Abstract
Background/Objectives
Energy expenditure (EE), as reflective of body energy demand, has been proposed to be the key driver of food intake, possibly influencing weight change in humans. Variation in this energy-sensing link (overeating relative to weight-maintaining energy requirements) may lead to weight gain over time.
Subjects/Methods
Sixty-one overweight otherwise healthy Native Americans (age: 34.0±7.9 years, body fat: 39.7±9.5%, 36 males) were admitted to our clinical research unit for measurements of body composition by dual-energy X-ray absorptiometry, and 24-h EE and respiratory quotient (RQ) in a whole-room indirect calorimeter during energy balance and weight stability. Following this, ad libitum food intake was assessed for three days using computerized vending machines. Body weight change under unrestricted free-living conditions was assessed at an outpatient follow-up visit (median follow-up time=1.7 years).
Results
Total ad libitum food intake (3-day average) was positively associated with 24-h EE (r=0.44, p<0.001), RQ (r=0.34, p=0.007), and fat free mass (r=0.38, p=0.002). A relatively greater food intake after accounting for 24-h EE, but not for RQ (p=0.30) or for fat free mass (p=0.23) nor total food intake (p=0.16), predicted weight gain at the outpatient follow-up visit (r=0.26, p=0.04), such that overeating 100 Kcal/d above the food intake predicted by 24-h EE at baseline was associated with an average weight gain of 0.22 Kg over the follow-up period (95% CI: 0.01 to 0.42 Kg). This was due to relatively greater dietary fat intake (r=0.32, p=0.01), but not carbohydrate (p=0.27) or protein (p=0.06) intake.
Conclusion
The individual propensity to overeating, particularly fat, in excess of the weight-maintaining energy requirements can be assessed and predicts long-term weight gain, suggesting that variation in energy sensing may influence appetite by favoring overeating thus promoting obesity development.
Key Terms: energy expenditure, energy intake, energy sensing, fat intake, food intake, overeating, overfeeding, weight change, weight gain
INTRODUCTION
The imbalance between energy intake and energy expenditure (EE) leads to obesity development but the relative role of each component on weight change is still not totally understood. In the last decade, studies investigating the crosstalk between EE, energy intake and body composition have suggested a novel and different formulation for appetite regulation and its effects on weight change [1–3]. Specifically, recent findings indicate that energy intake is mainly determined by fat free mass (FFM) [4, 5], which is also the strongest anthropometric determinant of EE [6], thus supporting a potential energy-sensing mechanism for appetite regulation driven by body energy demands [2, 7]. However, it is still debated whether FFM per se or EE drives energy intake in humans [8, 9]. In a previous study, both FFM and resting EE were predictors of daily energy intake, although the total effect of FFM on intake was entirely mediated by resting EE [9]. In line with those results, our research group has also shown that ad libitum food intake was independently predicted by 24-h EE and respiratory quotient (RQ) measured in a whole-room calorimeter during energy balance where, again, the relationship between FFM and food intake was almost entirely mediated by 24-h EE [8]. Taken together, these results indicate that EE might act as a specific signal of energy need that may drive daily food intake.
The effects of reciprocal influences between energy intake and EE determine daily energy balance, which ultimately influence body weight change [2]. To date, it is not clear whether a deviation in “energy sensing”, defined as the causal link between EE and energy intake [2, 7], may lead to a change in body weight over time. Therefore, the aim of this study was to investigate the relationship between 24-h EE measured in energy balance and food intake in ad libitum conditions (i.e., energy sensing), and to test whether a deviation from the EE-food intake relationship, as a measure of individual capability to sense metabolic demands in order to adjust daily food intake, may predict weight change after a long-term follow-up. We hypothesized that using reproducible gold standard measures of food intake and EE separated in time we could measure and assess variation in “energy sensing”, such as a positive deviation from the EE-food intake relationship, namely, overeating relative to what is predicted by energy requirements, may indicate the propensity of an individual to gain weight over time.
SUBJECTS AND METHODS
All the individuals who participated in this inpatient study investigating the determinants of EE and ad libitum food intake (clinicaltrials.gov identifier: NCT00342732) were healthy after examination of medical history and laboratory testing, and had no evidence of medical diagnoses other than overweight/obesity or impaired glucose tolerance. Before participation, all subjects signed written and informed consent. The experimental protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The present study included 61 Native Americans living in the Phoenix (AZ) metropolitan area, who had measures of both 24-h EE inside a whole-room indirect calorimeter during energy balance and ad libitum food intake assessed by computerized vending machine systems over 3 consecutive days while inpatient, and also had a follow-up outpatient visit after at least one month following discharge to assess their free-living weight change.
On admission to our clinical research unit, subjects were fed with a standardized weight-maintaining balanced diet for at least three days prior to any metabolic testing. The weight-maintaining energy needs (WMEN) of this diet was initially estimated based on body weight at admission and gender [10], and then adjusted daily by the research dietitian to assure weight stability within 1% of admission weight. The diet consisted of 50% carbohydrate, 30% fat, 20% protein with a food quotient (FQ) of 0.87 [11]. Body composition (percentage of body fat) was evaluated by dual energy x-ray absorptiometry (DPX-1; Lunar Radiation Corp, Madison, Wisconsin) to calculate body fat mass (FM) and FFM. Glucose tolerance was assessed by 75-g oral glucose tolerance test (OGTT) and diabetes diagnosis was ruled out based on the American Diabetes Association criteria [12]. After at least 3 days on the weight-maintaining diet, subjects resided for 24 hours inside a whole-room indirect calorimeter to measure their 24-h EE during energy balance. On the following day, subjects were asked to self-select all their food on a computer-operated vending machine system for the following 3 days on the metabolic ward, and then they were discharged from the unit.
