Abstract
BACKGROUND
For severe cutaneous adverse reactions (SCARs) associated with multiple antibiotics dosed concurrently, clinical causality is challenging and diagnostic approaches are limited, leading to constricted future antibiotic choices.
OBJECTIVE
To examine the combined utility of in vivo and ex vivo diagnostic approaches at assigning drug causality in a cohort of patients with antibiotic-associated (AA)-SCARs.
METHODS
Patients with AA-SCARs were prospectively recruited between April 2015 and February 2017. In vivo testing (patch testing or delayed intradermal testing) was performed to the implicated antibiotic(s) at the highest nonirritating concentration and read at 24 hours through 1 week. Ex vivo testing used patient peripheral blood mononuclear cells (PBMCs) stimulated with a range of pharmacologically relevant concentrations of implicated antibiotics to measure dose-dependent IFN-g release from CD4D and CD8D T cells via an enzyme-linked immunoSpot assay.
RESULTS
In 19 patients with AA-SCARs, combined in vivo and ex vivo testing assigned antibiotic causality in 15 (79%) patients. Ten patients (53%) with AA-SCARs were positive on IFN-g release enzyme-linked immunoSpot assay, with an overall reported sensitivity of 52% (95% CI, 29-76) and specificity of100% (95% CI, 79-100), with improved sensitivity noted in acute (within 1 day to 6 weeks after SCAR onset) testing (75%) and in patients with higher phenotypic scores (59%). There was increased use of narrow-spectrum beta-lactams and antibiotics from within the implicated class following testing in patients with a positive ex vivo or in vivo test result.
CONCLUSIONS
We demonstrate the potential utility of combined in vivo and ex vivo testing in patients with AA-SCARs to assign drug causality with high specificity.
Keywords: Antibiotic allergy, Delayed hypersensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms
Severe cutaneous adverse reactions (SCARs), such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS), are associated with significant mortality and short-term and long-term morbidity1,2 and may be caused by a range of medications including antibiotics.1 SJS and TEN are considered the same condition representing different severities across a spectrum. The hallmarks of SJS/TEN are skin detachment (1%-10% for SJS, 10%-30% for SJS/TEN overlap, and >30% for TEN) and blistering of mucous membranes accompanied by other serious manifestations of systemic involvement.3 Patients experiencing DRESS exhibit an exanthematous rash, fever, internal organ involvement, and possible eosinophilia.3 Acute generalized exanthematous pustulosis (AGEP), another SCAR, is an acute widespread erythematous reaction that is followed by a pustular eruption together with fever.3 To avoid the recurrence of SCARs, culprit drugs are traditionally avoided in the future.
Often in SCARs multiple antibiotics are prescribed concurrently, creating uncertainty in ascribing causality, which can lead to significant constriction of future therapeutic choices.1,2 Current diagnostic options such as in vivo patch testing (PT) or delayed intradermal skin testing (IDT) have been limited by lack of experience, lack of validated concentrations and approaches, limited availability to providers, and poor sensitivity.4–6 Ex vivo and in vitro testing using a range of research platforms including lymphocyte transformation testing and IFN-g release enzyme-linked immunoSpot (ELISpot) assay have been used in small cohorts of antibiotic-associated (AA) delayed hypersensitivities with varied success.7–12 Furthermore, there is scarce published literature on the utility of combination in vivo and ex vivo/in vitro approaches such as PT and/or delayed IDT with IFN-g release ELISpot assay or lymphocyte transformation testing in AA-SCARs. The objectives of this pilot study were to examine the potential combined utility of IFN-g release ELISpot assay and in vivo skin testing in defining antibiotic causality assessments in patients with AA-SCARs.
METHODS
Patient recruitment and definitions
Study patients were prospectively recruited at Austin Health, Alfred Health and Peter MacCallum Cancer Centre from April 2015 until February 2017. Inclusion criteria were patients 18 years or older with a history of AA-SCARs. Patients with AA-SCARs with an antibiotic identified as the primary implicated drug(s) and corresponding Naranjo adverse drug reaction score of 5 or more (probable adverse drug reaction)13 were recruited. For the SJS/TEN phenotypes, an ALDEN score of 4 or more was required (as per published definitions14), with an antibiotic having to carry the highest ALDEN (algorithm for assessment of drug causality for epidermal necrolysis) score. For phenotypes of DRESS and AGEP, a RegiSCAR score of 2 or more and an AGEP score of 2 or more, respectively, were required.15,16. All cases had the diagnosis and phenotype confirmed by a dermatologist and were reviewed in the respective hospital antibiotic allergy clinics (Austin Health and Peter MacCallum Cancer Centre). Patients with an alternative viral, bacterial, or autoimmune SCAR etiology were excluded, evidenced by any one of the following: (1) positive plasma PCR for herpesvirus (HSV1/2, cytomegalovirus, EBV) or Enterovirus, (2) positive Mycoplasma species PCR (respiratory specimen) or serology, or (3) detectable antinuclear antigen antibody titer of more than 1:64. Patients were also excluded if skin biopsy (histopathology or direct immunofluorescence) was not consistent with a drug reaction or clinical picture was consistent with an alternative diagnosis. There were 2 control groups: (1) antibiotic-tolerant controls, patients who had tolerated at least 4 consecutive weeks of single antibiotic at therapeutic intra-venous or oral dosing, and (2) healthy random donors, patients with AA-SCARs tested against antibiotics that previously resulted in a positive IFN-g release ELISpot assay.
Peripheral blood mononuclear cell (PBMCs) were isolated from whole heparinized blood of patients with AA-SCARs, tolerant controls, and healthy donors, washed, and counted. PBMCs were stored at 80oC in 90% heat-inactivated FBS and 10% dimethyl sulfoxide until use for IFN-g release ELISpot assay. Patients were followed for adverse events and antibiotic prescribing for 90 days after testing. Ingestion challenge was not performed as routine after ex vivo and in vivo testing; rather, it was based on acute antibiotic requirements. This study was approved by the Austin Health Ethics Committee (HREC/15/Austin/75) and laboratories where this testing was performed had independent review board approvals (Institute for Immunology & Infectious Diseases, Murdoch [Murdoch University HREC 2011/056] University and Vanderbilt University Medical Center).
Skin testing (in vivo)
IDT and PT were performed for all implicated antibiotics at least 6 weeks after AA-SCAR onset using previously recommended nonirritating antibiotic concentrations.17–19. In patients in whom an intravenous formulation of the implicated antibiotic was not available or incompatible with IDT and/or in the setting of SJS/TEN, PT was performed in isolation. IDT was performed on the volar forearm of the skin with 0.02 mL of antibiotic reagent or normal saline (negative control) and read after 24 and 48 hours. A positive IDT result was considered when there was evidence of dermal induration and erythema at the injection site that exceeded 5 mm from baseline. In PT, a patch was applied to the upper back and removed at 48 hours and re-read at 72 hours, using white petroleum jelly as drug carrier in all cases of the antibiotics tested and negative control (Figure 1).
Figure 1.
