to the departmental baseline. As a result, early termination of the study appeared to be the only ethically justifiable option.
It is important to emphasise that this study was not designed to have anastomotic leakage as an endpoint. It was a pilot study intended to assess applicability and safety with a view to performing a larger efficacy study at a later date. Mindful of the widespread use of these as well as other patches in non-gastrointestinal surgical specialties, their ever increasing distribution and availability, and the limitations posed by the small numbers of patients involved, it was felt that the potential serious deleterious outcomes to patients by applying such patches to gastrointestinal anastomoses justified wider distribution of our results so as to alert the surgical community to these possible concerns.
Conclusions
Our study suggests that the wrapping of colonic anastomoses with an adhesive patch is technically possible but likely dangerous and should therefore be avoided, especially outside the tightly controlled environment of a clinical trial.
References
- 1.Pahlman L, Williams N, Monson J. Surgical Management of Rectal Cancer : Keighley MR, Williams NS . 2nd edn Philadelphia: Saunders; 1999. p1,134. [Google Scholar]
- 2.Ang K, Oey I, Rathinam S. ‘Drain Dance’ technique for thoracoscopic application of Tissuepatch to reduce postoperative air-leak saves time and cost. 2013; (Suppl 1): O218. [Google Scholar]
- 3.von der Brelie C, Soehle M, Clusmann HR. Intraoperative sealing of dura mater defects with a novel, synthetic, self adhesive patch: application experience in 25 patients. 2012; : 231–235. [DOI] [PubMed] [Google Scholar]
- 4.Gatt M, Anderson AD, Reddy BS et al. Randomized clinical trial of multimodal optimization of surgical care in patients undergoing major colonic resection. 2005; : 1,354–1,362. [DOI] [PubMed] [Google Scholar]
- 5.Renz BW, Leitner K, Odermatt E et al. PVA gel as a potential adhesion barrier: a safety study in a large animal model of intestinal surgery. 2014; : 349–357. [DOI] [PubMed] [Google Scholar]
- 6.Zhou B, Ren J, Ding C et al. Protection of colonic anastomosis with platelet-rich plasma gel in the open abdomen. 2014; : 864–868. [DOI] [PubMed] [Google Scholar]
- 7.Vuocolo T, Haddad R, Edwards GA et al. A highly elastic and adhesive gelatin tissue sealant for gastrointestinal surgery and colon anastomosis. 2012; : 744–752. [DOI] [PubMed] [Google Scholar]
- 8.Sheldon HK, Gainsbury ML, Cassidy MR et al. A sprayable hyaluronate/carboxymethylcellulose adhesion barrier exhibits regional adhesion reduction efficacy and does not impair intestinal healing. 2012; : 325–333. [DOI] [PubMed] [Google Scholar]
- 9.Tsereteli Z, Sporn E, Geiger TM et al. Placement of a covered polyester stent prevents complications from a colorectal anastomotic leak and supports healing: randomized controlled trial in a large animal model. 2008; : 786–792. [DOI] [PubMed] [Google Scholar]
- 10.Erturk S, Yuceyar S, Temiz M et al. Effects of hyaluronic acid-carboxymethylcellulose antiadhesion barrier on ischemic colonic anastomosis: an experimental study. 2003; : 529–534. [DOI] [PubMed] [Google Scholar]
- 11.Mutter D, Aprahamian M, Tiollier J et al. Evaluation of human collagen biomaterials in the healing of colonic anastomoses in dogs. 1997; : 287–295. [PubMed] [Google Scholar]
- 12.Medina M, Paddock HN, Connolly RJ, Schwaitzberg SD. Novel antiadhesion barrier does not prevent anastomotic healing in a rabbit model. 1995; : 179–186. [DOI] [PubMed] [Google Scholar]
- 13.Chmelnik M, Lasch L, Weih S et al. Anastomotic sealing with a fibrin-coated collagen patch in small-diameter bowel. 2011; : 685–691. [DOI] [PubMed] [Google Scholar]
- 14.Giuratrabocchetta S, Rinaldi M, Cuccia F et al. Protection of intestinal anastomosis with biological glues: an experimental randomized controlled trial. 2011; : 153–158. [DOI] [PubMed] [Google Scholar]
- 15.van der Ham AC, Kort WJ, Weijma IM, Jeekel H. Transient protection of incomplete colonic anastomoses with fibrin sealant: an experimental study in the rat. 1993; : 256–260. [DOI] [PubMed] [Google Scholar]
- 16.Byrne DJ, Hardy J, Wood RA et al. Adverse influence of fibrin sealant on the healing of high-risk sutured colonic anastomoses. 1992; : 394–398. [PubMed] [Google Scholar]
- 17.Wang X, Ren J, Zhu W et al. Fibrin sealant prevents gastrointestinal anastomosis dehiscence in intra-abdominal sepsis. 2007; : 27–31. [PubMed] [Google Scholar]
- 18.Morks AN, Havenga K, ten Cate Hoedemaker H et al. Thirty-seven patients treated with the C-seal: protection of stapled colorectal anastomoses with a biodegradable sheath. 2013; : 1,433–1,438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakker I, Morks A, ten Cate Hoedemaker H et al. Randomized clinical trial of biodegradeable intraluminal sheath to prevent anastomotic leak after stapled colorectal anastomosis. 2017; : 1,010–1,019. [DOI] [PubMed] [Google Scholar]
- 20.Beck DE, Cohen Z, Fleshman JW et al. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. 2003; : 1,310–1,319. [DOI] [PubMed] [Google Scholar]
- 21.Shogan BD, Smith DP, Christley S et al. Intestinal anastomotic injury alters spatially defined microbiome composition and function. 2014; : 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shogan BD, Carlisle EM, Alverdy JC, Umanskiy H. Do we really know why colorectal anastomoses leak? 2013; : 1,698–1,707. [DOI] [PubMed] [Google Scholar]