All individuals had a follow-up outpatient visit after at least one month following discharge. At the follow-up visit, body weight and glucose tolerance (assessed by a 75-g OGTT) were recorded [13]. All subjects were free from diabetes and women were not pregnant at the time of the first admission and at the follow-up visit. Body weight change in Kg was defined as the difference between the weight at the follow-up (outpatient) visit minus the baseline (inpatient) weight.
Energy expenditure measurement
The measurements of 24-h EE and RQ were assessed in a whole-room indirect calorimeter (respiratory chamber) during eucaloric conditions, as previously described in detail [6]. Oxygen consumption, carbon dioxide production, RQ, and EE by Lusk’s formula [14] were measured and calculated every 15 min, averaged and extrapolated to the 24-hour interval. Carbohydrate (CARBOX) and fat (FATOX) oxidation rates were derived after accounting for protein oxidation calculated from 24-h urinary nitrogen [15], which was measured using a bichromatic (340/383 nm) rate technique (Dimension®clinical chemistry system). The total energy content of four meals given throughout the 24 hours inside the respiratory chamber was estimated by unit-specific equations [15] to closely achieve a perfect energy balance in this confined setting. All unconsumed food was returned to the metabolic kitchen through an air lock for weighing for accurate calculation of 24-h energy intake. The difference between actual 24-h energy intake minus 24-h EE was defined as the 24-h energy balance inside the respiratory chamber.
Ad libitum food intake assessment
Objective measurement of ad libitum food intake was performed over three days using a highly reproducible (intraclass correlation coefficient, ICC=0.90) computerized vending machine paradigm, as previously described and validated [16, 17]. Briefly, a food selection questionnaire (FSQ) was used to determine individual food preferences on the day of admission to the clinical research unit. Forty different types of food that the subjects had given an intermediate rating on the FSQ (i.e., scores between 4 and 8 on a 9-point Likert scale) were made available in the vending machine each day. Subjects were allowed to self-select any food item from the machines whenever they wanted for three days. Food leftovers were returned and weighed by the metabolic kitchen staff to correct the actual food intake. Total and individual macronutrient kilocalories consumed per day were calculated from the actual weights of the food and condiments consumed by using the CBORD Professional Diet Analyzer Program (CBORD, Inc., Ithaca, NY, USA) and the Food Processor database (ESHA version 10.0.0, ESHA Research, Salem, OR, USA). Total ad libitum food intake during the 3-day vending period was averaged and expressed as total kcals eaten per day, as well as the ratio to the weight-maintaining energy needs prior to the 3-day vending period (i.e., WMEN). Similar calculations were performed for each macronutrient intake.
Statistical Analysis
Statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC). A p-value <0.05 was considered statistically significant. Normally distributed data were presented as mean±SD, while data with skewed distributions were reported as median with interquartile range (IQR).
The Pearson’s correlation coefficient (r) was used to quantify associations between energy intake and EE (24-h EE and RQ, CARBOX and FATOX) and body composition measures (FFM and FM), as these were previously found to be determinants of ad libitum food intake [5, 8, 10]. Linear regression analysis was used to calculate residuals of total ad libitum food intake (i.e., the difference between observed minus predicted food intake using a linear equation as a function of the corresponding predictor) after separate adjustments for 24-h EE, RQ, FFM and FM. Similar analyses were performed to calculate residuals of individual macronutrient intakes. Linear regression models were then used to evaluate the relationships between body weight change and residuals of total ad libitum food intake and macronutrient (fat, carbohydrate, or protein) intake after adjustment for age, gender, and follow-up time. Sensitivity analyses were also conducted accounting for seasonality, and using follow-up weight as the dependent variable and weight at baseline as an additional covariate in the model (i.e., analysis of covariance, ANCOVA).
RESULTS
Characteristics of the study population including anthropometric, metabolic and food intake measurements are reported in Table 1. Measurements of EE inside the respiratory chamber were close to the prescribed intake (average 24-h EE-intake deviation= −7%, average RQ within 3% of FQ). The total ad libitum energy intake, expressed as daily average over the 3-day period, varied considerably between subjects with a range from 1502 to 6951 Kcal/d with an average of 4386±1144 Kcal/d, representing nearly a 60% increase compared to the average kcals of the weight-maintaining diet (WMEN).
Table 1.