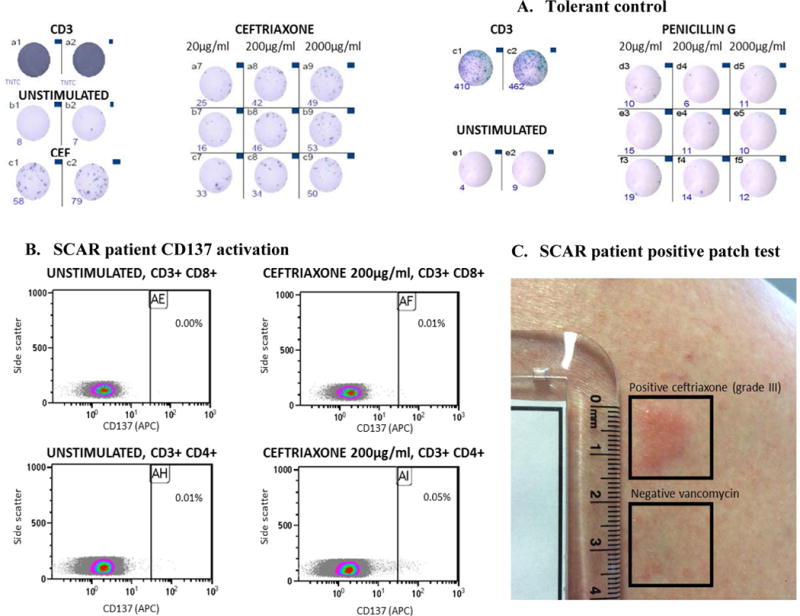
Representative positive (A. SCAR patient, S1) and negative (B. tolerant control) IFN-γ release ELISpot. Representative CD137 T-cell activation (C.) and positive patch test of (D.) of SCAR patient S1.
Abbreviations: CD3, anti-CD3 antibodies (polyclonal T-cell activator); CEF, peptide pool consisting of 23 viral peptides (EBV, CMV and influenza) which stimulated human CD8+ T cells.
A, IFN-γ release ELISpot of positive SCAR patient. B, IFN-γ release ELISpot of antibiotic tolerant control. Representative images of IFN-γ release spots in 96 well plate in the presence of, (i) CD3 & 200,000 cells/well, media and 200,000 cells/well (unstimulated) and implicated drug (A. SCAR patient) and tolerate drugs (B. Tolerant Control) [left to right of image]. In A, the addition of a physiological control (CEF) is also demonstrated. C, Drug-induced T-cell activation (flow cytometry) from patient demonstrated in A. D, Representation of positive patch test to ceftriaxone 10% for patient represented in 1A and 1C
IFN-g release ELISpot assay (ex vivo)
IFN-g release in response to overnight incubation with the implicated antibiotic(s) was performed by ELISpot assay in triplicate from thawed PBMCs (rested overnight) as previously described.20 PBMCs (200,000 cells per well) were incubated with investigated drugs at concentrations representative of peak serum concentrations (Cmax) and a level 10-fold higher than Cmax,21 avoiding concentrations associated with T-cell cytotoxicity (data not shown). Testing was also performed with a negative (unstimulated) and positive control (anti-CD3 antibody; Mabtech, Victoria, Australia) in duplicate. The mean number of spots for the test and unstimulated wells were calculated. A positive response was defined as greater than 50 spot-forming unit (SFU)/million cells after background (unstimulated control) removal as per previously published definitions20,22 (Figure 1).
Indeterminate results were defined by failure of the positive CD3 control. In addition to IFN-g release ELISpot assay, in patients with a positive result (SFU/million cells >50) and available PBMCs from successive time points, T-cell stimulation was assessed via flow cytometry by measuring upregulation of the early activation marker CD137, a member of the TNF receptor family, on viable CD3þCD8þ and CD3þCD4þ T cells (Figure 1).
Statistical analysis
Categorical variables were summarized and compared between groups using the chi-square test or Fisher exact test. Continuous variables were compared using a student t test or Wilcoxon signed-rank test. A P value of less than .05 (2-tailed) was deemed statistically significant. IFN-g release ELISpot assay’s diagnostic performance was expressed in terms of sensitivity and specificity, with indeterminate results included in the analysis. Statistical analyses were performed using Stata 13.0 (Statacorp, College Station, Texas).
RESULTS
Baseline demographic characteristics
Nineteen patients with AA-SCARs meeting the inclusion/exclusion criteria and 16 antibiotic-tolerant controls were recruited. There was no statistically significant difference (P > .05) in clinical characteristics between study patients and tolerant controls (Table I). The clinical characteristics, implicated antibiotics, and phenotypic scores are summarized in Table II. From the 19 study patients, there were 36 implicated antibiotics, with 12 patients (63%) with more than 1 implicated antibiotic. The most commonly implicated antibiotics were vancomycin (10 of 19 [52%]) and piperacillin-tazobactam (8 of 19 [42%]). The phenotypes encountered were DRESS (14 of 19 [73%]), SJS/TEN (4 of 19 [21%]), and AGEP (1 of 19 [5%]) (Table II).
Table 1.
Baseline characteristics of patients and controls
| Variable | Patients (n = 19) |
Tolerant Controls (n = 16) |
P value |
|---|---|---|---|
| Age, years, median (IQR) | 58 (51,71) | 67.5 (55,76) | 0.93 |
| Sex (M: F) | 12:7 | 10:6 | >0.99 |
| Immunocompromiseda | 6 (32) | 6 (38) | 0.73 |
| Caucasian | 17 (89) | 13 (81) | 0.64 |
| Age-adjusted CCI, median (IQR) | 3 (1,4) | 4 (2,5.75) | 0.23 |
| Lymphopeniab | 3 (16) | 2 (11) | >0.99 |
| Implicated antibiotic(s) present at time of blood drawn | 1 (5) | 15 (94) | 0.001 |
| Multiple implicated antibiotics | 12 (63) | NA | - |
| Multiple implicated drugs | 14 (74) | NA | - |
| Skin test latencyd, days, median, (IQR) [range] | 193 (69, 470) [53-3650] | NA | - |
| ELISpot latencyd, days, median (IQR) [range] | 138.5 (62, 504) [3, 3650] | NA | - |
Values are given as No. (%), unless otherwise stated.
Abbreviations: IQR, interquartile range; M, male; F, female; CCI, Charlson comorbidity index.
Immunocompromised– transplant recipient, haematological or oncological malignancy (last 5 years), steroids > 10 mg prednisolone equivalent per day, connective tissue or autoimmune condition.
Lymphopenia defined as a total white blood cell count < 1 units
A point within at least 5 drug half-lives of the last drug administration in patients (implicated antibiotic[s]) and controls (tolerated antibiotic).
This was taken as the time from onset of SCAR phenotype to skin testing being performed (“skin test latency”) or PBMC collection (“ELISpot latency”).
Table 2.
