Abstract
Background
Major depression is the fourth leading cause of disability worldwide and poses a socioeconomic burden worldwide. Transcutaneous vagus nerve stimulation (tVNS) is a promising noninvasive clinical device that may reduce the severity of major depression. However, the neural mechanism underlying continuous tVNS has not yet been elucidated.
Objective
We aimed to explore the effect of hypothalamic subregion functional connectivity (FC) changes during continuous tVNS treatment on major depressive disorder (MDD) patients and to identify the potential biomarkers for treatment outcomes.
Methods
Forty-one mild to moderate MDD patients were recruited and received either real or sham tVNS treatment for 4 weeks. We used a seed-to-whole brain approach to estimate the FC changes of hypothalamic subregions and their surrounding control areas during continuous tVNS treatment and explored their association with clinical outcome changes after 4 weeks of treatment.
Results
Of the thirty-six patients that completed the study, those in the tVNS group had significantly lower scores on the 24-item Hamilton Depression (HAM-D) Rating Scale compared to the sham tVNS group after 4 weeks of treatment. The FC between the bilateral medial hypothalamus (MH) and rostral anterior cingulate cortex (rACC) was significantly decreased during tVNS but not during sham tVNS. The strength of this FC was significantly correlated with HAM-D improvements after 4 weeks of tVNS.
Conclusion
The FC between the bilateral MH and rACC may serve as a potential biomarker for the tVNS state and predict treatment responses. Our results provide insights into the neural modulation mechanisms of continuous tVNS and reveal a potential therapeutic target for MDD patients.
Keywords: Major depressive disorder, Hypothalamus, Rostral anterior cingulate cortex, Functional connectivity, Biomarker, Transcutaneous vagus nerve stimulation
Introduction
Major depressive disorder (MDD), characterized by persistent sadness, pessimism, social withdrawal, low self-confidence, and compromised cognitive disabilities [1], has been recognized as the fourth leading cause of disability worldwide [2]. Despite the availability of a variety of treatments, around 30% of patients fail to respond to antidepressant medication [3] or psychological treatment [4]. Vagus nerve stimulation (VNS) is an effective Food and Drug Administration (FDA) approved method for treatment-resistant MDD [5–7]. However, the involvement of surgery as well as potential side effects have largely limited its application [8].
Recently, transcutaneous VNS (tVNS), a noninvasive and safe variant of traditional VNS, has drawn the interest of researchers for treating MDD [8–12]. tVNS stimulates the afferent auricular branch of the vagus nerve located on the surface of the ear and produces a similar effect to classical VNS in reducing depressive symptoms without any surgical intervention [11,13]. Although tVNS has shown promising results in treating MDD patients in several studies [8,10,11], its underlying mechanism remains poorly understood.
Using functional magnetic resonance imaging (fMRI), investigators have studied resting-state functional connectivity (rsFC) changes before and after four weeks of treatment [10,14] and fMRI signal changes evoked by intermittent real and sham tVNS using a block design [8,9,15]. Yet, the neural mechanism of tVNS during continuous stimulation, which is the way tVNS is applied in our clinical report [11] and others [13], has rarely been studied. In this manuscript, we propose that continuous electrical stimulation may bring the brain into a new state known as the “the continuous tVNS state” (for abbreviation, we call it the ‘tVNS state’ in the rest of the paper), offering researchers a new angle to explore the neural modulation and therapeutic effects of tVNS.
The hypothalamus is known to mediate many neuroendocrine and neurovegetative functions [16–18]. Through the hypothalamic-pituitary-adrenal (HPA axis), the hypothalamus drives both the acute cortisol response to stress and cortisol secretion in a circadian rhythm [19–21]. Although the maladaptive activation of the HPA axis is characterized across many studies of depressed patients, other hypothalamic functions and nuclei have remained largely unexplored. A recent study demonstrated decreased hypothalamic functional connectivity (FC) with the subgenual cortex in psychotic major depression, which suggests that hypothalamic communication with the rest of the brain is critically important for many physiological and psychological functions [22]. Therefore, studying how tVNS can modulate the FC of different hypothalamic subregions and its association with clinical improvement can provide us with a better understanding of the mechanism of tVNS and the pathophysiology of depression.
Recently, FC analyses have been widely used to explore the biomarkers for neurologic and psychiatric diseases in order to extend the focus from isolated regions to a network of regions [23–25]. Thus, in the present work, we explored baseline FC differences between the hypothalamus and other brain regions during continuous real and sham tVNS, as well as the no-stimulation resting state before tVNS stimulation. In addition, we explored the association between FC strength during baseline treatment and clinical improvement after four weeks. We hypothesized that real but not sham tVNS would significantly modulate the FC of the hypothalamus in MDD patients, and this FC may serve as a distinct biomarker for the tVNS state, as well as predict treatment outcomes.