Demographic, anthropometric, metabolic and food intake measures of the study group.
| Whole study group (n=61) | Men (n=36) | Women (n=25) | |
|---|---|---|---|
| Gender (M/F) | 36/25 | 36 | 25 |
| Age (years) | 34.0 ± 7.9 | 33.9 ± 8.2 | 34.0 ± 7.6 |
| Body weight (Kg) | 94.3 ± 26.1 | 96.1 ± 27.3 | 91.7 ± 24.5 |
| BMI (Kg/m2) | 33.6 ± 8.5 | 32.3 ± 7.9 | 35.4 ± 9.1 |
| Body fat (%) | 39.7 ± 9.5 | 33.9 ± 6.8 | 47.9 ± 6.5 |
| Fat mass (Kg) | 38.5 ± 16.8 | 33.8 ± 15.3 | 45.2 ± 16.9 |
| Fat free mass (Kg) | 55.8 ± 13.7 | 62.3 ± 13.1 | 46.4 ± 8.2 |
| Fasting glucose (mg/dL) | 90.8 ± 9.2 | 90.0 ± 10.1 | 91.7 ± 7.9 |
| 2-h glucose (mg/dL) | 127.7 ± 28.9 | 123.3 ± 25.4 | 133.3 ± 34.0 |
| Respiratory chamber | |||
| 24-h energy intake (Kcal/d) | 2238.0 ± 392.4 | 2377.5 ± 352.7 | 2037.2 ± 363.8 |
| 24-h EE (Kcal/d) | 2419.0 ± 410.0 | 2574.4 ± 363.2 | 2195.2 ± 373.5 |
| 24-h energy balance (Kcal/d)* | −180.9 ± 206.9 | −196.9 ± 198.5 | −157.9 ± 220.4 |
| 24-h energy balance (%) | −7.0 ± 8.6 | −7.1 ± 7.3 | −6.9 ± 9.1 |
| 24-h RQ (ratio) | 0.84 ± 0.02 | 0.85 ± 0.02 | 0.84 ± 0.03 |
| CARBOX (Kcal/d) | 1075.7 ± 271.2 | 1195.9 ± 262.6 | 902.5 ± 174.2 |
| FATOX (Kcal/d) | 1034.9 ± 325.6 | 1066.8 ± 306.0 | 989.0 ± 353.3 |
| Computerized vending machine systems† | |||
| Total food intake (Kcal/d) | 4385.7 ± 1143.9 | 4791.0 ± 1051.1 | 3802.2 ± 1003.8 |
| WMEN (Kcal/d) | 2790.2 ± 281.5 | 2905.6 ± 272.5 | 2624.0 ± 202.6 |
| Total food intake (% WMEN) | 157.5 ± 39.6 | 165.2 ± 35.8 | 146.4 ± 43.0 |
| Carbohydrate intake (Kcal/d) | 2209.5 ± 579.3 | 2414.9 ± 509.4 | 1913.6 ± 553.2 |
| Fat intake (Kcal/d) | 1726.1 ± 537.3 | 1890.0 ± 513.8 | 1490.0 ± 488.2 |
| Protein intake (Kcal/d) | 549.6 ± 155.8 | 585.8 ± 141.8 | 497.6 ± 237.3 |
| Follow-up visit | |||
| Median follow-up time (years) | 1.7 (IQR: 1.2–2.9) | 1.9 (IQR: 1.1–2.9) | 1.7 (IQR: 1.4–2.3) |
| Body weight change (Kg) | 3.4 ± 7.5 | 3.2 ± 8.1 | 3.6 ± 6.6 |
| Body weight change (% of baseline weight) | 4.2 ± 8.9 | 3.5 ± 8.7 | 4.8 ± 9.4 |
| Fasting glucose (mg/dL) | 93.8 ± 10.6 | 94.7 ± 11.1 | 92.5 ± 10.0 |
| 2-h glucose (mg/dL) | 108.6 ± 26.0 | 108.1 ± 27.0 | 109.3 ± 25.2 |
CARBOX: carbohydrate oxidation rate; EE: energy expenditure; FATOX: fat oxidation rate; RQ: respiratory quotient; WMEN: weight-maintaining energy needs.
Data are presented as mean±SD, unless otherwise indicated.
Twenty-four-hour energy balance in the respiratory chamber was calculated as the difference between 24-h energy intake (total caloric intake of the four meals given in the 24 hours accounting for leftovers) minus measured 24-hour energy expenditure.
Ad libitum food intake measures are reported as the average of three days on the vending machine
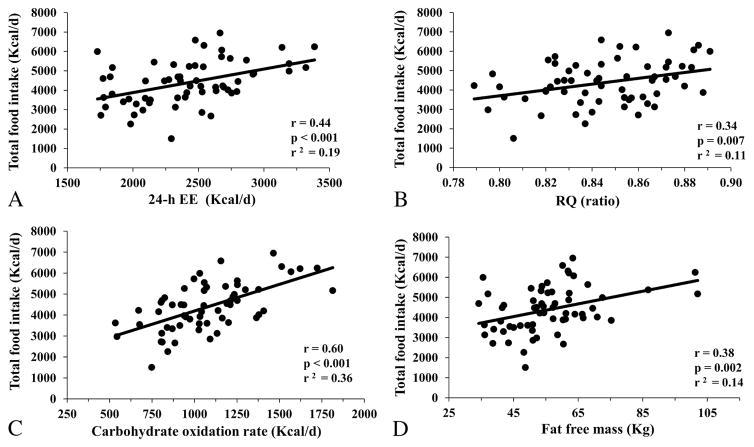
In a linear correlation analysis, unadjusted 24-h EE (r=0.44, p<0.001, Figure 1A), RQ (r=0.34, p=0.007, Figure 1B), CARBOX (r=0.60, p<0.001, Figure 1C) and FFM (r=0.38, p=0.002, Figure 1D) were positively correlated with total ad libitum energy intake. No associations were found between energy intake and FM (p=0.18) or FATOX (p=0.43).