Summary of cohort clinical characteristics, SCAR history and outcomes post testing
| Study No. | Age-Sex | ICHa | SCAR Phenotype |
Culprit antibiotics(s) | Ex vivo or in vivo positive | Antibiotic use post testing | ||
|---|---|---|---|---|---|---|---|---|
| Status | Immunosuppression | Antibiotic(s) | Phenotypic Scoreb | |||||
| S1 | 72F | N | DRESS | CEF, VAN | 6 | Yes | VAN, AMP, FLU | |
| S2 | 20M | N | TEN | TEIC | 4 | Yes | FLU | |
| MER | 4 | |||||||
| VAN | 4 | |||||||
| S3 | 43M | N | SJS | TMP-SMXc | 5 | No | NIL | |
| S4 | 48M | N | DRESS | TMP-SMX | 4 | No | DOX | |
| S5 | 83M | Y | ITP/Prednisolone | SJS | CEF | 4 | No | MER, DOX, CIP, VAN |
| PIP-TAZ | 3 | |||||||
| S6 | 38F | N | DRESS | PIP-TAZ, VAN | 7 | Yes | CEFU | |
| S7 | 74M | N | DRESS | CEFZ, MER, PENG | 5 | Yes | PENV, FLU | |
| S8 | 62M | Y | NHL | DRESS | PIP-TAZ | 4 | Yes | CEPH |
| S9 | 57M | Y | AML/chemotherapy | DRESS | VAN, PIP-TAZ, MER | 6 | Yes | PIP-TAZ, MER |
| S10 | 51F | N | DRESS | VANC, CEFZ | 6 | Yes | NIL | |
| S11 | 59F | N | DRESS | AMP | 4 | Yes | NIL | |
| S12 | 52F | N | DRESS | VAN, PIP-TAZ, ADF | 4 | Yes | ADF, MER | |
| S13 | 53F | N | AGEP1 | MET | 8 | Yes | PENV | |
| AGEP2 | VAN, CIP | |||||||
| S14 | 60M | Y | AML/chemotherapy | DRESS | VAN, PIP-TAZ | 4 | No | CIP |
| S15 | 58M | N | DRESS | VAN, CIP | 6 | Yes | PENV | |
| S16 | 75M | Y | DRESS/Prednisolone | DRESS | VAN, PIP-TAZ | 2 | Yes | CEPH |
| S17 | 66M | Y | HCL | DRESS | TMP-SMXc | 4 | Yes | NIL |
| S18 | 71M | N | DRESS | PIP-TAZ, AMP | 2 | Yes | AMP | |
| S19 | 54F | N | SJS | VAN | 4 | Yes | PENG | |
Abbreviations: ICH, immunocompromised host; SCAR, Severe Cutaneous Adverse Drug Reaction; N, no; Y, yes; ITP, immune mediated thrombocytopenia; NHL, non-Hodgkin lymphoma; AML, acute myeloid leukemia; DRESS, drug reaction with eosinophilia and systemic symptoms; HCL, hairy cell leukemia; M, male; F, female; AMP, ampicillin; ADF, amoxicillin clavulanate; CEF, ceftriaxone; CEFZ, cefazolin, CEFU, cefuroxime; CEFT, ceftazidime; CEPH, cephalexin; CIP, ciprofloxacin; FLU, flucloxacillin; MER, meropenem; MET, metronidazole; PENG, benzylpenicillin; PENV, penicillin VK; PIP-TAZ, piperacillin-tazobactam; TEIC, teicoplanin; VAN, vancomycin; 4NO, 4-Nitro sulfamethoxazole; TMP, trimethoprim, SMX, sulfamethoxazole; TMP-SMX, trimethoprim-sulfamethoxazole; FLU, flucloxacillin; Nil, no antibiotics required; DOX, doxycycline
ICH: Transplant recipient, haematological or oncological malignancy (last 5 years), steroids > 10mg prednisolone equivalent per day, connective tissue or autoimmune condition. Immunosuppression was defined as that the patient was receiving at time of PBMC collection.
Phenotypic scores as per published guidelines for SJS/TEN (ALDEN), DRESS (RegiSCAR) and AGEP.
Patient S3 had ibuprofen implicated in causality, ALDEN score (3) was lower than that of TMP-SMX. Patient S18 had cladribine and allopurinol implicated, also with lower ALDEN scores (3) that TMP-SMX.
Skin testing results (in vivo)
Seventeen patients (89%) underwent skin testing, PT only (21%; 4 of 19), IDT only (42%; 8 of 19), or combined PT/IDT (26%; 5 of 19). Two patients declined skin testing (S3 and S15), and of the 4 patients who underwent PT without IDT, 3 had SJS/TEN and 1 had multiorgan involvement DRESS. Fifty-two percent of patients (9 of 17) were positive on skin testing, 44% (4 of 9) on PT, and 100% (9 of 9) on IDT. All PT results were confirmed positive on follow-up IDT (4 of 4 [100%]) (Table III). All IDT positive results were positive within 24 hours of being administered and read. Of those skin tested, there was a higher rate of skin test positivity in DRESS compared with SJS/TEN phenotypes (8 of 13 [61%] vs 0 of 3 [0%], P .20). Of the skin test positive, beta-lactams predominated over non-ebeta-lactams (7 of 9 [77%] vs 2 of 9 [22%], P .05) and there were no positive in vivo test results to vancomycin, despite it being implicated in 58% (11 of 19) of the patients. There was a trend toward increased skin test positivity if performed more than 3 months after SCAR onset compared with less than 3 months (7 of 11 [63%] vs 2 of 6 [33%]; P .34). There was no difference in skin test positivity in immunocompromised versus nonimmunocompromised patients (6 of 11 [54%] vs 3 of 6 [50%]; P 1). The median time from SCAR onset to skin testing was longer in patients with positive skin test results than in patients with negative skin test results (301 days vs 100 days; P .07), even when patients who ultimately tested positive to vancomycin on ex vivo testing were excluded (301 days vs 146 days; P .33). There were no systemic events noted from PT or IDT in this cohort.
Table 3.
