Abstract
What is known and objective
Some public skepticism exists about generics in terms of whether brand and generic drugs produce identical outcomes. This study explores whether adverse event (AE) reporting patterns are similar between brand and generic drugs, using authorized generics (AGs) as a control for possible generic drug perception biases.
Methods
Events reported to the FDA Adverse Event Reporting System from the years 2004–2015 were analyzed. Drugs were classified as brand, AG, or generic based on drug and manufacturer names. Reports were included if amlodipine, losartan, metoprolol extended-release (ER), or simvastatin were listed as primary or secondary suspect drugs. Disproportionality analyses using the reporting odds ratio (ROR) assessed the relative rate of reporting labeled AEs compared to reporting these AEs with all other drugs. The Breslow Day test compared RORs across brand, AG, and generic. Interrupted time series analysis evaluated the impact of generic entry on reporting trends.
Results and discussion
Generics accounted for significant percentages of total U.S. reports, but AGs accounted for smaller percentages of reports, including for amlodipine (14.26%), losartan (1.48%), metoprolol ER (0.35%), and simvastatin (0.70%). While the RORs were significantly different for multiple brand vs. generic comparisons, the AG vs. generic comparisons yielded fewer statistically significant findings. Namely, only the ROR for AG differed from generic for amlodipine with peripheral edema (P < 0.01).
What is new and conclusion
Inconsistent reporting patterns were observed more between brand and generic compared with AG and generic. Use of AGs as a control for perception biases against generics is useful, but this approach can be limited by small AG report numbers. Requiring the manufacturer name to be printed on the prescription bottle or packaging could improve the accuracy of assignment for products being reported.
Keywords: Adverse event, surveillance, generic, authorized generic
1 | WHAT IS KNOWN AND OBJECTIVE
Some public skepticism exists about generic drugs. The U.S. FDA generic drug approval process is very rigorous and much of the public skepticism is believed to be simply a biased perception of generics because of factors such as lower cost, lack of generic product promotion (and heavy brand promotion), and simply a different look and feel of generics compared with brands.1–6 Given these potential public biases against generics, studying generic drug utilization patterns and outcomes in the real world poses methodological challenges. For instance, if an observational study comparing generic tolerability vs. brand tolerability were conducted in the real world, it is likely that patients taking generics would likely report more tolerability concerns than patients taking brands just by virtue of this generic bias. This phenomenon has been illustrated in prior studies where patients taking “generic” placebo reported fewer benefits and more adverse events than patients taking “brand” placebo for headaches.7
One possible way to overcome this challenge is by studying authorized generic (AG) drugs. AG drugs contain the exact active and inactive ingredients as the branded drugs, are marketed under the brand drug’s New Drug Application (NDA), but are sold and distributed with a generic label.8 The marketing of the AG drugs began early in the1990s as a strategy for brand-name companies to maintain market share, but become a widespread practice in 2003.9 In fact, from 2003–2006 the number of AG drugs that entered the market were between 19 and 21 per year compared to only 7 per year in 2001–2002.9 The therapeutic efficacy and safety profile of the brand drug and AG are identical, but patients perceive the AG to be similar to other generics which are approved via Abbreviated New Drug Applications (ANDA). Therefore, it may be feasible to use AG drugs as “control drugs” in postmarking surveillance research.
This study explored whether adverse event (AE) reporting patterns are similar between brand and generic drugs, using AG drugs as a control for possible generic drug biases. We hypothesized that if the adverse event reporting patterns were different between brand and AG, this could signal possible bias against generics. Whereas, if adverse event reporting patterns were shown to be different between the AG and other generics, this could indicate possible differences across drug product types. Amlodipine (Norvasc), losartan (Cozaar), metoprolol extended-release (metoprolol ER -- Toprol-XL) and simvastatin (Zocor) were chosen as example drugs since these drugs are used to prevent cardiovascular events and had a generic and an AG introduced during the past decade. Amlodipine, losartan, and metoprolol ER are indicated for cardiovascular disease and high blood pressure and simvastatin is indicated for high cholesterol.10,11 We believe these drugs are good candidates for such evaluation because they are commonly used and have adequate historical reporting data to evaluate trends over time. Further, we are unaware of potential environmental factors that could have artificially enhanced sensitivity for reporting specific drugs or events that we are studying (e.g., changes in labeling, news coverage, lawsuits, etc.) and this minimizes the risk of stimulated reporting.