Figure 1.
Relationships between total ad libitum food intake during the 3-day vending period (expressed as the average over 3 days) and 24-h EE (panel A), 24-h respiratory quotient (RQ, panel B), carbohydrate oxidation rate (panel C) and fat free mass (panel D). In each panel, the Pearson’s correlation coefficient (r) is reported along with its significance (p) and goodness of fit (r2).
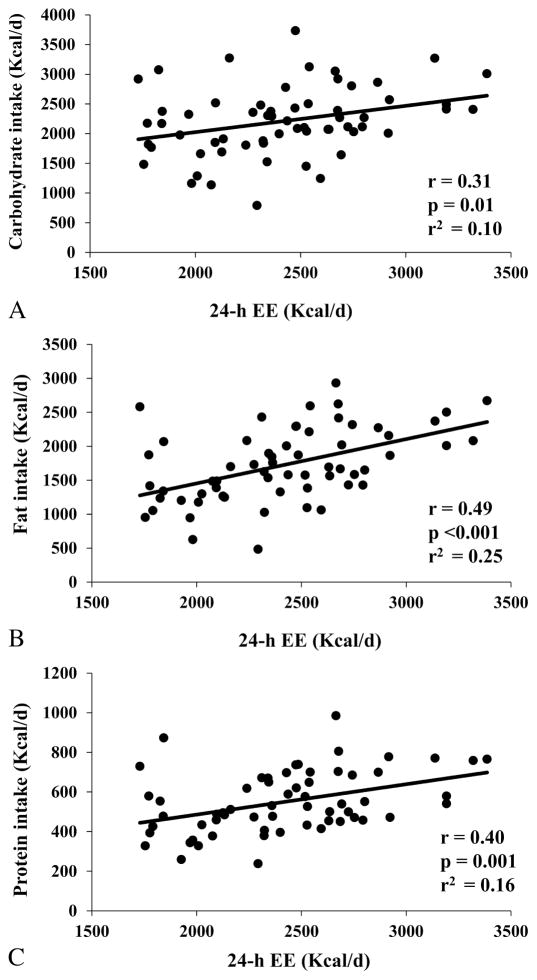
The relationship between 24-h EE and total ad libitum food intake was linear across the entire range of 24-h EE values (r2=0.19) and had an intercept of 1446 Kcal/day (95% CI: −143 to 3036, p=0.07). On average, a 100-kcal difference in 24-h EE was associated with an increase in total ad libitum food intake of approximately 121 Kcal/d (95% CI: 57 to 186, p<0.001), of which 65 Kcal/d were fat (95% CI: 35 to 95 Kcal/d, p<0.001), 44 Kcal/d were carbohydrate (95% CI: 9 to 79 Kcal/d, p = 0.01) and 15 Kcal/d were protein (95% CI: 9 to 79 Kcal/d, p=0.01) (Supplemental Figure 1). Residuals of total ad libitum food intake after adjustment for 24-h EE ranged from −2730 to 2443 Kcal/d (SD=1019 Kcal/d). After adjustment for 24-h EE, residuals of macronutrient intakes ranged from −1361 to 1501 Kcal/d (r2=0.10, SD=550 Kcal/d) for carbohydrate intake, from −1161 to 1304 Kcal/d (r2=0.25, SD=466 Kcal/d) for fat intake and from −293 to 410 Kcal/d (r2=0.16, SD=143 Kcal/d) for protein intake.
Weight change prediction
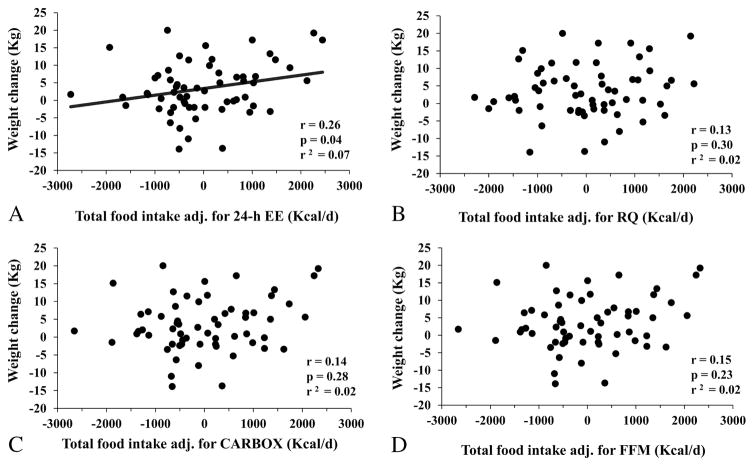
In the longitudinal analysis (median follow-up time: 1.7 years, IQR: 1.2–2.9), body weight at the outpatient visit increased an average of 3.4±7.5 Kg (range: −13.9 to 20.0, p=0.001), or 4.0±9.0% (range: −16.4 to 33.2%) of baseline weight, with no difference between sexes (p=0.82) or according to seasonality (p=0.50). There was no correlation between total ad libitum food intake and body weight change (p=0.16). However, the residuals of total ad libitum food intake after adjustment for 24-h EE at baseline (i.e., the actual food intake minus the food intake predicted by 24-h EE based on linear regression analysis) were positively associated with body weight change (r=0.26, p=0.04, Figure 2A). This association remained significant (partial r=0.27, adj. p=0.04) after controlling for age, gender, and follow-up time, such that an increase of 100 Kcal/d in ad libitum food intake over that predicted by 24-h EE at baseline was associated with an average weight gain of 0.22 Kg at follow-up (95% CI: 0.01 to 0.42 kg). The same result was obtained by ANCOVA considering follow-up weight as dependent variable and including baseline weight as an additional covariate (β=0.23 Kg, p=0.04). Similar results (r=0.30, p=0.02) for weight change were also obtained when residuals of total ad libitum food intake were calculated after concomitant adjustment for both 24-h EE and WMEN to further adjust for the degree of overeating.
