Abstract
Background
Limited prospective information exists regarding spectral-domain optical coherence tomography (SD-OCT) in secondary progressive multiple sclerosis (SPMS). Objective: Document cross-sectional and longitudinal retinal nerve fiber layer (RNFL) and macular ganglion cell plus inner plexiform layer (GCIPL) features of an SPMS clinical trial cohort.
Methods
Prospective, observational study using a 2-year randomized placebo-controlled SPMS trial cohort with yearly SD-OCT testing. Post-hoc analysis determined influences of optic neuritis (ON), disease duration, and baseline SD-OCT on annualized atrophy rates and on correlations between OCT and brain atrophy.
Results
Mean RNFL and GCIPL values of patients (n=47, mean age 59, mean disease duration 30 years) were significantly lower among eyes with prior ON than those without (NON). Annualized RNFL (−0.31 μm/year) and GCIPL (−0.29 μm/year) atrophy rates did not differ between ON and NON eyes. Baseline RNFL thickness >75 microns was associated with greater annualized RNFL atrophy (−0.85 μm/year). Neither RNFL nor GCIPL atrophy correlated with whole brain atrophy.
Conclusion
This study suggests that eyes with and without ON history may be pooled for atrophy analysis in SPMS clinical trials utilizing SD-OCT. Low baseline RNFL, small retinal atrophy rates, and lack of correlation with whole brain atrophy in this population are important trial design considerations.
Keywords: optical coherence tomography (OCT), multiple sclerosis, progressive, longitudinal, macular ganglion cell, retinal nerve fiber layer
Introduction
Optical coherence tomography (OCT) is a powerful tool used to measure axonal integrity in eyes of patients with multiple sclerosis (MS) and is an increasingly used outcome measure in relapsing-remitting MS (RRMS) clinical trials (1, 2). OCT provides quantifiable, reliable, in vivo information regarding thickness of the peripapillary retinal nerve fiber layer (RNFL) at the optic nerve head, as well as the combined ganglion cell and inner plexiform layer (GCIPL) thickness within the macula. The ability to quantify RNFL and GCIPL in both a cross-sectional and longitudinal fashion allows researchers to establish convenient structural measures of disease burden in MS, including measures of functional disability such as low-contrast letter acuity and visual quality of life (3). As such, efforts have been made to identify specific threshold RNFL values that associate with visual recovery after optic neuritis and risk of MS disability progression (4, 5). Furthermore, RNFL and GCIPL thinning have been correlated with whole brain and brain substructure volume loss on MRI, suggesting that optic atrophy reflects central nervous system MS disease burden, with faster GCIPL thinning in the early, active stages (6–9). While OCT reliably reflects damage from optic neuritis (ON) (4, 10–12), peripapillary RNFL and macular thinning in eyes of MS patients unaffected by ON is also apparent (11, 13–15), corroborating tissue evidence of eventual axonal and ganglion cell loss in almost all MS patients at autopsy (16).
Relative to relapsing-remitting MS, OCT studies in secondary progressive MS (SPMS) are underrepresented, and few are longitudinal (3, 6, 10, 17–19). Because patients with SPMS are often older with a longer disease duration, one might infer that OCT interpretation in this population is limited due to accumulated disease burden at baseline, but this has not been proven.
The goals of this study are to describe the RNFL and GCIPL SD-OCT baseline features and longitudinal atrophy rates in a 2-year clinical trial cohort of long-standing SPMS. We investigate the effects of ON history, disease duration, and baseline values of RNFL and GCIPL on measures of optic atrophy, and determine OCT atrophy correlates with brain atrophy rates.