Summary of number of in vivo, ex vivo and combined testing results in patients with a history of antibiotic-associated SCAR
| Patient study No. | Skin test results (in vivo) | IFN-γ ELISpot results (ex vivo)b | Combined results | ||||
|---|---|---|---|---|---|---|---|
| Modality positive: Antibiotic(s)a | Concentration (mg/ml) | Interpretation | Antibiotics positive | Concentrations (μg/ml)c | Interpretation | In vivo or ex vivo | |
| S1 | PT/IDT: CEF | 10%/2.5 | Positive (CEF) | CEF | 20,200,2000 | Positive (CEF) | Positive |
| S2 | Nil | Negative | TEIC | 25, 50, 100 | Positive (TEIC) | Positive | |
| S3 | NP | NP | Nil | - | Negative | Negative | |
| S4 | Nil | Negative | Nil | - | Negative | Negative | |
| S5 | Nil | Negative | Nild | - | Indeterminate | Negative | |
| S6 | IDT: AMP, PIP-TAZ | 25, 4.5 | Positive (PIP-TAZ, AMP) |
PIP-TAZ | 37.5/300, 375/3000 | Positive (PIP-TAZ) | Positive |
| S7 | IDT: CEFZ, MERO | 1, 2.5 | Positive (CEFZ, MERO) | CEFZ MERO |
20, 200, 200 20, 200 |
Positive (CEFZ, MERO) | Positive |
| S8 | IDT: AMP, FLU, PENG, PIP-TAZ | 25, 2, 10, 4.5 | Positive (AMP, FLU, PENG, PIP-TAZ) |
AMP FLUC PENG PIP-TAZ |
200, 2000 200 20, 200, 2000 18.75/150, 187.5/1500 |
Positive (PENICILLINS) | Positive |
| S9 | Nil | Negative | VAN | 5, 50, 500 | Positive (VAN) | Positive | |
| S10 | Nil | Negative | VAN | 5, 50, 500 | Positive (VAN) | Positive | |
| S11 | IDT: AMP | 25 | Positive (AMP) | Nil | - | Negative | Positive |
| S12 | Nil | Negative | VAN | 5, 50, 500 | Positive (VAN) | Positive | |
| S13 | PT/IDT: MET | 3 | Positive (MET) | Nil | - | Negative | Positive |
| S14 | Nil | Negative | Nil | - | Negative | Negative | |
| S15 | NP | NP | VAN | 5, 50, 250 | Positive | Positive | |
| S16 | IDT: PIP-TAZ | 4.5 | Positive (PIP-TAZ) | Nil | - | Negative | Positive |
| S17 | PT: TMP | 5% | Positive (TMP) | Nil | - | Negative | Positive |
| S18 | IDT: PIP-TAZ | 4.5 | Positive (PIP-TAZ) | Nil | - | Negative | Positive |
| S19 | Nil | Negative | VAN | 5, 50, 500 | Positive | Positive | |
Abbreviations: IDT, delayed intradermal testing; PT, patch testing; NP, not performed; Nil, nil positive. AMP, ampicillin; CEF, ceftriaxone; CEFZ, cefazolin; FLU, flucloxacillin; MER, meropenem; MET, metronidazole; PIP-TAZ, piperacillin-tazobactam; TEIC, teicoplanin; VAN, vancomycin; TMP, trimethoprim.
In patients beta-lactam implicated in SCAR causality a standard panel employed for IDT (DAP-major, DAP -minor, PENG, FLU, AMP, PIP-TAZ, CEFX, CEF) in addition to implicated beta-lactam at recommended concentrations1. For all other drugs the implicated antibiotic was tested via either patch testing (PT) or delayed intradermal testing (IDT). For PT, expressed as %. In patient S3 ELISpot testing was also performed to ibuprofen and for S18 PT was also performed to allopurinol.
A SFU/million cells of > 50 was interpreted as positive result by subtracting the spot count in the negative control (no drug) from the spot count in the wells with drugs.
Performed in triplicate (or duplicate if thawed PBMC numbers low).
Negative CD3 control.
IFN-g release ELISpot assay responses (ex vivo)
Ten patients (53%) exhibited a positive IFN-g release ELISpot assay to at least 1 implicated antibiotic (median SFU/ million cells, 102; interquartile range [IQR], 71.46-147.3), 8 patients were negative (median SFU/million cells, 0; IQR, 0-15.42), and 1 patient indeterminate (Table III). Seven patients (70%) were positive to more than 1 antibiotic concentration of the same drug and 9 patients (90%) were positive at the highest tested antibiotic concentration (see Figure E1 in this article’s Online Repository at www.jaci-inpractice.org). No tolerant controls exhibited a positive result (median SFU/million cells, 0; IQR, 0-8.953) (Figure E1), nor did healthy random donors at highest tested antibiotic concentrations (data not shown). The IFN-g release ELISpot assay results for all tested antibiotics in patients with SCARs and controls are provided in this article’s Online Repository at www.jaci-inpractice.org.
A summary of IFN-g release ELISpot assay results stratified for phenotype and timing is demonstrated in Table IV. From the 10 positive IFN-g release ELISpot assays, 40% (4 of 10) were to a beta-lactam and 60% (6 of 10) a non-ebeta-lactam (P .66). All the non-ebeta-lactam positive ELISpot assays were to a glycopeptide (6 of 6 [100%]). There was no difference in ex vivo positivity with DRESS compared with SJS/TEN phenotypes (8 of 13 [61%] vs 2 of 3 [75%]; P .60). The median time from SCAR onset to ELISpot assay testing was shorter in ex vivo positive than in ex vivo negative patients (115 days vs 140 days; P .66). A trend toward a higher number of ex vivo positives was noted if performed within 1 year of SCAR onset (7 of 10 vs 3 of 10; P 0.17) and if the patient was immunocompetent compared with immunocompromised (8 of 10 [80%] vs 5 of 9 [55%]; P .34). In the 3 IFN-g release ELISpot assay positive patients with subsequent time point PBMCs available, 2 (2 of 3 [67%]) remained positive out to a median time of 552 days after AA-SCAR onset (IQR, 548-556) (Figure 2). In these 2 patients, CD137 activation on CD4 and/or CD8 T cells was also noted at the corresponding time points (Figure 2).
Table 4.
Summary of results for IFN-γ release ELISpot for patients and tolerant controls
| Result of IFN-γ release ELISpot | Patients | Tolerant controls, n = 16 |
|||||
|---|---|---|---|---|---|---|---|
| SJS/TEN, n = 4 | DRESS, n = 14 | AGEP, n = 1 | Acute patientsa, n = 4 | Convalescent patientsb, n = 15 | All patients, n = 19 | ||
| Positive | 2 (50) | 8 (57) | 0 (0) | 3 (75) | 7 (47) | 10 (53) | 0 (0) |
| Negative | 1 (25) | 6 (43) | 1 | 1 (25) | 7 (47) | 8 (42) | 16 (100) |
| Indeterminatec | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 1 (5) | 0 (0) |
Values are given as No. (%)
Abbreviations: SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; DRESS, drug reaction with eosinophilia and systemic symptoms; AGEP, acute generalized exanthematous pustulosis.
Acute patients were defined as within 6 weeks of SCAR onset
Convalescent patients were defined as > 6 weeks’ post SCAR onset
In an indeterminate results being the CD3 response was not present; this was included in analysis as a ‘false negative’.
Figure 2. IFN-γ release ELISpot and CD137 T-cell activation over time in SCAR patients.