Material and Methods
The present study is based on a brain imaging study nested in a clinical trial (ChiCTR-TRC-11001201). The inclusion criteria (e.g., comorbidities, medications) and intervention details (e.g., equipment, MRI environmental setup, electrical stimuli) can be found in the clinical report [26]. In our prior imaging studies, a subset of 35 patients was used to investigate changes in the resting-state default mode network (DMN) after four weeks of tVNS treatment [10], and a subset of 38 patients was used to study the changes in fMRI signals induced by intermittent tVNS [9]. In this study, we focus on how 6 minutes of continuous tVNS modulates the FC of the hypothalamus, a key region in the limbic system, using a seed-to-whole-brain approach. This continuous tVNS dataset at baseline has never been analyzed or published before.
Participants
The study was approved by the Institutional Ethics Committee of the China Academy of Chinese Medical Sciences. All experiments were performed in accordance with approved guidelines. Due to safety and ethical concerns and to increase the homogeneity of the study, we only included participants with mild or moderate depressive symptoms. All patients were recruited using advertisements and by sending flyers to the four hospitals involved in the study. Forty-one patients with mild or moderate MDD were recruited for the trial. ICD-10 classification of mental and behavioral disorders was used for the diagnosis of MDD. Patients who voluntarily provided informed consent and met inclusion criteria were enrolled in this study.
Inclusion criteria
1) Meets ICD-10 diagnosis standards for a depressive episode: mild (2 typical + 2 other core symptoms), moderate (2 typical + 3 other core symptoms); 2) 18-70 years of age; 3) Willing to stop taking anti-depressive or other psychiatric medications 2 weeks before beginning the intervention; 4) Junior high or higher level education; 5) Exhibit symptoms for 2 weeks to 2 years.
Exclusion criteria
1) Ongoing addiction to drugs and/or alcohol; 2) Bipolar disorder; 3) Organic mental disorder; 4) Drug-induced depression; 5) Seasonal affective disorder; 6) Severe medical disorders; 7) Pregnant women; 8) Postpartum depression; 9) Dementia or other cognitive disorders; 10) Patients who do not agree to sign the consent form.
Procedures
A single-blinded clinical trial was used to investigate the antidepressant effects of real tVNS treatment. The first cohort of patients all received real tVNS treatment for 12 weeks. After demonstrating the effect of tVNS, we recruited a second cohort of patients who received four weeks of sham tVNS before shifting to real tVNS for eight weeks. At the beginning of the treatment (baseline), the fMRI scans were applied during both resting state and 6-minutes of continuous real or sham tVNS. In this manuscript, we compared FC differences during continuous real tVNS and sham tVNS on the right ear at baseline, as well as predicted treatment outcomes after four weeks (Figure 1, A).
Figure 1.
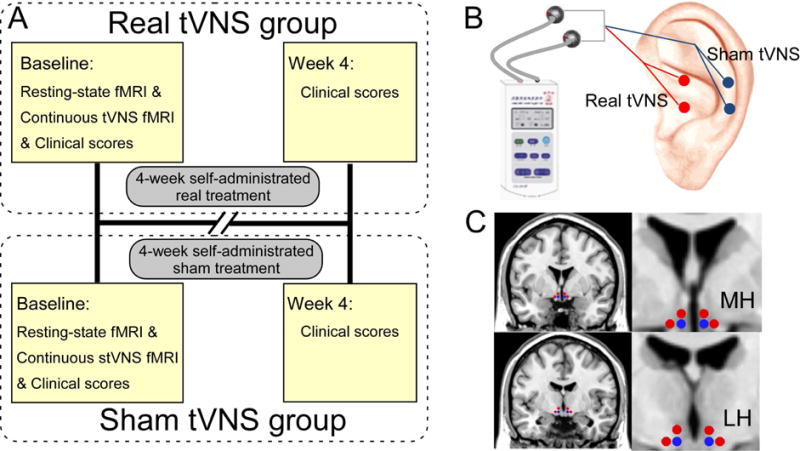
The study protocols. (A) Procedures and data used for the study. In baseline, a resting-state fMRI, a continuous tVNS fMRI and clinical scores were collected and used for the patient in the real tVNS group. All subsequent 4-week treatments were self-administered by the patients at home, except for the 6-mintue tVNS during the MRI scan. The investigators collected the patient’s clinical scores at the end of the 4-week treatment. All procedures performed in the sham tVNS treatment group were identical to the procedures for the real tVNS group, except for the location of stimulation. (B) Locations for real tVNS and sham tVNS stimulation. (C) Selected medial hypothalamus (MH) and lateral hypothalamus (LH) for seed-base whole-brain connectivity analyses (seeds marked in blue). Red dots represent the positions of controlled null seeds.
Intervention
The stimulation points for real tVNS are located in the auricular concha area where there is rich vagus nerve branch distribution (Figure 1, B). Real tVNS was applied on the concha area of both ears simultaneously during the treatments except during the MRI scan in which tVNS was applied on the right ear. After being disinfected, electrodes were attached to the ear area (i.e. auricular concha) at the stimulation site. Stimulation parameters included: 1) density wave adjusted to 20 Hz with a wave width less than 1 millisecond and 2) intensity adjusted based on the tolerance of the patient (typically between 4 – 6 mA). Each treatment lasted 30 minutes and was carried out twice a day (i.e. once in the morning and once again in the evening), at least 5 days per week, for the duration of the treatment period (4 weeks). Patients were also instructed to record any side effects of treatment in a diary each day.