Figure 2.
Relationships between body weight change and residuals of total ad libitum food intake after adjustment for 24-h EE (panel A), 24-h respiratory quotient (RQ, panel B), carbohydrate oxidation rate (CARBOX, panel C) and fat free mass (panel D). Residuals were calculated via linear regression analysis using 24-h EE as predictor. In each panel, the Pearson’s correlation coefficient (r) is reported along with its significance (p) and goodness of fit (r2).
No significant association was observed between residuals of total ad libitum intake after adjustment for RQ and body weight change (p=0.30, Figure 2B), even after adjustment for age, gender, and follow-up time. Similar results were obtained for residuals of total ad libitum intake after separate adjustments for CARBOX (p=0.28, Figure 2C), FFM (p=0.23, Figure 2D), FM (p=0.17) or FATOX (p=0.13).
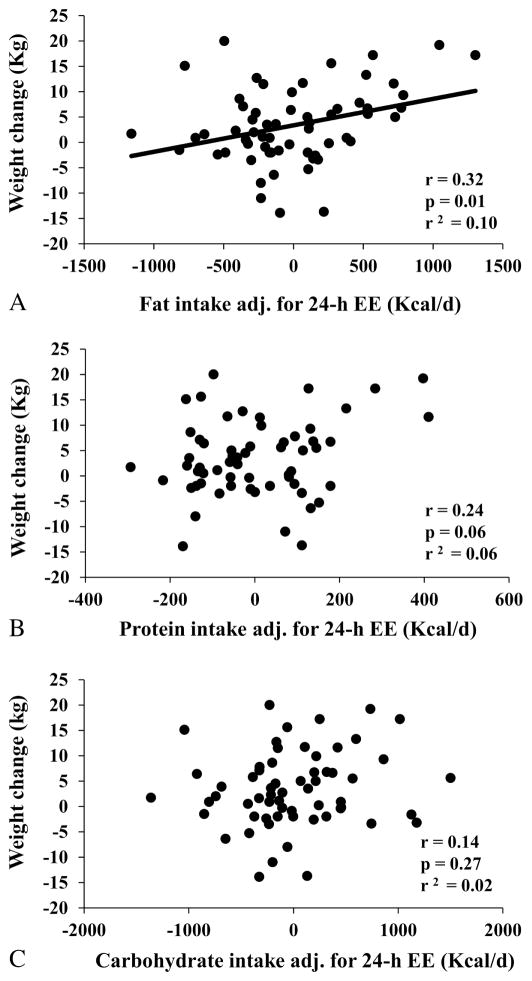
Residuals of fat intake adjusted for 24-h EE were directly correlated with body weight change (r=0.32, p=0.01, Figure 3A), even after adjustment for age, gender, and follow-up time (partial r=0.33, p=0.01). On average, overeating 100 Kcal/d from fat (approximately 11 g per day) was associated with an average weight gain of 0.56 Kg at the outpatient follow-up visit (95% CI: 0.13 to 0.99 kg). No associations were found between weight change and residuals of protein (p=0.06, Figure 3B) or carbohydrate (p=0.27, Figure 3C) intake.
Figure 3.
Relationships between body weight change and residuals of macronutrients intake during the 3-day vending period after adjustment for 24-h EE. Panels show the correlation between body weight change and residuals of fat intake (panel A), protein intake (panel B) and carbohydrate intake (panel C). Residuals were calculated via linear regression analysis using 24-h EE as predictor. In each panel, Pearson’s correlation coefficient (r) is reported along with its significance (p) and goodness of fit (r2).
DISCUSSION
In the present study, we confirmed positive associations between ad libitum energy intake and body FFM and metabolic measures including 24-h EE, RQ and CARBOX measured during energy balance and reflective of body energy demand. We found that normalizing ad libitum food intake to 24-h EE, but not to FFM, RQ or CARBOX, predicted long-term weight change, indicating that the individual propensity to overeating (particularly fat) relative to measured daily energy requirements, rather than absolute assessed caloric intake, is a determinant of weight gain over time.
Previous studies have shown positive associations between FFM, EE and energy intake in humans [4, 5, 18, 19]. The mechanism underlying the association between FFM and energy intake could be a plausible physiological demand created by the energy required to preserve body lean tissue. Yet, whether it is FFM per se or ultimately EE driving food intake is still a matter of discussion. Hopkins et al. demonstrated that daily food intake is predicted by both FFM and resting EE, with FFM indirectly influencing food intake through its effect on resting metabolism [9]. In line with the hypothesis that EE is the key regulator of food consumption in humans, our group demonstrated that both 24-h EE and RQ were predictors of ad libitum food intake, independently of FFM [8]. Moreover, in the same study the direct effect of FFM per se on food intake was negligible, as 80% of the total effect of FFM on energy intake was accounted by 24-h EE [8]. Taken together, these findings imply that EE is the foremost driver of daily food intake, as a physiological need to preserve energy balance.