2 | METHODS
2.1 | Study Design and Data Source
The FDA Adverse Event Reporting System (FAERS) is a post-marketing surveillance database composed of adverse events reported from the U.S. and other countries. Reporting is voluntary for patients and health care providers, but it is required for pharmaceutical manufacturers.12,13 This is a retrospective analysis of public release data from FAERS for amlodipine, losartan, metoprolol ER, and simvastatin. Data covered the period from January, 2004 through March, 2015. We compared reporting rates for events associated with brand, AG, and generic products for each drug. We removed all duplicate reports prior to analyses based on the FDA’s recommendation for using the most recent case number.14
2.2 | Reports Identification
We included reports when amlodipine (Norvasc), metoprolol ER (Toprol XL), losartan (Cozaar), and simvastatin (Zocor) were listed as the primary or secondary suspect drugs. We excluded reports when these drugs were listed as interacting or concomitant. We used text string searches for each drug by generic names, brand names, and abbreviations. Since drug name misspellings are possible among reported events, we used complete name and shorter character strings to identify a subset of reports. Drug names in these reports were then recoded by one reviewer, and a second reviewer verified the accuracy of the recoding.
Reports were classified as brand, AG, or generic based on the manufacturer making the report. Manufacturers were identified based on the filer of the New Drug Application (NDA) for brand, based on manufacturers other than the brand company marketing the drug under the NDA for AGs, and based on filers of Abbreviated New Drug Applications (ANDAs) for generics. While generally this approach works, the manufacture making the report might not be the actual manufacturer of the reported product. For instance, a patient might report the adverse event of a generic drug to the brand-name company out of familiarity or vice versa. Reports made directly to the FDA were excluded since these reports usually do not include manufacturer name. For amlodipine, brand reports and AG reports were from Pfizer. Because the brand and AG were coded from the same manufacturer, we differentiated between the two using verbatim name. If the drug was reported by the brand name (Norvasc) then it was classified to be brand and if it was reported by the generic name amlodipine then it was classified to be an AG. For losartan, brand reports were from Merck Sharp Dohme, AG reports were from Sandoz, and generic reports were from all other manufacturers. For metoprolol ER, brand reports were from AstraZeneca, AG reports were from Par Pharmaceuticals, and generic reports were from all other manufacturers. For simvastatin, brand reports were from Merck Sharp Dohme, AG reports were from Dr. Reddy’s Lab, and generic reports were from all other manufacturers.
2.3 | Measures
We estimated the total number of overall AE reports and the number of serious AE reports, categorized as death, disability, and other serious outcomes. Previously documented adverse events were identified based on the most recent product label and drug reference databases (Micromedex and Lexi-Comp). The events investigated for each product are listed in table 1 for each drug. These adverse events were identified in FAERS reports from the REAC files using the Medical Dictionary for Regulatory Activities (MedDRA) preferred terms (PTs) noted in the supplemental data. We employed existing categorization approaches to group specific PTs into broader AE terms. We grouped all PTs listed in the Standardized MedDRA Query (SMQ) when the adverse events of interest reasonably corresponded with an SMQ.15 When SMQs were considered to be an inadequate definition of the event of interest, we used a hierarchical search process.16 We first examined High Level Group Terms (HLGTs), then High Level Terms (HLTs), and then individual PTs. The broadest of these definitions was selected to define the event of interest.
Table 1.