The treatment procedure of sham tVNS was the same as real tVNS except that the stimulation points for sham tVNS were located at the superior scapha (outer ear margin midpoint) where there is no vagus nerve distribution [26,27] (Figure 1, B).
After the baseline MRI scan, all subsequent treatments were self-administered by the patients at home after adequate training. Patients were also instructed to complete a patient diary booklet each day to describe any side effects corresponding with or temporally related to treatment. The investigators checked the patient diaries at the end of the 4-week treatment.
Clinical outcomes
The primary clinical outcome measure of this study was the 24-item Hamilton Depression Rating Scale (HAM-D); the secondary outcomes included the 14-item Hamilton Anxiety Rating Scale (HAM-A), the Self-Rating Anxiety Scale (SAS), and the Self-Rating Depression Scale (SDS), which were measured at baseline and week 4.
Data acquisition
fMRI data was acquired with a 1.5 Tesla GE Sigma MRI system (GE Healthcare, Buckinghamshire, United Kingdom) equipped with a standard two-channel birdcage head coil. Subjects were asked to stay awake and to keep their heads still during the scan. Prior to the functional run, T1-weighted high-resolution structural images were acquired with the three-dimensional fast spoiled gradient-echo sequence (matrix 192×256, field of view 200 mm, flip angle 15°, slice thickness 1.4 mm). T2-weighted functional images encompassing the whole brain were acquired with the gradient echo echo-planar imaging sequence (echo time 30 ms, repetition time 2500 ms, matrix 64×64, field of view 240 mm, flip angle 90°, slice thickness 3.0 mm, gap 0.5 mm, 41 slices, parallel to the anterior commissure-posterior commissure line). Image collection was preceded by four dummy scans to allow for equilibration of the MRI signal.
A 6-minute resting-state fMRI session was acquired prior to treatment. During treatment, the right ear was stimulated continuously for 6 minutes with either real or sham tVNS. To avoid the noise evoked by the electrical current of the tVNS, the connecting line between the stimulator and the ear was electromagnetically shielded. A round tin electrode, about 5 mm in diameter, was installed at the distant end of the line. The electrodes were fixed by tape during the 6-minute fMRI scan.
fMRI data analysis
fMRI data preprocessing
The fMRI data were preprocessed and analyzed using SPM12 (Wellcome Trust Center for Neuroimaging, London, UK). The first ten volumes were discarded to allow for signal equilibration. Images were slice timing corrected, head motion corrected, co-registered to respective structural images for each subject, segmented, regressed out 6 head motion parameters, white matter signals, and cerebrospinal fluid (CSF) signals, normalized by using Montreal Neurological Institute (MNI) space [28], and spatially smoothed using a Gaussian kernel of 8 mm full width at half maximum (FWHM = 8 mm). The resulting images were detrended and bandpass filtered (0.01 – 0.08 Hz). In the last step, global signal regression (GSR) was applied to reduce head motion-related signal changes [29].
Seed-based FC
The bilateral medial hypothalamus (MH) (MNI coordinates: x = ±4; y = −2; z = −12 plus 2 mm sphere) and lateral hypothalamus (LH) (MNI coordinates: x = ±6; y = −9; z = −10 plus 2 mm sphere) [30] were selected as seeds using WFU-Pick Atlas software [31] (Figure 1, C). FCs were computed between the time courses of the seed and time courses of every other voxel using Pearson’s correlation coefficients. Correlation coefficients were Fisher z-transformed to increase normality and allow for statistical analyses. We also selected two null seeds next to the MH (x = ±8; y = −2; z = −12 plus 2 mm sphere; x = ±4; y = −2; z = −8 plus 2 mm sphere; Figure 1, C) and two null seeds next to the LH (x = ±10; y = −9; z = −10 plus 2 mm sphere; x = ±6; y = −9; z = −6 plus 2 mm sphere; Figure 1, C), in order to study the specific phenomenon of the hypothalamus.
Statistical analyses
Clinical data analyses
All statistical analyses of clinical scores were performed using SPSS 22 (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). A two-sample t-test (the normality of the data was tested using a Shapiro-Wilk test) was applied to compare the baseline characteristics between the patients in the real and sham groups. A repeated measurement analysis (the homogeneity of variance was tested using a Levene’s test) was applied to compare the treatment effects (baseline vs. week 4) between the real and sham groups.
Functional data analyses
MH/LH seed-to-voxel FC between-group analyses (continuous real tVNS vs. sham tVNS) were performed using a two-sample t-test. A threshold of a voxel-wise p < 0.001 (uncorrected) and a cluster-level p < 0.05 (cluster-based false discovery rate [FDR] corrected) were applied for the correction of multiple comparisons [32].