Methods
Patients
This prospective, observational study is a post-hoc analysis of data obtained from 51 patients with SPMS enrolled in a randomized placebo-controlled trial of 1200mg daily lipoic acid and followed for 2 years. Full study details have been presented (20). Institutional review board approvals were obtained from the Veterans Affairs Portland Health Care System and the Oregon Health & Science University, Portland, Oregon, and the study was carried out in accordance with the principals of the Helsinki Accordance. Inclusion criteria pertinent to this analysis included ages 40–70 years, established MS diagnosis (2005 revised McDonald criteria), and SPMS as defined by clinical worsening in the prior 5 years from MS, independent of MS relapse (21). Self-reported histories of ON were confirmed as available via medical records. Neurologic disability was determined by the Expanded Disability Status Scale (EDSS) (22). Interferon and glatiramer acetate disease-modifying therapies were permitted. Excluded were patients with reported ocular disease that would affect OCT interpretation or who could not position adequately. High contrast visual acuity (VA) was tested with ETDRS charts at 3 meters.
OCT acquisition
Baseline spectral-domain OCT (Cirrus HD-OCT, Model 5000; software version 7.0.1.290, Carl Zeiss Meditec, Inc., Dublin, California) was collected in a dark room from each eye after pharmacologic dilation. A single operator (RS) or trained photographer obtained all optic nerve and macula scans from both eyes of each patient on the same day on a single machine at the baseline, year 1, and year 2 visits. High contrast logMAR VA was collected just prior to dilation. Peripapillary and macular scans were obtained in triplicate with Optic Disc Cube 200 × 200 and Macular Cube 512 × 128 protocols, respectively. All scans were reviewed for quality with the best quality scan selected by a separate OCT reader (KW), using standards from quality control OCT clinical trial reports and OSCAR-IB criteria (23, 24). Final OCT scans containing confounding retinal or optic nerve findings (i.e. macular edema or glaucomatous optic nerve cupping), artifact, misalignment, or signal strength less than 7, were excluded from post-hoc analysis.
MRI acquisition and analysis
Details of the MRI acquisition and analysis have been previously presented (20). All MRI scans were obtained on a single Philips Achieva 3.0T X-series with Quasar Dual gradient systems. Whole brain atrophy was estimated from the 3D sagittal T1-weighted MPRAGE images using SIENA (Structural Image Evaluation, using Normalisation, of Atrophy) from the FSL package (25), while deep gray matter atrophy was estimated utilizing the FreeSurfer package (surfer.nmr.mgh.harvard.edu/). SIENA is a robust registration-based longitudinal atrophy measure that is fully automated, is less sensitive to image acquisition errors, and balances multi-site reproducibility with length agreement (16).
Statistical analysis
Primary intervention analysis observed no effect of lipoic acid treatment on rate of change in the principle OCT outcomes of RNFL or GCIPL thickness, regardless of a prior history of ON. Additionally, no significant cross-sectional differences between intervention groups were observed in the OCT measures at any study time point. In addition, there was no interaction or moderation effect of lipoic acid treatment on the relationships between the OCT and MRI outcomes, with no observed difference in those model associations between treatment arms. Accordingly, the primary study treatment arms were combined for this post-hoc analysis.
Cross-sectional models compared the difference in RNFL or GCIPL thicknesses between ON and NON eyes at baseline and at study completion. Longitudinal analysis compared annualized rate of atrophy in the OCT measures between ON eyes and NON eyes. In both methods, linear mixed-effects regression models were used, including a random effects portion to account for pooling of left and right eyes from single participants. Additional random effects modeling adjusted the linear rate of change across the entire study period to account for within-participant serial correlation of repeated OCT measures. Rates of RNFL and GCIPL atrophy were considered under an intention-to-treat framework to allow for missing data. Under this mixed-model design, rates of retinal and brain atrophy were first separately determined for individual subjects, then distinct rates were evaluated for linear associations.
Relationships between selected baseline characteristics and subsequent changes in OCT measures were investigated by plotting annualized rates of change in RNFL or GCIPL against baseline RNFL, GCIPL, and MS disease duration, then applying regression lines of best fit. A natural threshold for subsequent RNFL atrophy was observed at a baseline RNFL of 75 μm. Subsequently, participants were binned into either >75 μm or <75 μm baseline RNFL cohorts and underwent rate of atrophy contrast. OCT measures were correlated with whole brain atrophy using Pearson’s correlation coefficient within a repeated measures framework. All regression models were corrected for covariates as defined in the primary study design, including age, gender, and MS duration, along with additional covariates of baseline RNFL and GCIPL thicknesses. In instances of normality violations, covariates were log transformed.