Drugs dosage form and FDA approval date
| Amlodipine | Losartan | Metoprolol ER1 | Simvastatin | |
|---|---|---|---|---|
| Dosage forms/strengths | 2.5, 5, and 10 mg Tablet | 25, 50, 100mg Tablet | 25, 50, 100, 200 mg Extended Release Tablet | 5, 10, 20, 40, 80 mg Tablet |
| 1st Innovator approval date | 07/31/1992 | 04/14/1995 | 01/10/1992 | 12/23/1991 |
| 1st Generic approval date | 03/23/2007 | 04/06/2010 | 11/21/2006 | 06/23/2006 |
| Known adverse events evaluated in this study | Flushing, liver injury, palpitation, and peripheral edema | Pregnancy fetal disorder, angioedema, hyperkalemia, and acute renal failure | Vertigo/dizziness, shortness of breath, peripheral edema, and bradycardia | Rhabdomyolysis /myopathy, liver injuries, upper respiratory tract infection, and gastritis |
| Total US reports | 10,297 | 2,841 | 6,921 | 7,137 |
| Brand n (%) | 3042 (29.54%) | 1596 (56.18%) | 1934 (27.94%) | 3941 (55.22%) |
| Authorized Generic n (%) | 1468 (14.26%) | 42 (1.48%) | 24 (0.35%) | 50 (0.70%) |
| Generic n (%) | 5787 (56.20%) | 1203 (42.34%) | 4963 (71.71%) | 3146 (44.08%) |
Metoprolol Extended-release
2.4 | Data Analysis
Descriptive analyses were performed to summarize the sources of AE reports for each drug. Reporting odds ratios (RORs) with 95% confidence intervals were estimated with disproportionality analyses, which evaluated the likelihood of documented events to be reported with a specific type of product (i.e. brand, AG, or generic) in comparison to all other drugs in FAERS. In particular, cases were reports of the AE of interest and non-cases were all reports of adverse events other than the event of interest. The ROR estimates the odds of the event of interest in those exposed to each target drug product of interest divided by the odds of the event of interest in those exposed to all other drugs in FAERS. We calculated the ROR for each drug-product type (i.e., brand, AG, generic).
Because possible measurement error with our approach to classifying brand vs. AG vs. generic reports was considered to be non-constant over time, we only analyzed the period after the introduction of the generic into the U.S. market (Table 1). The magnitude and direction of the RORs were compared across drug-product types. Additionally, Breslow Day tests were used to examine homogeneity of the RORs for AG and generic compared to the brand product, as well as compared with each other. To minimize potential type 1 error, we used a Bonferroni correction to adjust for multiple comparisons with a critical p-value < 0.01. Interrupted time series with segmented regression was performed to evaluate the impact of generic entry on reporting trends.17 In a series of linear regression models, the change in the slope and intercept of the number of the worldwide reported adverse event per quarter was estimated from the pre-generic period to post-generic period for each drug. We used the FDA received date (i.e. FDA_DT) to define the corresponding quarter for each report. All statistical analyses were performed using SAS version 9.4. This study was determined to be exempt non-human subjects research by the Auburn University Institutional Review Board and the FDA Research in Human Subjects Committee.
3 | RESULTS
As shown in Table 1, we identified 27,196 reports, including 10,297 for amlodipine, 6,921 for metoprolol ER, 2,841 for losartan, and 7,137 for simvastatin. Of these reports, 29.54%, 14.26%, and 56.20% were reported for brand, AG, and generic amlodipine, respectively; 56.18%, 1.48%, and 42.34% were reported for brand, AG, and generic losartan, respectively; 27.94%, 0.35%, and 71.71% were reported for brand, AG, and generic metoprolol ER, respectively; and 55.22%, 0.70%, and 44.08% were reported for brand, AG, and generic simvastatin, respectively.
We examined the impact of AG and generic entry on reporting trends by performing an interrupted time series analysis. We performed the analysis for all drugs except for metoprolol ER due to the low sample size. Only losartan showed a significant increase in the reporting trend in the post generic period (p-value = 0.0001). Sensitivity analyses were conducted by using manufacturer submitted date and event date instead of FDA received date. The losartan results showed a significant increase in AE reports when using the event date (p-value = 0.02).