To identify the distinct FC biomarkers for the tVNS state and predict treatment effects, we computed partial correlations, adjusting for age and gender, between HAM-D improvements (post-treatment vs. pre-treatment) and extracted FCs from seed-to-voxel between-group analyses. In addition, we also extracted FCs from respective resting-state fMRI scans and correlated them with HAM-D improvements.
Results
Clinical outcome
There were 20 participants in the tVNS group and 21 participants in the sham tVNS group that participated in the baseline MRI scan. Three participants from the tVNS group and two participants from the sham tVNS group were dropped from the study at the end of week four. Baseline characteristics of the two groups are shown in Table 1. No significant differences were observed between the two groups at baseline.
Table 1.
Clinical outcome of different measurements in tVNS group and sham tVNS group (pre-treatment and post-treatment).
| Clinical Outcome | tVNS Group | Sham tVNS Group | Effect of Treatment | Effect of Interaction | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |||
|
|
||||||
| HAM-D | 30.47±4.38 | 17.29±5.72 | 28.42±3.49 | 23.16±4.65 | F(1,34)=16.73, p < 0.001 | F(1,34)=16.73, p < 0.001 |
| HAM-A | 21.41±5.71 | 11.65±4.77 | 17.37±4.21 | 13.63±3.82 | F(1,34)=76.94, p < 0.001 | F(1,34)=15.34, p < 0.001 |
| SAS Score | 58.53±7.04 | 47.76±11.64 | 58.79±10.66 | 54.68±6.38 | F(1,34)=16.57, p < 0.001 | F(1,34)=3.32, p = 0.08 |
| SDS Score | 70.35±8.23 | 53.29±14.35 | 67.00±8.44 | 61.47±9.31 | F(1,34)=37.64, p < 0.001 | F(1,34)=9.82, p = 0.004 |
HAM-D: Hamilton Depression Rating Scale; HAM-A: Hamilton Anxiety Rating Scale; SAS: Self-Rating Anxiety Scale; SDS: Self-Rating Depression Scale.
Post-treatment clinical outcomes are also summarized in Table 1. Overall, tVNS treatment (pre vs. post) had significant effects on all measurements. We also observed significant interactions between treatment and group (real vs. sham) in HAM-D, HAM-A and SDS (HAM-D: F(1,34) = 16.73, p < 0.001; HAM-A: F(1,34) = 15.34, p < 0.001; SDS: F(1,34) = 9.82, p = 0.004), indicating the real tVNS group had significantly stronger treatment effects than the sham tVNS group.
Functional connectivity results
The strength (represented by z scores) of MH based FCs was significantly lower (real < sham) in the real tVNS group compared to the sham tVNS group in the rostral anterior cingulate cortex (rACC; pcluster-corr=0.022) and right medial frontal gyrus (MFG; pcluster-corr=0.003), and significantly higher (real > sham) in the bilateral cerebellum (LCB; pcluster-corr=0.020 and RCB: pcluster-corr=0.036; Figure 2, First column and Table 2). No significant region was observed in the null seeds next to the MH.
Figure 2.
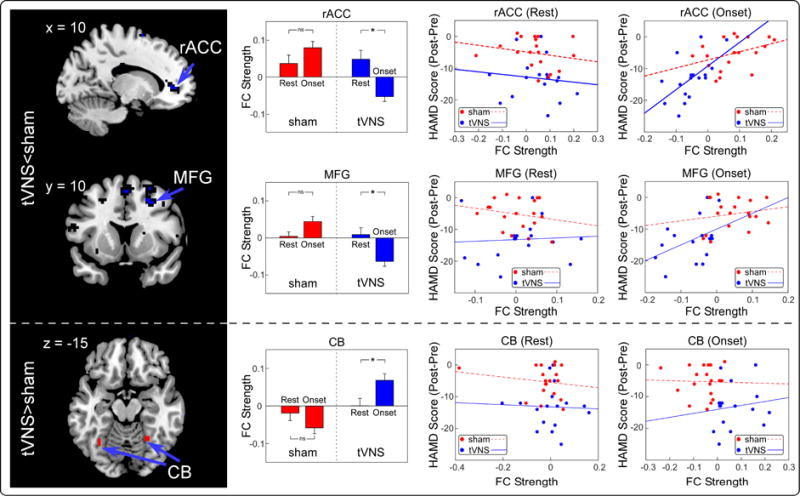
Identified significantly different MH FCs and their relationships with HAM-D improvements. First column: Compared to sham group, tVNS group had significantly lower FCs in rACC and MFG, while higher FCs in CB. Second column: real but not sham tVNS significantly modulated the strength of MH-rACC, MH-MFG and MH-CB. Third column: All connections in sham and real groups during resting-state were not significantly correlated with HAM-D improvements. Fourth column: Only the strength of MH-rACC in real tVNS group during treatment was significantly correlated with HAM-D improvements. ns: no significance; *: p < 0.05.
Table 2.