The role of EE in determining weight gain remains unclear, and studies in the literature concerning the association between EE and body weight change are mixed [2]. A relatively lower 24-h EE as measured during energy balance predicts long-term weight gain in Native Americans [20–22]. Conversely, a relatively higher resting EE is associated with weight gain in a Nigerian population [23]. These contradictory results could be explained by differences in energy intake response to basal energy requirements, thus influencing daily energy balance and ultimately weight change [2]. In the present study, we assessed the individual ability to sense energy needs in relation to spontaneous weight change by using precise and reproducible measures of both EE and food intake, obtained separately and in a controlled setting, focusing on their causal link (energy sensing) [2, 7]. Despite overall overeating while on the vending machines as previously reported [10, 17], there was a large inter-subject variability in ad libitum overeating at all levels of adiposity and 20% of this variability in intake was explained by 24-h EE. For instance, we identified leaner subjects who overate with respect to that predicted by their absolute 24-h EE (data points above the regression line on the left side of Figure 1A), as well as heavier subjects who instead overate less than predicted by their actual higher 24-h EE (data points below the regression line on the right side of Figure 2A). Not only did this provide an actual measure of energy sensing using two independent measures but, importantly, this residual (unexplained) variance in ad libitum food intake after accounting for 24-h EE (our measure of energy sensing) was informative for the propensity of a person to gain weight in free-living conditions. This indicates that we have established a meaningful method for identifying individuals whose energy intake deviate from daily energy demands. Although the variance in weight change explained by EE-adjusted food intake was only 7%, this is comparable to the variance explained by other metabolic predictors of weight change in Native Americans including 24-h EE [20] and RQ [24]. It is important to note that the actual degree of overeating without adequate adjustment for 24-h EE was not a predictor of future weight change, indicating that cross-sectional assessment of energy intake relative to daily energy expenditure may be more important than assessment of actual energy intake alone to identify subjects predisposed to weight gain.
Relatively greater ad libitum food intake was positively associated with weight change only when food intake was adjusted for 24-h EE, but not for the other anthropometric or metabolic measures which have been related to food intake in our previous analyses [5, 10]. Specifically, we did not observe any association between ad libitum food intake after adjustment for FFM and weight change, suggesting that FFM may have an indirect effect on food intake only via its influence on metabolic demand. Our findings indicate that the relationship between higher EE and weight gain could be driven by a disproportional increase in food intake with respect to weight-maintaining energy requirements, hence advocating energy sensing as one of the mechanisms underlying the pathophysiology of weight gain. The energy-sensing mechanism may serve to achieve daily energy balance while also protecting against a fall of energy intake, thus representing an inherent mechanism to preserve body weight. This mechanism, as proposed by Blundell et al. [1], may modulate hunger to ultimately influence feeding behavior and the drive to eat.
The physiologic mechanisms of energy sensing in humans are still not well understood. The central nervous system likely plays an important role by enhancing peripheral signals from the gastrointestinal tract such as ghrelin, glucagon-like peptide 1, cholecystokinin, and insulin, as well as leptin from adipose tissue [25, 26]. These peptides have been associated with appetite and body weight regulation and may also represent biomarkers of eating behavior [27]. It is plausible that the physiological request for energy, together with inhibitory signals from adipose tissue and signals from the gastrointestinal tract, give rise to a tonic drive for food [1].
The basic principle of energy balance states that dynamic changes in food intake and EE regulate body weight change. Food intake is composed mainly of three macronutrient groups, which are carbohydrate, protein and fat, and total energy balance is achieved when each macronutrient intake matches the relative substrate oxidation. In our macronutrient intake analysis after accounting for 24-h EE, we showed that fat intake was the only macronutrient associated with weight change, indicating that regulation of fat intake by energy sensing, rather than protein or carbohydrate intake, is a determinant of weight gain. Over the past years, many studies have compared different diets for the maintenance of body weight, weight loss and even weight gain. To date, dietary macronutrient composition and its influence on weight change is still highly debated. In recent years, the “carbohydrate restriction theory” has been proposed as an effective dietary treatment for weight loss [28] and previous studies have shown that a greater body fat loss is achieved with reduced carbohydrate intake rather than fat intake, due to the decreased insulin secretion with carbohydrate restriction, which in turn leads to release of free fatty acids from adipose tissue, increased fat oxidation and EE [28, 29]. Conversely, other studies have shown that diets with a higher proportion of energy derived from fat lead to weight gain, presumably because fat has higher energy density in comparison to carbohydrate and protein [30, 31]. Hall et al. [32] showed a greater body fat loss after restricting dietary fat as compared to carbohydrate, although total weight loss was higher with the carbohydrate restriction diet. A meta-analysis of 48 randomized trials showed that both low-carbohydrate and low-fat diet produced equal weight loss [33]. Other studies using different diets have showed small and inconsistent differences on weight loss between each diet [34] and in recent years other meta-analyses have confirmed the same findings [35, 36]. We found that a relatively higher fat intake with respect to the intake predicted by 24-h EE led to weight gain, supporting the fat intake-weight gain link, and indicating that energy sensing may have a stronger effect on fat metabolism and eating behavior with regard to consumption of high-density fatty food.