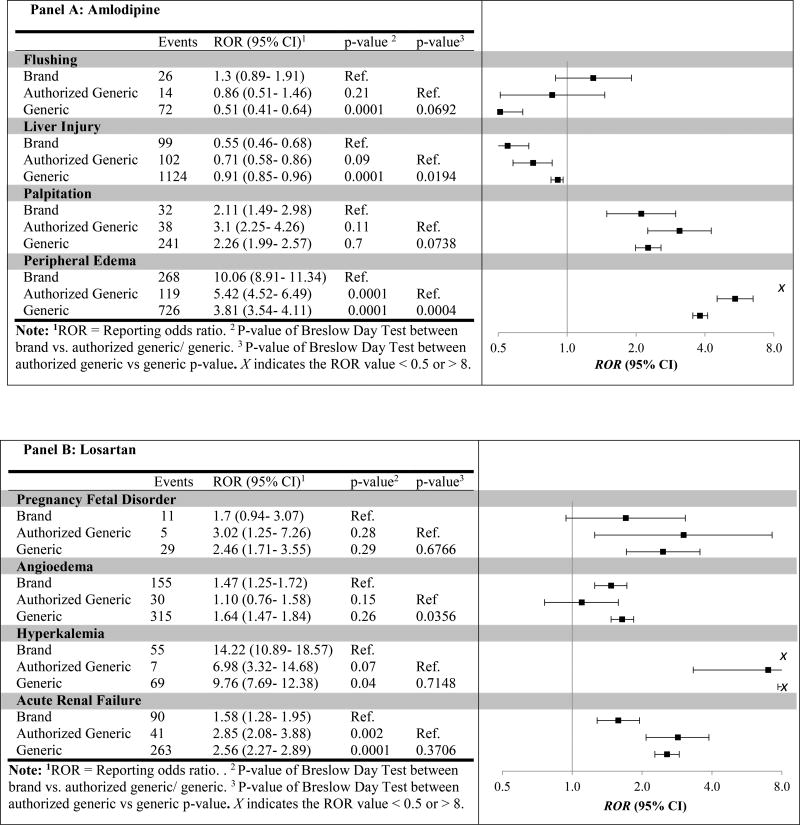
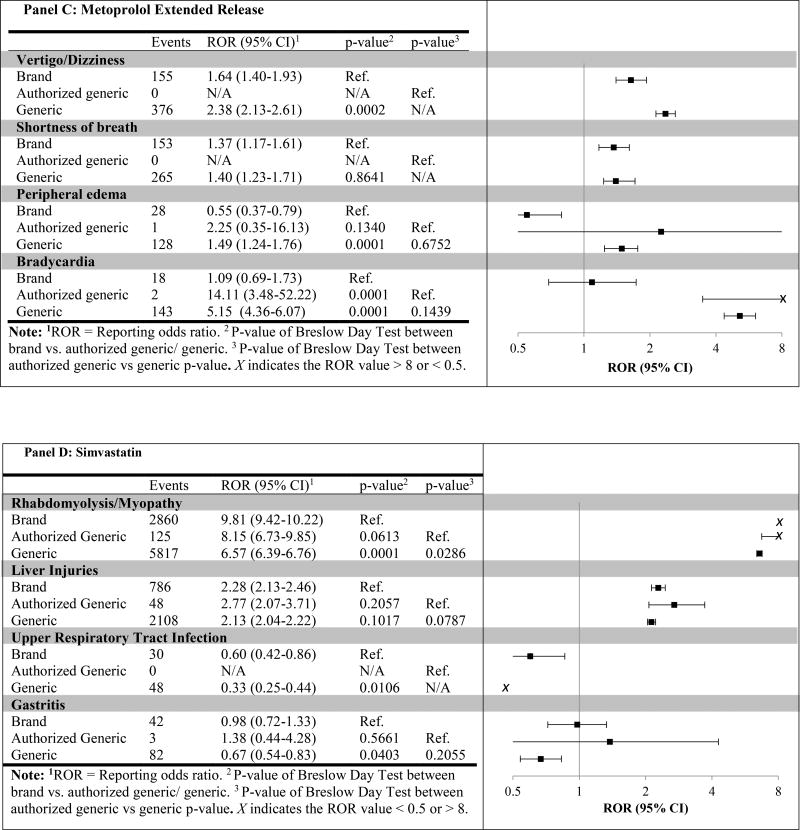
We then compared the RORs across drug types for known adverse events on the package label and in drug reference databases (Figure 1). We observed multiple significant differences in RORs when comparing brand vs. generic, but fewer when comparing brand vs. AG. For example, the ROR for generic amlodipine (ROR = 0.91; 95%CI 0.85–0.96) for liver injury was significantly higher than the corresponding ROR for brand (ROR = 0.55; 95%CI 0.46–0.68; generic versus brand p-value = 0.0001), yet no significant difference between brand and AG RORs was found (ROR = 0.71; 95%CI 0.58–0.86; AG versus brand p-value = 0.09). Further, when comparing RORs for AG vs. generic, which we hypothosized would reduce the effect of public perception bias against generic products, only the ROR for AG versus generic amlodipine with peripheral edema was significant (P = 0.0004).