Identified significantly different brain regions between real and sham tVNS groups.
| Brain Regions | Hemisphere | MNI coordinates
|
k | T | Z | Peak p (unc) | Cluster p (FDR-corr) | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Sham>Real | |||||||||
| rACC | L | −15 | 41 | 5 | 37 | 4.30 | 3.82 | p < 0.001 | p = 0.022 |
| MFG | R | 27 | 14 | 44 | 70 | 4.51 | 3.96 | p < 0.001 | p = 0.003 |
| Real>Sham | |||||||||
| Left CB | L | −33 | −55 | −13 | 39 | 4.05 | 3.64 | p < 0.001 | p = 0.020 |
| Right CB | R | 33 | −43 | −19 | 56 | 3.90 | 3.52 | p < 0.001 | p = 0.036 |
Previous studies showed that rACC activity predicts treatment responses for MDD patients [33,34], we thus extracted the FCs between the hypothalamus and identified rACC during real and sham tVNS treatment, as well as during resting state fMRI scans applied before the tVNS scans. We found tVNS significantly reduced the MH-rACC FC (p = 0.004; paired t-test) compared to resting state FC, while sham tVNS did not (p = 0.17; paired t-test). The MH-rACC FC during tVNS was significantly correlated with HAM-D improvements (r = 0.58, p = 0.02; Figure 2, Top right panel, Blue line), but not during sham tVNS (r = 0.39, p = 0.10; Figure 2, Top right panel, Red dashed line). In addition, the resting-state MH-rACC FCs of MDD patients in both real and sham groups were not significantly correlated with HAM-D improvements (Figure 2, Top middle panel and Table S1).
As an exploratory aim, we also investigated the modulation effects of tVNS between the MH and the other two brain regions. Results showed that tVNS significantly modulated MH-MFG and MH-cerebellum FCs compared to resting state FC and that there were significant differences between the real and sham tVNS groups during treatment. These differences were not significantly correlated with HAM-D improvements during rest or treatment (Figure 2, middle/bottom panel and Table S1).
Functional connectivity analysis using LH as a seed showed that FCs in the mid-cingulate cortex (MCC) and putamen were significantly lower in the real tVNS group compared to the sham tVNS group (Figure 3 and Table 2). Compared to resting state data, both real and sham tVNS significantly modulated the strength of two connections (Table S2). We also explored the association between FC and clinical improvement and found LH-MCC and LH-putamen FCs were not significantly correlated with HAM-D improvements in both real and sham tVNS groups during rest and treatment (Table S1). No significant region was observed in the null seeds next to the LH.
Figure 3.
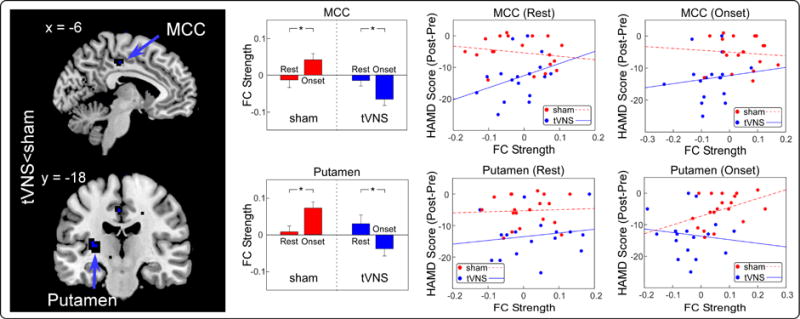
Identified significantly different LH FCs and their relationships with HAM-D improvements. First column: Compared to sham group, tVNS group had significantly lower FCs in MCC and Putamen. Second column: Both real and sham tVNS significantly modulated the strength of LH-MCC and LH-MFG. Third column: All connections in sham and real groups during resting-state were not significantly correlated with HAM-D improvements. Fourth column: All connections in sham and real groups during treatment were not significantly correlated with HAM-D improvements. *: p < 0.05.
Discussion
In the present study, we explored the FC of the subregions of the hypothalamus during real and sham tVNS treatment in MDD patients, as well as the respective resting state FC. We found real tVNS showed significantly higher FC between the MH and cerebellum and lower FC between the MH and both rACC and MFG and decreased FC between the LH and both MCC and putamen compared to sham tVNS. In addition, compared to resting state FC, the FC between the MH and rACC was significantly modulated by real tVNS but not by sham tVNS. The strength of this connection in the tVNS state was significantly correlated with treatment effects after four weeks, suggesting that MH-rACC FC may be a distinct biomarker for the tVNS state and may predict treatment outcomes for depression.
The mechanism of VNS treatment remains unclear. Hypotheses are based on the impact of anatomy and function/neurotransmitter changes evoked by VNS/tVNS in mood control. Studies suggest that the anti-depressant effect of tVNS may be due to the vagal projections from the nucleus tractus solitarius (NTS) to brain structures that are associated with the regulation of mood and emotion [35]. Neuroimaging studies have demonstrated that VNS/tVNS can modulate BOLD responses in widespread brain regions including the hypothalamus, orbitofrontal cortex (OFC), parieto-occipital cortex, temporal lobe, amygdala, insula, thalamus, hippocampus, postcentral gyrus, nucleus accumbens (NAc), and brainstem [15,36–40]. Some of these brain regions are also believed to be involved in the pathogenesis and remission of MDD, particularly the hypothalamus [18,22].