The strength of our present study is the uniformity of our study group as all the individuals are Native Americans and we were able to obtain follow weights on these volunteers on average after 2 years following their initial assessment, without observing any seasonal variation in weight change. Furthermore, both EE and ad libitum food intake were measured in a carefully controlled inpatient setting using very accurate and highly reproducible methods including measurements of EE over 24 hours in a whole-room indirect calorimeter during weight maintenance and both total and macronutrient balance, as well as measurements of ad libitum food intake for three days using computerized vending machines and accounting for food left over in the metabolic kitchen. Nonetheless, several limitations should also be acknowledged. As the study cohort includes only Native Americans, an overweight population prone to weight gain [37] as this population may have distinctive metabolic characteristics, generalization to other ethnicities with different body habitus, lifestyle, and genetics is not straightforward. For instance, the association between EE and intake estimated in the current overweight cohort (mean BMI=33.6 kg/m2) may be influenced by obesity, therefore different results could be obtained in ethnic groups with smaller body size. More importantly, the high degree of overeating observed on the vending machines in our inpatient setting represents the major limitation of this study as these estimates of food intake, albeit highly accurate and reproducible (ICC=0.90)[17], may not be reflective of those observed in free-living conditions. Nevertheless, the inter-subject variance in ad libitum overeating explained by 24-h EE in our current study (19%) was similar to that (20%) explained by resting EE in a recent study including ad libitum food intake measurements closer to subject’s weight-maintaining energy needs [19], suggesting that the energy sensing relationship linking EE to food intake may be independent of the degree of overconsumption. More importantly, even in a context of widespread overeating as in our current study, we were still able to identify subjects who overate more than that predicted by their weight-maintaining EE, and these subjects were also those more prone to gain weight over time as opposed to those individuals that overate less than predicted by their 24-h EE.
CONCLUSIONS
We demonstrated for the first time that the variance in independent measures of energy intake and EE can provide a measure of energy sensing and, importantly, that this measure is relevant as the individual propensity to overeat relative to metabolic demands, mainly fat, is a predictor of weight change over a long-term follow-up. These results suggest that variation in energy sensing which underlies the link between EE and intake may be involved in appetite regulation by promoting overeating, thus eventually leading to future weight gain. The ability to measure energy sensing may shed light into the pathophysiology of obesity and might lead to novel weight loss therapies acting on reducing food intake via EE intervention.
Supplementary Material
Figure 4.
Acknowledgments
The authors wish to thank the volunteers who participated in our studies. They also thank the clinical staff of the Phoenix Epidemiology and Clinical Research Branch for conducting the examinations. The authors have nothing to disclose.
Funding. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- CARBOX
carbohydrate oxidation rate
- EE
energy expenditure
- FATOX
lipid oxidation rate
- FFM
fat free mass
- FM
fat mass
- FQ
food quotient
- ICC
intraclass correlation coefficient
- OGTT
oral glucose tolerance test
- RQ
respiratory quotient
- WMEN
weight-maintaining energy needs
Footnotes
Conflict of interest: The authors have nothing to disclose.
ClinicalTrials.gov identifier: NCT00342732.
Prior presentation. This study was presented in abstract form at the 2017 Obesity Week, Washington, DC, October 29th-November 2nd, 2017.
Author contributions
A.B. analyzed and interpreted data, and wrote the manuscript. S.H. assisted with the interpretation of the data and reviewed the manuscript. S.V. and J.K. designed the study, assisted with the interpretation of the data, and reviewed the manuscript. P.P. designed the study, performed data analysis, assisted with the interpretation of the data, revised the manuscript, and had primary responsibility for final content. All authors read and approved the final manuscript. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blundell JE, Caudwell P, Gibbons C, Hopkins M, Naslund E, King N, et al. Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Disease models & mechanisms. 2012;5:608–13. doi: 10.1242/dmm.009837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piaggi P, Vinales KL, Basolo A, Santini F, Krakoff J. Energy expenditure in the etiology of human obesity: spendthrift and thrifty metabolic phenotypes and energy-sensing mechanisms. Journal of endocrinological investigation. doi: 10.1007/s40618-017-0732-9. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam YY, Ravussin E. Variations in energy intake: it is more complicated than we think. The American journal of clinical nutrition. 2017;106:1169–70. doi: 10.3945/ajcn.117.167742. [DOI] [PubMed] [Google Scholar]
- 4.Blundell JE, Caudwell P, Gibbons C, Hopkins M, Naslund E, King NA, et al. Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. The British journal of nutrition. 2012;107:445–9. doi: 10.1017/S0007114511003138. [DOI] [PubMed] [Google Scholar]
- 5.Weise CM, Hohenadel MG, Krakoff J, Votruba SB. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. International journal of obesity. 2014;38:243–51. doi: 10.1038/ijo.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. The Journal of clinical investigation. 1986;78:1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dulloo AG, Jacquet J, Miles-Chan JL, Schutz Y. Passive and active roles of fat-free mass in the control of energy intake and body composition regulation. Eur J Clin Nutr. 2017;71:353–7. doi: 10.1038/ejcn.2016.256. [DOI] [PubMed] [Google Scholar]