Figure 1.
Post-generic specific event reporting odds ratio for brand, authorized generic, and generic between 2004–2015
4 | DISCUSSION
Generic drugs reduce health care costs, and both payers and providers recommend their use.18,19 Given that the market share of generic drugs is more than 80%,20,21 ongoing attention needs to be paid to monitor their safety and efficacy and promote public confidence in their use. The FAERS database is a tool used broadly for safety signal detection, but it has not been widely used to assess differences in adverse events reporting between brand and generic drugs. While we recognize that the FAERS is not designed to consistently capture specific brand vs. generic drug product signals, it could play a role as a generic drug safety surveillance tool. Further, employing the use of AG drugs as a “control” to account for generic drug perception bias is a novel approach to generic drug safety surveillance.
We explored reports of known adverse events of amlodipine, losartan, metoprolol ER, and simvastatin. We compared the RORs of brand vs. generic, brand vs. AG, and AG vs. generic to explore potential differences in the reporting rates. Our results showed that no significant differences were found for many adverse events when comparing AGs and generics. Assuming the AG represents the brand product in terms of safety and effectiveness but is perceived as a generic, this would suggest that the reporting differences between brand and generic are at least in part related to perception bias. For example, the generic losartan ROR for acute renal failure was significantly higher than the corresponding brand ROR (p-value < 0.01), yet no significant difference between AG and generic RORs was found (p=0.37). This finding was consistent across most drugs; however, there were a few cases that trended towards a difference, and one case where we found a statistically significant difference between AG and generic. Namely, the ROR for AG was significantly higher than generic for amlodipine with peripheral edema. Our results demonstrate that the public perception bias against generics is found in AE reporting.
A number of different factors could have influenced our findings. First, the FAERS is not designed to identify specific drug products (i.e. brand, AG, or generic) due to inconsistent capture of manufacturer information and imperfect methods for product identification. A possible solution would be to require printing the NDA and ANDA numbers on the drug bottles or printing the manufacturer’s name on the packaging to facilitate accurate reporting by consumers and physicians to either the FDA or manufacturers. Also, the voluntary reporting system does not ensure perfect information capture in a report. The reporting system requires patients and providers to supply as much information as they can regarding the person, event, drug, and other background information but this may be incomplete due to limitations in patient knowledge about their drugs. The voluntary nature of FAERS also results in a database that contains only a subset of patient AE reports. The implementation of programs to promote and expand healthcare provider and consumer direct reporting to FAERS could help increase the proportion of events documented in the database.
While generic drugs accounted for the majority of dispensed prescriptions for drugs we studied during the study period, brand drugs accounted for a high number of reports even after generic drug market entry. Similarly, the Institute for Safe Medication Practices (ISMP) reported that brand drugs accounted for 1% of dispensed prescriptions of the most widely used drugs, but brand drug manufacturers submitted around 68% of all serious adverse reports.22 This indicates that the quality of FAERS data depends on how patients and health care providers recognize and report on their medications and how manufacturers collect and follow-up on reported adverse events with their products. ISMP reported multiple reasons why generic manufacturers’ reports are low compared to the brand. For instance, the providers as well as the consumers usually do not have access to the manufacturer’s name of their drugs, so they likely report to the brand name manufacturer out of familiarity.