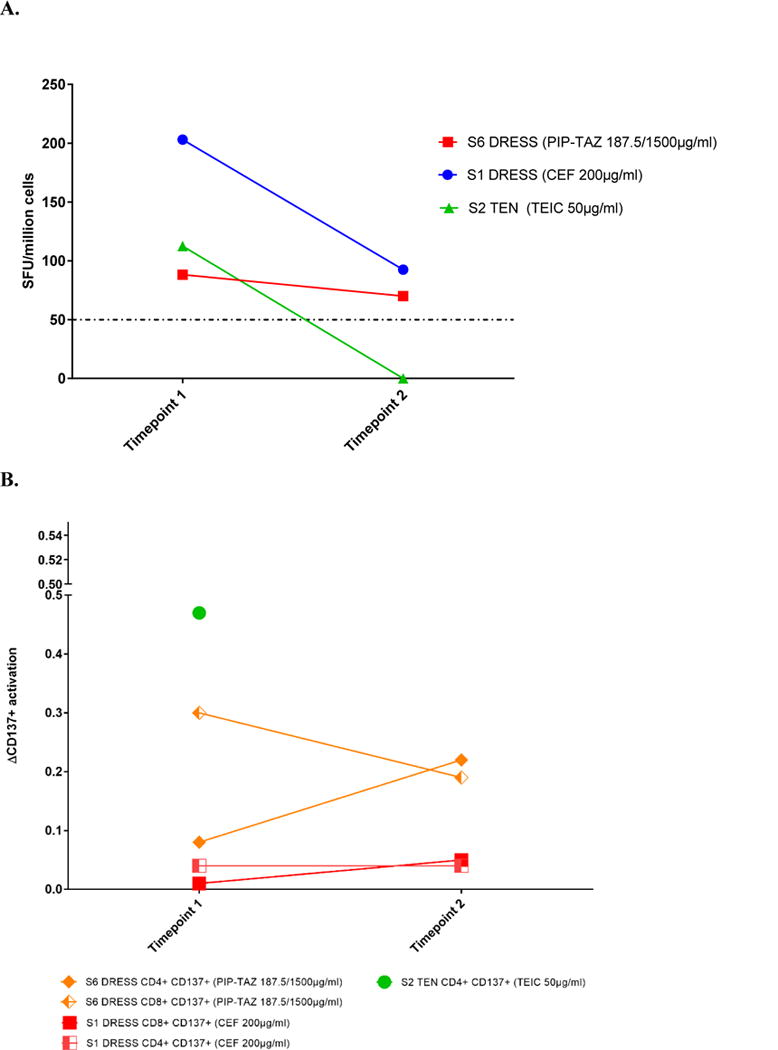
A. IFN-γ release ELISpot for three patients with positive timepoint 1 results and subsequent timepoints.
B. CD137 T cell activation by flow cytometry of the three patients with positive timepoint 1 results above.
Abbreviations: PIP-TAZ, piperacillin-tazobactam; CEF, ceftriaxone; TEN, toxic epidermal necrolysis; SFU, spot forming units; DRESS, drug reaction with eosinophilia system symptoms.
Demonstration of IFN-y release responses in patients with an initial positive result (> 50 SFU/million cells) with a subsequent bleed at least 9 months’ post SCAR onset.
Timepoints post SCAR onset: S1 DRESS (Timepoint 1, 66 days; Timepoint 2, 548 days)
S2 DRESS (Timepoint 1, 312 days; Timepoint 2, 556 days)
S6 DRESS (Timepoint 1, 4 days; Timepoint 2, 286 days)
Correlation and utility of combined in vivo and ex vivo testing
Using combined testing (in vivo and ex vivo), 15 patients (79%) with AA-SCARs were positive to an implicated antibiotic (Table III). The proportion of patients who are positive on ex vivo, in vivo, or a combination of modalities is outlined in Figure 3. In patients who were positive on both ELISpot assay and skin testing, there was a 100% (4 of 4) correlation. On examining in vivo negative and ex vivo positive patients (n 5), we found that all patients were positive on IFN-g release ELISpot assay to a glycopeptide (vancomycin 4, teicoplanin 1) with a short median time from SCAR onset to ELISpot assay (20 days; IQR, 3.5-115). In those in vivo positive and ex vivo negative patients (n 5), a longer median time from SCAR onset to ELISpot assay was noted (301 days; IQR, 100.5-1443), longer than in the in vivo negative and ex vivo positive patients above (P .055).
Figure 3.
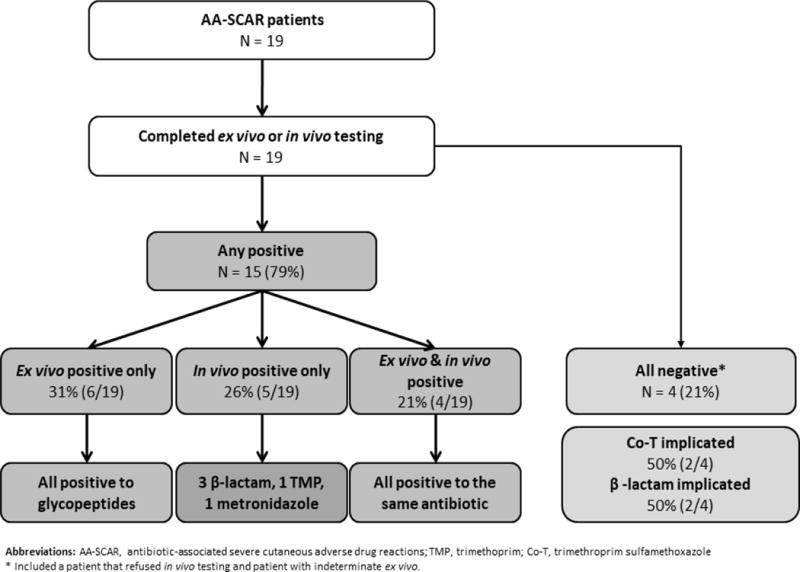
Flow chart of testing results in antibiotic-associated SCAR patients
Of the most commonly encountered phenotype, DRESS, 86% (12 of 14) had a positive in vivo or ex vivo test result (Table II): 66% (8 of 12) in vivo, 66% (8 of 12) ex vivo, and 33% (4 of 12) both. For those positive on both in vivo and ex vivo testing, they were all toward beta-lactams, with a 100% correlation (4 of 4). For those with a positive in vivo testing result, 50% (4 of 8) were toward piperacillin-tazobactam (2 piperacillin-tazobactam alone, 2 piperacillin-tazobactam with an additional penicillin) (Table III). Of the in vivo positives, 88% (7 of 8) were positive on IDT and 25% (2 of 8) on PT (1 trimethoprim, 1 ceftriaxone). In all patients who were ex vivo positive and in vivo negative, the IFN-g release ELISpot assay was positive for vancomycin (4 of 4). The sensitivity and specificity of IFN-g release ELISpot assay for patients with DRESS was 57.74% and 100%, respectively (Table V).
Table 5.
Sensitivity and specificity of IFN-γ release ELISpot
| Result (N = 19) |
Phenotype | Timing | Phenotype scoringa | All patients | |||
|---|---|---|---|---|---|---|---|
| SJS/TEN | DRESS | AGEP | Acute | Convalescent | ≥ Probable or Definitive only | ||
| Sensitivity, % (CI) | 50 (6.76-93.24) | 57.14 (28.86-82.34) | 0 (0 – 97.50) | 75 (19.41-99.37) | 50 (23.04-76.96) | 58.82 (39.92-81.56) | 52 (28.86-75.55) |
| Specificity, % (CI) | 100 (79.41-100) | 100 (79.41-100) | 100 (79.41-100) | 100 (79.41-100) | 100 (79.41-100) | 100 (79.41-100) | 100 (79.41-100) |
Abbreviations: PPV, Positive Predictive Value; NPV, Negative Predictive Value
Patients were stratified based on their phenotypic score - “Possible” or “Probable or Definitive” (n = 17).