In a previous study, we investigated DMN resting state FC changes at baseline and after four weeks of tVNS and sham tVNS treatment [10]. We found that FC of the DMN within the precuneus and orbital prefrontal cortex increased, and these FC increases were associated with improvements in HAM-D scores. This result reveals how tVNS can modulate intrinsic brain functional connectivity and consequently reduce the severity of major depression. More recently, we studied fMRI signal changes evoked by intermittent real and sham tVNS using a block design to explore potential biomarkers for tVNS longitudinal treatment [9]. We found the activation level in the left anterior insula was enhanced by tVNS compared with sham tVNS, and this enhancement was associated with an improvement in longitudinal treatment outcomes. The identification of a biomarker to characterize the tVNS state could promote our understanding of treatment mechanisms and help in the development of therapeutic targets for MDD.
FC allows researchers to study the pathophysiology of neurologic diseases and to translate fMRI into clinically relevant information. In the present study, we found that (1) the FC between the MH and rACC was significantly modulated by tVNS but not by sham tVNS; and (2) the strength of MH-rACC FC during treatment at baseline was significantly associated with the treatment effect of tVNS in the following four weeks but not the treatment effect of sham tVNS. Therefore, MH-rACC FC could serve as a distinct biomarker for the tVNS state and predict the therapeutic performance of MDD patients after four weeks of tVNS treatment.
Literature suggests that hypothalamic communication with other cortical and subcortical regions of the brain is critical for maintaining neuroendocrine and affective processes [18]. In addition, activity in the anterior cingulate cortex (ACC), particularly the pregenual ACC, has been recognized as a predictor of treatment responses in depression [33]. Mayberg et al. first reported that increased resting glucose metabolism in the rACC before the onset of pharmacological treatment predicted better treatment responses in MDD patients [41]. Similar findings have been reported using EEG [34,42,43], positron emission tomography (PET) [44,45], and fMRI [46–49]. The rACC represents one of the main ‘hubs’ within the default network (DN), an intrinsically organized functional network that has been associated with a variety of self-referential processes, including introspective processing, remembering the past, and planning the future [50–53]. Dysregulation of the hypothalamus and hyperactivity of the rACC are both frequently observed in depression, but how these two regions interact with one another is poorly understood [22,33].
Our results extended previous findings and found that decreased MH-rACC FC during tVNS may reveal the antidepressant effect of tVNS treatment based on bottom-up processing. Specifically, continuous tVNS modulates the activity of nucleus of the solitary tract (NTS), a series of nuclei that relay information to the hypothalamus and indirectly project information to higher brain structures, such as the rACC. The strength of the connection of the hypothalamus and rACC thus can be a useful marker indicating the treatment effect of tVNS. In addition, depression is also a common psychiatric comorbidity of chronic pain disorders [54]. Studies suggest that rACC plays an important role in the pathophysiology of chronic pain and pain modulation [55–59]. The enhanced connectivity between the hypothalamus-rACC may also implicate the potential of tVNS for the treatment of chronic pain disorders.
We also found that other brain connections, including the MH-MFG and MH-cerebellum, were significantly modulated by tVNS but not by sham tVNS. However, an exploratory analysis showed there were no significant associations between these FCs and clinical outcomes, and further studies are needed to explore the role of these regions in tVNS. Compared to resting state data, sham tVNS also significantly modulated (1) connections between the LH and MCC which may be due to epiphenomenal sensory activation and (2) connections between the LH and putamen which may be associated with the placebo effect of sham tVNS [60].
Previous studies highlighted the functional importance of the interaction between the hypothalamus and ACC, as well as prefrontal cortices [61,62], and reported abnormal FC between the hypothalamus and subgenual cortex in major depression [22]. Although both MH and LH are involved in the regulation of reward and mood and modulate anxiety-like behavior [18], they may belong to different neural circuitries. For example, using the seeds applied in our study, Kullmann et al. suggested that MH and LH are tapped into different neural networks. Specifically, the MH network reflects major components of the reward system which is responsible for salience and learning and emotion, while LH is integrated into the dorsal striatum network which has been proposed as a translational network linking cognition and emotion or salience processing [30]. In this study, we demonstrated specific neural modulation pathways between the hypothalamus and ACC during tVNS treatment of MDD. Further studies are needed to validate our findings.