Our study has several limitations. We limited our analysis to four cardiovascular drugs with different safety profiles. The observed difference in reporting between product types may not generalize to other drugs. Also, it is important to note that duplicative reports may exist if an AE is reported multiple times by different entities (e.g. directly reported by patients or by pharmaceutical companies). This issue was minimized by removing duplicative reports to keep the most recent case number, but some duplicates may still exist. Overall, the impact of such duplication is expected to be minimal. Also, we found that no reports originated from Greenstone Pharmaceuticals, which is the AG manufacturer of amlodipine. We assumed this happened because Greenstone Pharmaceuticals is a subsidiary of Pfizer. Therefore, we differentiated between brand and AG Pfizer reports using verbatim name.
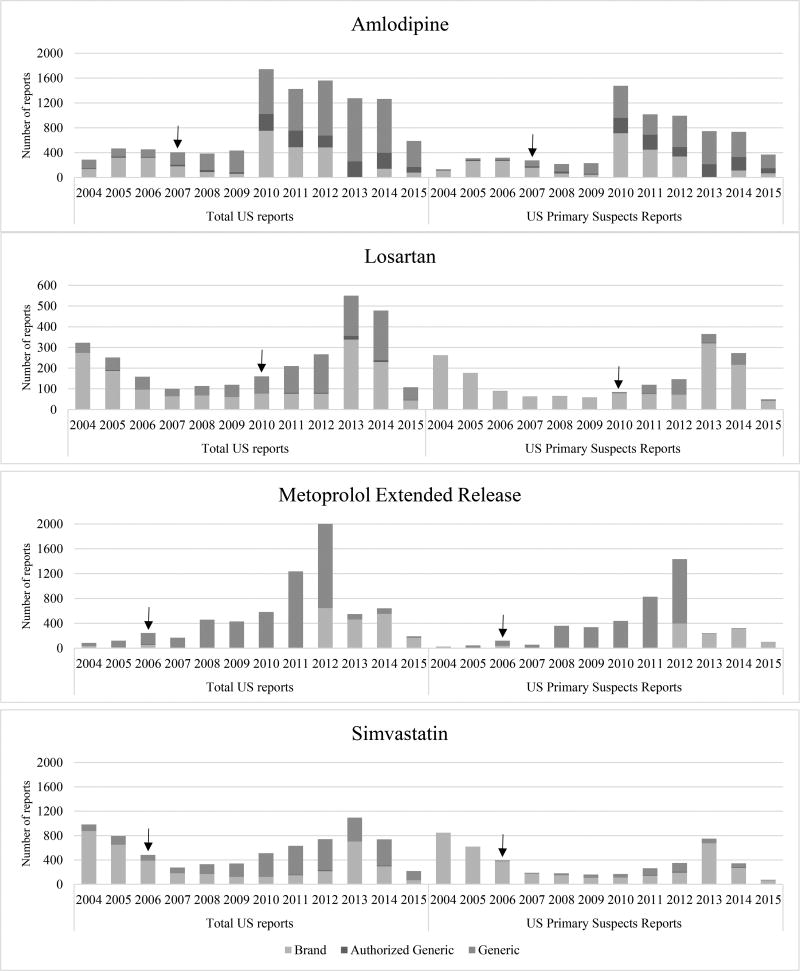
Furthermore, misclassification of adverse events for generic drugs to branded drugs also is a concern. One prior study used NDA numbers to differentiate generic drugs from brand drugs in the FAERS database.23 But, this approach would fail to identify AGs since they are marketed under the brand NDA. We classified product types (i.e. brands, generics, and AG) based on their manufacturer’s name, which is the most feasible and generalizable approach with the data currently available in the FAERS. To minimize bias introduced by product misclassification, we examined multiple restrictive approaches. When we restricted reports to primary suspects and valid serious primary suspects, the misclassification was minimized, but at the cost of sample size. Restricting to just US primary suspect reports seemed to be a reasonable compromise to maintaining sample size while minimizing misclassification. This is illustrated in Figure 2, whereby a relatively high number of reports are assigned to generic even before the generic was available in the market (i.e., these are brand reports misclassified as generic) when we looked at total US reports. When we restricted to US primary suspect reports, the proportion of reports that are obviously misclassified is significantly reduced.
Figure 2.