Overall sensitivity and specificity of IFN-g release ELISpot assay
Using the phenotypic causality assessments as the reference, the sensitivity and specificity for varied phenotypes and timing of ex vivo testing are outlined in Table V. The highest sensitivity and specificity was achieved from acute bleeds (<6 weeks after SCAR onset), 75% and 100%, respectively. When only including SCAR cases with a probable or definite phenotypic score (n ¼ 17), the sensitivity was 58.82% and specificity 100%.
AA-SCAR follow-up and posttesting prescribing
In the 90 days following combined testing, on comparing those with a positive ex vivo or in vivo test result to those without, we found that there was increased use of class-related antibiotics (9 of 15 [60%] vs 0 of 4 [0%]; P .086) and narrow-spectrum beta-lactams (10 of 15 [66%] vs 0 of 4 [0%]; P .032). In patients with DRESS and a positive in vivo or ex vivo test result to a beta-lactam (n 8), 75% (6 of 8) were able to tolerate an alternative beta-lactam posttesting. There were no adverse events reported or recurrent SCARs in the follow-up period, including in those who were prescribed an antibiotic that was implicated in causality but negative on in vivo or ex vivo testing (n ¼ 4).
DISCUSSION
Delayed hypersensitivity reactions related to antibiotics are confounded by multiple drugs or classes of drug implicated in causality and high morbidity, not only related to the sequelae of the acute hypersensitivity reaction but due to uncertainty and constriction of future antibiotic choices.1,2,23–27 At present, PT and delayed skin testing in isolation are hampered by uncertain availability and poor sensitivity.23,24 Ex vivo techniques such as ELISpot assay have shown promise in small cohorts; however, their availability and performance have not been validated in large-scale high-throughput clinical laboratories against a clinical probability score.7,10,11 Our pilot study notably demonstrated that the combination of a clinical causality algorithm in combination with in vivo and ex vivo diagnostics could aid causality in 79% of patients with AA-SCARs, higher in cases of the most predominate phenotype, DRESS. Furthermore, in these patients with a positive combined result, particularly to a beta-lactam antibiotic, this had a clinical impact by allowing the safe prescribing of both narrow-spectrum beta-lactams and drugs from within implicated antibiotic classes after SCARs. The use of alternative beta-lactams even in those with positive beta-lactam testing is likely to significantly aid safe and appropriate antibiotic prescribing in this cohort.
Neither IDT nor PT (in vivo) in our patients with AA-SCARs was associated with systemic events, supporting similar findings by Barbaud et al.4 This is an important finding because the use of skin testing, in particular IDT in SCARs, often remains absent from local and international clinical guidelines due to safety concerns.25,26. In vivo testing was positive in 52% of patients, falling within the wide range reported in the literature (6.6%-100%),23 with greater success evident in beta-lactam DRESS.27,28 Of interest was the persistence of skin testing responses beyond the acute period, where ex vivo diagnostics appeared to lack sensitivity. Pinho et al 29 have recently demonstrated the long-term reproducibility of positive patch test reactions in patients with delayed hypersensitivities to antibiotics, mainly maculopapular exanthems to beta-lactams.29 This maintenance of skin test positivity at a time where antibiotic antigen-specific T cells are diminishing in the blood may be due to the sensitivity for some implicated drugs (ie, glycopeptides) may also relate to the absence of well-supported testing concentrations and constraints of locally induced mast cell activation, which have been suggested to independently upregulate IL-10 and suppress the local hypersensitivity reaction.31.
The use of antigen-specific IFN-g production using ELISpot assay is a well-established principle in the diagnosis of latent tuberculosis,32,33 yet the utility in AA-SCARs has been limited to small studies. Porebski34 recently reviewed the literature of in vitro and ex vivo testing in SCARs, which demonstrated (1) variations in clinical phenotyping methods and measurable in vitro cytokine outputs, (2) grouping of SCARs with other non-SCAR T-cellemediated hypersensitivity phenotypes, and (3) use primarily in nonantibiotic cases.34. In comparison, we present one of the largest single cohorts of AA-SCARs with extended patient follow-up, using strict phenotypic scoring algorithms and IFN-g release ELISpot assay. IFN-g release ELISpot assay was able to aid the diagnostic algorithm in 53% of patients, picking up an additional 5 cases that were missed with skin testing alone. The benefit of ELISpot assay was apparent in acute samples and glycopeptide-associated cases. Tanvarasethee et al.35 demonstrated improved responses if performed within 2 years of reaction, supporting our findings of increased positives within 1 year postonset. Although the overall sensitivity and specificity of 52% and 100%, respectively, is consistent with previous reports for ex vivo T-cell diagnostics (27%-70%),12,23 we postulate that improved sensitivity is likely to require early case ascertainment and PBMC collection and an understanding of the role of drug metabolites for antibiotics that proved problematic ex vivo (eg, sulfamethoxazole and trimethoprim).36,37 Previous reports have demonstrated drug-specific T-cell responses for up to 20 years after drug exposure,9,38 and both long-lived patch test and ex vivo responses particularly in patients with a history of the HLA-B*57:01erestricted CD8 T-celledependent abacavir hypersensitivity reaction.39–41 In the absence of reexposure to drugs or structurally unrelated drugs, the pathway to such long-lived memory T-cell responses and whether there are drug-specific memory T-cell responses that cross-react with a chronic prevalent pathogen is currently unclear.42 Although we and others have clearly shown long-lived ex vivo positivity, we also highlight the apparent loss of reactivity in a teicoplanin TEN patient11 and the paucity of literature demonstrating robust peripheral T-cell responses to antibiotics over time. Furthermore, a trend was noted of less ex vivo positivity in immunocompromised hosts, and the role of costimulation (ie, IL-2, IL-7) in such patients may be worth exploring.43
There are limitations to this pilot study, including the small study numbers, single-center experience, use of frozen PBMCs, and absence of standardized testing concentrations (especially for ex vivo). Furthermore, for delayed hypersensitivity reactions, the criterion standard for testing sensitivity and specificity is multiple dose oral or ingestion challenge, which is rightly discouraged in clinical practice because it is neither evidence nor guideline based and causes potential harm to patients.19,23,26 Our small study however suggests that improved patient outcomes may be obtained through the use of strict causality assessment (to ascertain pretest clinical probability) in combination with in vivo and ex vivo testing and postprescribing follow-up. Although laboratory testing is not readily available to many clinicians, the development of centralized ex vivo and in vitro testing centers could allow the transfer of acute patient PBMCs (frozen) for assessment, to supplement traditional PT and IDT, and progress toward a personalized approach to drug hypersensitivity and aid safe antibiotic prescribing after AA-SCARs. For the future development of such testing, a number of key questions remain including the following: (1) the ideal range of antibiotic concentrations used for both in vivo and ex vivo testing at and above the physiological Cmax concentration; (2) the ideal timing of ex vivo testing and how this relates to causal antibiotics; (3) the concentration-dependent role of parent antibiotic versus antibiotic metabolite; and (4) the utility of an enhanced spectrum of ex vivo tests that may include the use of flow cytometry with intracellular cytokine staining to examine for T-cell activation,44 and other mechanisms that may improve the sensitivity of ex vivo testing.12.