- 8.Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher Daily Energy Expenditure and Respiratory Quotient, Rather Than Fat-Free Mass, Independently Determine Greater ad Libitum Overeating. The Journal of clinical endocrinology and metabolism. 2015;100:3011–20. doi: 10.1210/jc.2015-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkins M, Finlayson G, Duarte C, Whybrow S, Ritz P, Horgan GW, et al. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. International journal of obesity. 2016;40:312–8. doi: 10.1038/ijo.2015.155. [DOI] [PubMed] [Google Scholar]
- 10.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. The American journal of clinical nutrition. 2007;86:625–32. doi: 10.1093/ajcn/86.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annual review of nutrition. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 12.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Pettitt DJ, Saad MF, Charles MA, Nelson RG, Howard BV, et al. Obesity in the Pima Indians: its magnitude and relationship with diabetes. The American journal of clinical nutrition. 1991;53:1543S–51S. doi: 10.1093/ajcn/53.6.1543S. [DOI] [PubMed] [Google Scholar]
- 14.Lusk G. Animal calorimetry: analysis of oxidation of mixtures of carbohydrates and fat. J Biol Chem. 1924;59:41–2. [Google Scholar]
- 15.Abbott WG, Howard BV, Christin L, Freymond D, Lillioja S, Boyce VL, et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. The American journal of physiology. 1988;255:E332–7. doi: 10.1152/ajpendo.1988.255.3.E332. [DOI] [PubMed] [Google Scholar]
- 16.Rising R, Alger S, Boyce V, Seagle H, Ferraro R, Fontvieille AM, et al. Food intake measured by an automated food-selection system: relationship to energy expenditure. The American journal of clinical nutrition. 1992;55:343–9. doi: 10.1093/ajcn/55.2.343. [DOI] [PubMed] [Google Scholar]
- 17.Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. The American journal of clinical nutrition. 2010;91:343–8. doi: 10.3945/ajcn.2009.28315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caudwell P, Finlayson G, Gibbons C, Hopkins M, King N, Naslund E, et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. The American journal of clinical nutrition. 2013;97:7–14. doi: 10.3945/ajcn.111.029975. [DOI] [PubMed] [Google Scholar]
- 19.McNeil J, Lamothe G, Cameron JD, Riou ME, Cadieux S, Lafreniere J, et al. Investigating predictors of eating: is resting metabolic rate really the strongest proxy of energy intake? The American journal of clinical nutrition. 2017;106:1206–12. doi: 10.3945/ajcn.117.153718. [DOI] [PubMed] [Google Scholar]
- 20.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. The Journal of clinical endocrinology and metabolism. 2013;98:E703–7. doi: 10.1210/jc.2012-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. The New England journal of medicine. 1988;318:467–72. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 22.Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62:4043–51. doi: 10.2337/db13-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luke A, Durazo-Arvizu R, Cao G, Adeyemo A, Tayo B, Cooper R. Positive association between resting energy expenditure and weight gain in a lean adult population. The American journal of clinical nutrition. 2006;83:1076–81. doi: 10.1093/ajcn/83.5.1076. [DOI] [PubMed] [Google Scholar]
- 24.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. The American journal of physiology. 1990;259:E650–7. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 25.Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. International Journal of Obesity. 2001;25:S56. doi: 10.1038/sj.ijo.0801915. [DOI] [PubMed] [Google Scholar]
- 26.Keesey RE, Powley TL. Body energy homeostasis. Appetite. 2008;51:442–5. doi: 10.1016/j.appet.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blundell J, Levin F, King NA, Barkeling B, Gustafson T, Hellstrom P, et al. Overconsumption and obesity: peptides and susceptibility to weight gain. Regulatory peptides. 2008;149:32–8. doi: 10.1016/j.regpep.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Westman EC, Feinman RD, Mavropoulos JC, Vernon MC, Volek JS, Wortman JA, et al. Low-carbohydrate nutrition and metabolism. The American journal of clinical nutrition. 2007;86:276–84. doi: 10.1093/ajcn/86.2.276. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? Jama. 2014;311:2167–8. doi: 10.1001/jama.2014.4133. [DOI] [PubMed] [Google Scholar]
- 30.Fogelholm M, Anderssen S, Gunnarsdottir I, Lahti-Koski M. Dietary macronutrients and food consumption as determinants of long-term weight change in adult populations: a systematic literature review. Food & nutrition research. 2012;56:19103. doi: 10.3402/fnr.v56i0.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: shaped by global drivers and local environments. The Lancet. 2011;378:804–14. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 32.Hall KD, Bemis T, Brychta R, Chen KY, Courville A, Crayner EJ, et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell metabolism. 2015;22:427–36. doi: 10.1016/j.cmet.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston BC, Kanters S, Bandayrel K, Wu P, Naji F, Siemieniuk RA, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. Jama. 2014;312:923–33. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 34.Pagoto SL, Appelhans BM. A call for an end to the diet debates. Jama. 2013;310:687–8. doi: 10.1001/jama.2013.8601. [DOI] [PubMed] [Google Scholar]
- 35.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. The American journal of clinical nutrition. 2012;96:1281–98. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 36.Hu T, Mills KT, Yao L, Demanelis K, Eloustaz M, Yancy WS, et al. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. American journal of epidemiology. 2012;176:S44–S54. doi: 10.1093/aje/kws264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Votruba SB, Thearle MS, Piaggi P, Knowler WC, Hanson RL, Krakoff J. Weight maintenance from young adult weight predicts better health outcomes. Obesity. 2014;22:2361–9. doi: 10.1002/oby.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.