Summary of FAERS reports for brand, authorized generic, and generic between 2004–2015
↓ Indicates the date of the introduction of generic drugs to the U.S. market.
Also, the small number of reports for AGs limits the usefulness of our approach to controlling for generic drug perception bias. It is likely that patients and providers are more aware of brand manufacturer name than the generic manufacturer, so they report to the brand name manufacturer out of familiarity. Promoting the importance of reporting to the correct manufacturer may help increase the proportion of generic and AG reports. Also, printing the manufacturer name on prescription drug bottles or packaging could increase the generic and AG reporting rate accuracy.
FAERS is prone to several limitations due to its nature as a spontaneous reporting system. Reported adverse events for a specific drug do not necessarily mean that the drug was causally responsible for that event. Also, we do not have an actual denominator that represents the drug utilization in the U.S. or information regarding a patient’s past medical history. Similar to other spontaneous reporting systems, FAERS is prone to reporting biases. Reporting rates may be stimulated due to factors such as media coverage, FDA warnings, or advertisements from law firms. However, we do not know of any major issues that might have impacted reporting with these drugs during the study period. It is important to note that FAERS is suited for hypothesis generation and further study of any possible signals detected should employ other data sources and study designs, such as the FDA Sentinel Initiative.24
5 | WHAT IS NEW AND CONCLUSION
Although brand vs. generic comparisons showed higher AE report rates for generics, AG vs generic comparisons showed few significant differences in reporting rates, suggesting biases exist against generics when reporting in FAERS. The small number of cumulative AG reports over time could have influenced these findings. The reliability of using AGs for post-marketing surveillance could be improved through policy changes that require both the manufacturer and NDA or ANDA numbers on prescription bottles to facilitate accurate reporting by consumers and physicians to either the FDA or manufacturers. Our innovative approach of using AGs to account for perception biases against generics can be useful when AG use and reporting are relatively common and should be further investigated for post-marketing surveillance.
Table 2.
Segmented regression analyses of adverse event reports comparing the change in reporting rates pre and post -generic drugs.
| Trend Pre-Generic Period β1 (p-value) |
Level Change Post-Generic Period β2 (p-value) |
Trend change Post-Generic Period β3 (p-value) |
|
|---|---|---|---|
| Amlodipine | |||
| Total Adverse Events worldwide | 5 (0. 72) | 18 (0. 88) | 19 (0.20) |
| Losartan | |||
| Total Adverse Events worldwide | −1 (0. 09) | 9 (P= 0. 45) | 10 (0.0001) |
| Simvastatin | |||
| Total Adverse Events worldwide | −8 (0.5124) | −72 (P=0.3414) | 23 (0.631) |
Metoprolol ER is not included because of insufficient sample size during some quarters of analyses
Acknowledgments
The authors thank Wenlei Jiang, PhD (Food and Drug Administration) and Saranrat Wittayanukorn, PhD (Food and Drug Administration) for their thoughtful contributions to study design and data analysis, and Brandy Davis (Auburn University) for her contributions to manuscript preparation.
Sources of Funding
Funding for this work was made possible, in part, by the Food and Drug Administration through grant 1U01FD005272. Views expressed do not necessarily reflect the official policies of the Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Footnotes
Disclosures: In the past 3 years, Richard Hansen has provided expert testimony for Boehringer Ingelheim and Daiichi Sankyo. No other authors declare a potential conflict of interest.