This study encompassing one of the largest tested cohorts of AA-SCARs was carried out in a population of patients with high antibiotic needs. It demonstrates the potential utility of combined safe in vivo and novel ex vivo testing for patients with AA-SCARs, aiding the global causality assessment in a disease associated with significant morbidity, mortality, and high-risk prescribing. In the future, improving antibiotic appropriateness is likely to be aided by combined testing programs in patients with AA-SCAR. Personalization of such testing based on phenotype, implicated antibiotic, and timing postonset may improve sensitivity, specificity, and the negative predictive value of such testing, leading to safer options for patients and overall improvement in their care.
Supplementary Material
What is already known about this topic?
The individual use of in vivo skin testing and ex vivo IFN-g release enzyme- linked immunoSpot (ELISpot) assay for assigning drug causality in severe cutaneous adverse reactions (SCARs) shows promise, yet the joint utility in antibiotic-associated SCARs remains ill-defined.
What does this article add to our knowledge?
The combined use of in vivo and ex vivo diagnostics in antibiotic- associated SCARs assigned causality safely in 79% of cases, and IFN-g release ELISpot assay demonstrated good sensitivity and high specificity.
How does this study impact current management guidelines?
Skin testing (in vivo) and IFN-g release ELISpot assay (ex vivo) are complementary approaches that may prove safe and effective in ascertaining antibiotic causality and improve, often difficult antibiotic prescribing, after SCARs.
Acknowledgments
We thank the staff of the Infectious Diseases Departments of Austin Health, Alfred Health and Peter MacCallum Cancer Centre for supporting this study. Special thanks to infectious diseases fellows, Dr Victoria Hall, Dr Abby Douglas, and Dr Rekha Pai Mangalore, for patient recruitment. Thanks also to Trudi Bannam for laboratory assistance and Dr Michelle Goh for dermatological assessment.
J.A.T. is supported by the National Health and Medical Research Council (NHMRC) postgraduate research scholarship and Austin Medical Research Foundation grant.
K.C.K. is supported by the National Institutes of Health (NIH) (grant nos. 1P50GM115305 and 2T32GM7347). E.J.P. is supported in part by the NIH (grant nos. 1P50GM115305-01 and 1R01AI103348-01), the NIH-funded Tennessee Center for AIDS Research (grant no. P30 AI110527), National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant number 1R13 AR071267-01, the NHMRC, ACH2, and The Angela Anderson Foundation.
R. Pavlos has received research support from the National Health and Medical Research Council (NHMRC). K. C. Konvinse has received research support from the National Institute of General Medical Sciences (grant nos. P50GM115305 and T32GM07347) and the National Institute of Allergy and Infectious Diseases (grant no. F30AI131780). E. J. Phillips has received research support from NHMRC Australia, the National Institutes of Health, and ACH2 Australia; has received consultancy fees and honoraria from Biocryst; receives royalties from UpToDate; is codirector of the company holding the patent for HLA-B*57:01; and has received consultancy fees from Aicuris.
Abbreviations used
- AA
Antibiotic-associated
- AGEP
Acute generalized exanthematous pustulosis
- DRESS
Drug reaction with eosinophilia and systemic symptoms ELISpot-Enzyme-linked immunospot
- IDT
Intradermal testing IQR-Interquartile range PT-Patch testing
- SCAR
Severe cutaneous adverse reaction SFU-Spot-forming unit
- SJS
Stevens-Johnson syndrome TEN-Toxic epidermal necrolysis
Footnotes
Conflicts of interest: The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Trubiano JA, Aung AK, Nguyen M, Fehily SR, Graudins L, Cleland H, et al. A comparative analysis between antibiotic- and nonantibiotic-associated delayed cutaneous adverse drug reactions. J Allergy Clin Immunol Pract. 2016;4:1187–93. doi: 10.1016/j.jaip.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Lin YF, Yang CH, Sindy H, Lin JY, Rosaline Hui CY, Tsai YC, et al. Severe cutaneous adverse reactions related to systemic antibiotics. Clin Infect Dis. 2014;58:1377–85. doi: 10.1093/cid/ciu126. [DOI] [PubMed] [Google Scholar]
- 3.Pirmohamed M, Friedmann PS, Molokhia M, Loke YK, Smith C, Phillips E, et al. Phenotype standardization for immune-mediated drug-induced skin injury. Clin Pharmacol Ther. 2011;89:896–901. doi: 10.1038/clpt.2011.79. [DOI] [PubMed] [Google Scholar]
- 4.Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555–62. doi: 10.1111/bjd.12125. [DOI] [PubMed] [Google Scholar]
- 5.Cordoba S, Navarro-Vidal B, Martinez-Moran C, Borbujo J. Reactivation of skin lesions after patch testing to investigate drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Actas Dermosifiliogr. 2016;107:781–3. doi: 10.1016/j.ad.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181–3. doi: 10.1001/archderm.139.9.1181. [DOI] [PubMed] [Google Scholar]
- 7.Bensaid B, Rozieres A, Nosbaum A, Nicolas JF, Berard F. Amikacin-induced drug reaction with eosinophilia and systemic symptoms syndrome: delayed skin test and ELISPOT assay results allow the identification of the culprit drug. J Allergy Clin Immunol. 2012;130:1413–4. doi: 10.1016/j.jaci.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 8.El-Ghaiesh S, Monshi MM, Whitaker P, Jenkins R, Meng X, Farrell J, et al. Characterization of the antigen specificity of T-cell clones from piperacillin-hypersensitive patients with cystic fibrosis. J Pharmacol Exp Ther. 2012;341:597–610. doi: 10.1124/jpet.111.190900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozieres A, Hennino A, Rodet K, Gutowski MC, Gunera-Saad N, Berard F, et al. Detection and quantification of drug-specific T cells in penicillin allergy. Allergy. 2009;64:534–42. doi: 10.1111/j.1398-9995.2008.01674.x. [DOI] [PubMed] [Google Scholar]
- 10.Porebski G, Pecaric-Petkovic T, Groux-Keller M, Bosak M, Kawabata TT, Pichler WJ. In vitro drug causality assessment in Stevens-Johnson syndrome— alternatives for lymphocyte transformation test. Clin Exp Allergy. 2013;43:1027–37. doi: 10.1111/cea.12145. [DOI] [PubMed] [Google Scholar]
- 11.Trubiano JA, Redwood A, Strautins K, Pavlos R, Woolnough E, Chang CC, et al. Drug-specific upregulation of CD137 on CD8 T cells aids in the diagnosis of multiple antibiotic toxic epidermal necrolysis. J Allergy Clin Immunol Pract. 2017;5:823–6. doi: 10.1016/j.jaip.2016.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato K, Kawase A, Azukizawa H, Hanafusa T, Nakagawa Y, Murota H, et al. Novel interferon-gamma enzyme-linked immunoSpot assay using activated cells for identifying hypersensitivity-inducing drug culprits. J Dermatol Sci. 2017;86:222–9. doi: 10.1016/j.jdermsci.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]; Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88:60–8. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 14.Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88:60–8. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 15.Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction: results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 16.Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)ea clinical reaction pattern. J Cutan Pathol. 2001;28:113–9. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 17.Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. 2002;57:45–51. [PubMed] [Google Scholar]