There are several limitations in this study. First, the treatment was only four weeks in duration. Thus, the results obtained only represent short-term to mid-term effects. Further studies are needed to evaluate the long-term effects of tVNS treatment. Second, we did not have detailed medical histories of these patients; thus we could not include these factors as covariates in the analyses. Third, the treatment effect of tVNS may have been influenced by the short washout period (i.e. the period of time when patients stop taking antidepressants or other psychiatric medications prior to intervention). Our experimental setup has minimized this potentially confounding factor by comparing real and sham treatment groups with an identical washout time. Fourth, all patients were recruited from four participating hospitals through advertisements and flyers, and both real and sham groups were recruited using identical criteria. We believe this convenience sample method should not influence the validity of this study, but we cannot ensure that the sample selected in this study represents the entire population. Future randomized studies in different populations are needed to further replicate our findings. Finally, this is not a randomized study. Being the first study to use only tVNS on patients suffering from mild and moderate depression, we thought it would be wise to first test the effectiveness of tVNS. We first completed the real tVNS cohort and then collected data for the sham tVNS cohort. Since the baseline characteristics were similar in the two cohorts of patients and the study was completed within 1 year, we do not expect the design to largely influence the validity of the study. Nevertheless, future studies with a randomized study design are needed.
Conclusion
In summary, we found that continuous tVNS, but not sham tVNS, significantly modulated the strength of MH-rACC FC, and the strength of this connection during treatment was significantly associated with tVNS treatment effects. Our findings provide a biomarker for the tVNS state and a better understanding of the neural mechanism underlying tVNS treatment in MDD patients.
Supplementary Material
Highlights.
Transcutaneous vagus nerve stimulation (tVNS) reduced depression symptoms.
The hypothalamus-rACC connectivity was modulated by real but not sham tVNS.
The strength of hypothalamus-rACC connectivity was predictive to treatment outcome.
Acknowledgments
The work is supported by the Special Program of Chinese Medicine of the National Basic Research Program of China (973 Program 2012CB518503), the “Twelfth Five-year Plan” National Science and Technology Support Program of China (2012BAF14B10), the National Natural Science Foundation of China (81471389, 81473780, 81273674, 81303056), and the Beijing Natural Science Foundation of China (7111007). Jian Kong is supported by R01AT006364, R01AT008563, R21AT008707, R61AT009310, and P01AT006663 from NIH/NCCIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
J.K has a disclosure to report (holding equity in a startup company (MNT)), but declares no conflict of interest.
References
- 1.Belmaker RH, Agam G. Major Depressive Disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 2.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–86. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of Outcomes With Citalopram for Depression Using Measurement-Based Care in STAR*D: Implications for Clinical Practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 4.DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, et al. Cognitive Therapy vs Medications in the Treatment of Moderate to Severe Depression. Arch Gen Psychiatry. 2005;62:409–16. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- 5.Schlaepfer T, Frick C, Zobel A, Maier W. Vagus nerve stimulation for depression: efficacy and safety in a European study. Psychol Med. 2008;38:651–61. doi: 10.1017/S0033291707001924. [DOI] [PubMed] [Google Scholar]
- 6.Rush A, George M, Sackeim H, Marangell L. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47:276–86. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 7.Sackeim H, Rush A, George M. Vagus nerve stimulation (VNS™) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–28. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 8.Kraus T, Hösl K, Kiess O, Schanze A. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm. 2007;114:1485–93. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- 9.Fang J, Egorova N, Rong P, Liu J, Hong Y, Fan Y, et al. Early cortical biomarkers of longitudinal transcutaneous vagus nerve stimulation treatment success in depression. NeuroImage Clin. 2017;14:105–11. doi: 10.1016/j.nicl.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, et al. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol Psychiatry. 2016;79:266–73. doi: 10.1016/j.biopsych.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong P, Fang J, Wang L. Transcutaneous vagus nerve stimulation for the treatment of depression: a study protocol for a double blinded randomized clinical trial. BMC Complement Altern Med. 2012;12:255. doi: 10.1186/1472-6882-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trevizol AP, Shiozawa P, Taiar I, Soares A, Gomes JS, Barros MD, et al. Transcutaneous Vagus Nerve Stimulation (taVNS) for Major Depressive Disorder: An Open Label Proof-of-Concept Trial. Brain Stimul. 2016;9:453–4. doi: 10.1016/j.brs.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Hein E, Nowak M, Kiess O, Biermann T. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm. 2013;120:821–7. doi: 10.1007/s00702-012-0908-6. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Fang J, Wang Z, Rong P, Hong Y, Fan Y. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J Affect Disord. 2016;205:319–26. doi: 10.1016/j.jad.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Frangos E, Ellrich J, Komisaruk B. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8:624–36. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman J, Ostrander M, Mueller N. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Mogenson G, Jones D, Yim C. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 18.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of Depression. Neuron. 2002;34:13–25. doi: 10.1016/S0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 19.Pariante C, Lightman S. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–8. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Young E, Abelson J, Cameron O. Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biol Psychiatry. 2004;56:113–20. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Pariante C, Miller A. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 22.Sudheimer K, Keller J, Gomez R, Tennakoon L, Reiss A, Garrett A, et al. Decreased hypothalamic functional connectivity with subgenual cortex in psychotic major depression. Neuropsychopharmacology. 2015;40:849–60. doi: 10.1038/npp.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;24:424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 24.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rong P, Liu J, Wang L, Liu R, Fang J, Zhao J. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172–9. doi: 10.1016/j.jad.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang F, Dong J, Kong J, Wang H, Meng H, Spaeth RB, et al. Effect of transcutaneous auricular vagus nerve stimulation on impaired glucose tolerance: a pilot randomized study. BMC Complement Altern Med. 2014;14:203. doi: 10.1186/1472-6882-14-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashburner J, Friston K. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–41. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullmann S, Heni M, Linder K, Zipfel S, Häring H-U, Veit R, et al. Resting-state functional connectivity of the human hypothalamus. Hum Brain Mapp. 2014;35:6088–96. doi: 10.1002/hbm.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 32.Woo C, Krishnan A, Wager T. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–9. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizzagalli DA, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, et al. Anterior Cingulate Activity as a Predictor of Degree of Treatment Response in Major Depression: Evidence From Brain Electrical Tomography Analysis. Am J Psychiatry. 2001;158:405–15. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 35.Mohr P, Rodriguez M, Slavíčková A, Hanka J. The application of vagus nerve stimulation and deep brain stimulation in depression. Neuropsychobiology. 2011;64:170–81. doi: 10.1159/000325225. [DOI] [PubMed] [Google Scholar]
- 36.Bohning D, Lomarev M, Denslow S. Feasibility of vagus nerve stimulation–synchronized blood oxygenation level–dependent functional MRI. Invest Radiol. 2001;36:470–9. doi: 10.1097/00004424-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Lomarev M, Denslow S, Nahas Z, Chae J. Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res. 2002;36:219–27. doi: 10.1016/s0022-3956(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 38.Nahas Z, Teneback C, Chae J, Mu Q. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology. 2007;32:1649–60. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 39.Narayanan J, Watts R, Haddad N, Labar D, Li P. Cerebral activation during vagus nerve stimulation: a functional MR study. Epilepsia. 2002;43:1509–14. doi: 10.1046/j.1528-1157.2002.16102.x. [DOI] [PubMed] [Google Scholar]
- 40.Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, et al. VNS Therapy in Treatment-Resistant Depression: Clinical Evidence and Putative Neurobiological Mechanisms. Neuropsychopharmacology. 2006;31:1345–55. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 41.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 42.Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, et al. Prediction of treatment response in major depression: integration of concepts. J Affect Disord. 2007;98:215–25. doi: 10.1016/j.jad.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Korb AS, Hunter AM, Cook IA, Leuchter AF. Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. Clin Neurophysiol. 2009;120:1313–9. doi: 10.1016/j.clinph.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LR. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatry. 2003;160:522–32. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- 45.Holthoff VA, Beuthien-Baumann B, Zundorf G, Triemer A, Ludecke S, Winiecki P, et al. Changes in brain metabolism associated with remission in unipolar major depression. Acta Psychiatr Scand. 2004;110:184–94. doi: 10.1111/j.1600-0447.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 46.Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The Neural Substrates of Affective Processing in Depressed Patients Treated With Venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- 47.Chen C-H, Ridler K, Suckling J, Williams S, Fu CHY, Merlo-Pich E, et al. Brain Imaging Correlates of Depressive Symptom Severity and Predictors of Symptom Improvement After Antidepressant Treatment. Biol Psychiatry. 2007;62:407–14. doi: 10.1016/j.biopsych.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, et al. Increased Anterior Cingulate Cortical Activity in Response to Fearful Faces: A Neurophysiological Biomarker that Predicts Rapid Antidepressant Response to Ketamine. Biol Psychiatry. 2009;65:289–95. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, et al. Frontal and Limbic Activation During Inhibitory Control Predicts Treatment Response in Major Depressive Disorder. Biol Psychiatry. 2007;62:1272–80. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimura S, Ueda K, Suzuki S, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cogn. 2009;69:218–25. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 52.van Buuren M, Gladwin TE, Zandbelt BB, Kahn RS, Vink M. Reduced functional coupling in the default-mode network during self-referential processing. Hum Brain Mapp. 2010;31:1117–27. doi: 10.1002/hbm.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 54.Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, et al. Chronic spinal pain and physical-mental comorbidity in the United States: Results from the national comorbidity survey replication. Pain. 2005;113:331–9. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–75. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 56.Yu R, Gollub RL, Spaeth R, Napadow V, Wasan A, Kong J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. NeuroImage Clin. 2014;6:100–8. doi: 10.1016/j.nicl.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Z, Liu M, Lan L, Zeng F, Makris N, Liang Y, et al. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep. 2016;6:20298. doi: 10.1038/srep20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egorova N, Gollub RL, Kong J. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. NeuroImage Clin. 2015;9:430–5. doi: 10.1016/j.nicl.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, et al. Brain Activity Associated with Expectancy-Enhanced Placebo Analgesia as Measured by Functional Magnetic Resonance Imaging. J Neurosci. 2006;26:381–8. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 2010;16:1277–83. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- 61.Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–8. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- 62.Seminowicz D, Mayberg H, McIntosh A, Goldapple K, Kennedy S, Segal Z, et al. Limbic–frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–18. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