References
- 1.Sabaté E. Adherence to long-term therapies: evidence for action. World Health Organization; 2003. [PubMed] [Google Scholar]
- 2.Cutler DM, Everett W. Thinking outside the pillbox--medication adherence as a priority for health care reform. N Engl J Med. 2010;362(17):1553–1555. doi: 10.1056/NEJMp1002305. [DOI] [PubMed] [Google Scholar]
- 3.Sansgiry SS, Bhosle M, Pope N. Consumer perceptions regarding generic drug substitution: an exploratory study. Journal of Pharmaceutical Marketing & Management. 2005;17(1):77–91. [Google Scholar]
- 4.Donohue JM, Cevasco M, Rosenthal MB. A Decade of Direct-to-Consumer Advertising of Prescription Drugs. New England Journal of Medicine. 2007;357(7):673–681. doi: 10.1056/NEJMsa070502. [DOI] [PubMed] [Google Scholar]
- 5.Kesselheim AS, Gagne JJ, Franklin JM, et al. Variations in Patients' Perceptions and Use of Generic Drugs: Results of a National Survey. Journal of general internal medicine. 2016;31(6):609–614. doi: 10.1007/s11606-016-3612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kesselheim AS, Gagne JJ, Eddings W, et al. Prevalence and Predictors of Generic Drug Skepticism Among Physicians: Results of a National Survey. JAMA Intern Med. 2016;176(6):845–847. doi: 10.1001/jamainternmed.2016.1688. [DOI] [PubMed] [Google Scholar]
- 7.Faasse K, Cundy T, Gamble G, Petrie KJ. The effect of an apparent change to a branded or generic medication on drug effectiveness and side effects. Psychosomatic medicine. 2013;75(1):90–96. doi: 10.1097/PSY.0b013e3182738826. [DOI] [PubMed] [Google Scholar]
- 8.The Henry J. Kaiser Family Foundation. [Accessed May 17, 2014];Health insurance coverage of the total population. 2012 http://kff.org/other/state-indicator/total-population/?state=al.
- 9.Commission FT. Authorized generic drugs: short-term effects and long-term impact. Retrieved September. 2011;10:2014. [Google Scholar]
- 10.Gabb GM, Mangoni AA, Anderson CS, et al. Guideline for the diagnosis and management of hypertension in adults — 2016. The Medical Journal of Australia. 2016;205(2):85–89. doi: 10.5694/mja16.00526. [DOI] [PubMed] [Google Scholar]
- 11.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad SR. Adverse drug event monitoring at the Food and Drug Administration. Journal of general internal medicine. 2003;18(1):57–60. doi: 10.1046/j.1525-1497.2003.20130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA. [Accessed 2 March 2016];Questions and Answers on FDA's Adverse Event Reporting System (FAERS) http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/
- 14.Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2013;10(7):796–803. doi: 10.7150/ijms.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medical Dictionary for Regulatory Activities. [Accessed January 3, 2015];Standardised MedDRA Queries. 2015 http://www.meddra.org/standardised-meddra-queries.
- 16.Medical Dictionary for Regulatory Activities. [Accessed January 3, 2015];MedDRA Hierarchy. 2015 http://www.meddra.org/how-to-use/basics/hierarchy.
- 17.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 18.Sarpatwari A, Choudhry NK, Avorn J, Kesselheim AS. Paying physicians to prescribe generic drugs and follow-on biologics in the United States. PLoS medicine. 2015;12(3):e1001802. doi: 10.1371/journal.pmed.1001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett L. Physicians' attitudes and practices regarding generic drugs. Paper presented at: GERONTOLOGIST. 2005 [Google Scholar]
- 20.Grabowski H, Long G, Mortimer R. Recent trends in brand-name and generic drug competition. Journal of medical economics. 2014;17(3):207–214. doi: 10.3111/13696998.2013.873723. [DOI] [PubMed] [Google Scholar]
- 21.News DS. [Accessed October 10, 2016];Generic Report. 2016 http://www.drugstorenews.com/sites/drugstorenews.Com/files/GenericReport_2016.Pdf.
- 22.Moore T. ISMP quarter watch: monitoring FDA MedWatch reports. Philadelphia, PA: ISMP Quarter Watch: 2014 Quarter 1; 2015. [Google Scholar]
- 23.Geetha Iyer SPM, Segal Jodi B, Singh Sonal. Presented as a poster in the 32nd International Conference on Pharmacoepidemiology & Therapeutic Risk Management. Dublin, Ireland: Aug 25–28, 2016. Identification of Generic Drugs in the FDA Adverse Event Reporting System. 2016. [Google Scholar]
- 24.Robb MA, Racoosin JA, Sherman RE, et al. The US Food and Drug Administration's Sentinel Initiative: expanding the horizons of medical product safety. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):9–11. doi: 10.1002/pds.2311. [DOI] [PubMed] [Google Scholar]