- 18.Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, et al. Skin test concentrations for systemically administered drugs e an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68:702–12. doi: 10.1111/all.12142. [DOI] [PubMed] [Google Scholar]
- 19.Barbaud A, Goncalo M, Bruynzeel D, Bircher A, European Society of Contact Dermatitis Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001;45:321–8. doi: 10.1034/j.1600-0536.2001.450601.x. [DOI] [PubMed] [Google Scholar]
- 20.Keane NM, Roberts SG, Almeida CA, Krishnan T, Chopra A, Demaine E, et al. High-avidity, high-IFNgamma-producing CD8 T-cell responses following immune selection during HIV-1 infection. Immunol Cell Biol. 2012;90:224–34. doi: 10.1038/icb.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert DN, Chambers HF, Eliopoulos GM, et al. The Sanford Guide to Antimicrobial Therapy. 47th. Sperryville, VA: Antimicrobial Therapy, Inc.; 2017. [Google Scholar]
- 22.Keane NM, Pavlos RK, McKinnon E, Lucas A, Rive C, Blyth CC, et al. HLA Class I restricted CD8 and class II restricted CD4 T cells are implicated in the pathogenesis of nevirapine hypersensitivity. AIDS. 2014;28:1891–901. doi: 10.1097/QAD.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 23.Konvinse KC, Phillips EJ, White KD, Trubiano JA. Old dog begging for new tricks: current practices and future directions in the diagnosis of delayed antimicrobial hypersensitivity. Curr Opin Infect Dis. 2016;29:561–76. doi: 10.1097/QCO.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rive CM, Bourke J, Phillips EJ. Testing for drug hypersensitivity syndromes. Clin Biochem Rev. 2013;34:15–38. [PMC free article] [PubMed] [Google Scholar]
- 25.ASCIA guidelines for the acute management of anaphylaxis. 2016 Available from: https://www.allergy.org.au/images/stories/pospapers/ASCIA_SPT_Manual_March_2016.pdf. Accessed June 1, 2017.
- 26.Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–73. doi: 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Cabanas R, Calderon O, Ramirez E, Fiandor A, Prior N, Caballero T, et al. Piperacillin-induced DRESS: distinguishing features observed in a clinical and allergy study of 8 patients. J Investig Allergol Clin Immunol. 2014;24:425–30. [PubMed] [Google Scholar]
- 28.Barbaud A. Skin testing and patch testing in non-IgE-mediated drug allergy. Curr Allergy Asthma Rep. 2014;14:442. doi: 10.1007/s11882-014-0442-8. [DOI] [PubMed] [Google Scholar]
- 29.Pinho A, Marta A, Coutinho I, Goncalo M. Long-term reproducibility of positive patch test reactions in patients with non-immediate cutaneous adverse drug reactions to antibiotics. Contact Dermatitis. 2017;76:204–9. doi: 10.1111/cod.12720. [DOI] [PubMed] [Google Scholar]
- 30.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 31.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 32.Huo ZY, Peng L. Accuracy of the interferon-gamma release assay for the diagnosis of active tuberculosis among HIV-seropositive individuals: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:350. doi: 10.1186/s12879-016-1687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auguste P, Tsertsvadze A, Pink J, Court R, McCarthy N, Sutcliffe P, et al. Comparing interferon-gamma release assays with tuberculin skin test for identifying latent tuberculosis infection that progresses to active tuberculosis: systematic review and meta-analysis. BMC Infect Dis. 2017;17:200. doi: 10.1186/s12879-017-2301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porebski G. In vitro assays in severe cutaneous adverse drug reactions: are they still research tools or diagnostic tests already? Int J Mol Sci. 2017;18:1737. doi: 10.3390/ijms18081737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanvarasethee B, Buranapraditkun S, Klaewsongkram J. The potential of using enzyme-linked immunospot to diagnose cephalosporin-induced maculopapular exanthems. Acta Derm Venereol. 2013;93:66–9. doi: 10.2340/00015555-1386. [DOI] [PubMed] [Google Scholar]
- 36.Goldman JL, Leeder JS, Van Haandel L, Pearce RE. In vitro hepatic oxidative biotransformation of trimethoprim. Drug Metab Dispos. 2015;43:1372–80. doi: 10.1124/dmd.115.065193. [DOI] [PMC free article] [PubMed] [Google Scholar]; Castrejon JL, Berry N, El-Ghaiesh S, Gerber B, Pichler WJ, Park BK, et al. Stimulation of human T cells with sulfonamides and sulfonamide metabolites. J Allergy Clin Immunol. 2010;125:411–418.e4. doi: 10.1016/j.jaci.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 37.Castrejon JL, Berry N, El-Ghaiesh S, Gerber B, Pichler WJ, Park BK, et al. Stimulation of human T cells with sulfonamides and sulfonamide metabolites. J Allergy Clin Immunol. 2010;125:411–418.e4. doi: 10.1016/j.jaci.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Beeler A, Engler O, Gerber BO, Pichler WJ. Long-lasting reactivity and high frequency of drug-specific T cells after severe systemic drug hypersensitivity reactions. J Allergy Clin Immunol. 2006;117:455–62. doi: 10.1016/j.jaci.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 39.Phillips EJ, Sullivan JR, Knowles SR, Shear NH. Utility of patch testing in patients with hypersensitivity syndromes associated with abacavir. AIDS. 2002;16:2223–5. doi: 10.1097/00002030-200211080-00017. [DOI] [PubMed] [Google Scholar]
- 40.Lucas A, Lucas M, Strhyn A, Keane NM, McKinnon E, Pavlos R, et al. Abacavir-reactive memory T cells are present in drug naive individuals. PLoS One. 2015;10:e0117160. doi: 10.1371/journal.pone.0117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips EJ, Wong GA, Kaul R, Shahabi K, Nolan DA, Knowles SR, et al. Clinical and immunogenetic correlates of abacavir hypersensitivity. AIDS. 2005;19:979–81. doi: 10.1097/01.aids.0000171414.99409.fb. [DOI] [PubMed] [Google Scholar]
- 42.White KD, Chung WH, Hung SI, Mallal S, Phillips EJ. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: the role of host, pathogens, and drug response. J Allergy Clin Immunol. 2015;136:219–34. doi: 10.1016/j.jaci.2015.05.050. quiz 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinuzzi E, Scotto M, Enee E, Brezar V, Ribeil JA, van Endert P, et al. Serum-free culture medium and IL-7 costimulation increase the sensitivity of ELISpot detection. J Immunol Methods. 2008;333:61–70. doi: 10.1016/j.jim.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Teraki Y, Shiohara T. IFN-gamma-producing effector CD8 T cells and IL-10-producing regulatory CD4 T cells in fixed drug eruption. J Allergy Clin Immunol. 2003;112:609–15. doi: 10.1016/s0091-6749(03)01624-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