Significance of contrast results were established at p<0.05 after Holm-Sidak adjustments for comparisons among multiple outcomes within each of the investigated model frameworks, to account for the secondary nature of this post-hoc study. All statistical analyses were carried out using R 3.3 with additional utility from the lme4 package (26, 27).
Results
Patients
Four of the 51 patients enrolled in the parent study were excluded from the present analysis due to post-hoc OCT findings of glaucomatous optic nerve head cupping (n=4 patients, 8 eyes). Baseline demographics of the 47 participants analyzed are shown in Table 1. Notable demographics are mean age of 59 (SD 6.4) years, MS duration of 30 (SD 9.4) years, and median EDSS of 6.0 (IQR 2.0) (Table 1). Mean logMAR VA was 0.145 (SD 0.263). Twenty-two subjects (47%) reported a history of ON in at least one eye, including 11 subjects with a history of ON in both eyes. No significant differences were found between ON and NON groups with regard to age, gender, or median baseline EDSS scores. Nearly half were taking a disease modifying therapy (21 patients, 45%). There was a significantly longer duration of MS in the ON group than the NON group (32.6 vs. 27.3 years respectively, p=0.048).
Table 1.
Patient Demographics
| All Subjects | NON Subjects | ON Subjects | ON vs NON p value | |
|---|---|---|---|---|
| Number of subjects | 47 | 25 (53%) | 22 (47%) | – |
| Age (SD), years | 59 (6.4) | 59 (6.2) | 58.9 (6.8) | 0.92a |
| Female (%) | 28 (59.6%) | 13 (52%) | 15 (68.2%) | 0.37b |
| MS duration (SD), years | 30 (9.4) | 27.28 (10.05) | 32.59 (7.8) | 0.048a |
| Median EDSS {range} | 6.0 {4.3–6.3} | 5.0 {4–6} | 6.0 {4.5–6.9} | 0.12c |
| LogMAR visual acuity | 0.145 (0.263) | 0.102 (0.260) | 0.231 (0.252) | 0.065d |
| On disease modifying therapy | 21 (45%) | 12 (48%) | 9 (41%) | 0.77b |
| Number of eyes with history ON (%) | 32/94 (34%) | – | 32/44 (72.7%) | – |
Bold values indicate p <0.05.
Significance testing:
2-sample t-test on means;
Fisher’s exact test;
Wilcox test on medians;
mixed-model with repeated measures correction
EDSS=expanded disability status score; MS=multiple sclerosis; NON=no history of optic neuritis; ON=history of optic neuritis in at least one eye; SPMS=secondary progressive multiple sclerosis; SD=standard deviation.
Cross-sectional peripapillary RNFL and macular GCIPL thicknesses
Table 2 shows the cross-sectional baseline RNFL and GCIPL thicknesses for all, ON, and NON eyes at baseline. SD-OCT inclusion criteria were met by 80 of the 94 eyes in RNFL analysis and by 74 of the 94 eyes in GCIPL analysis. In all eyes, mean RNFL was 77.3 (SD 0.3) μm. RNFL was thickest in the inferior quadrant (100.6 SD 17.2 μm) and thinnest in the temporal quadrant (48.6 SD 11.5 μm). While average RNFL was significantly lower in ON than NON eyes (74.0 μm vs. 78.7 μm respectively; p=0.012), quadrant RNFL analysis did not find differences between ON and NON eyes, including the temporal quadrant (p=0.214). Mean GCIPL thickness for all eyes was 67.4 (SD 9.0) μm and was also significantly thinner in ON eyes than NON eyes (62.1 μm vs. 69.7 μm respectively; p<0.001). At 2-year follow-up, the same pattern of significant cross-sectional differences persisted (supplementary Table 1).
Table 2.
Comparison of SD-OCT Measures in SPMS Eyes With and Without a History of Optic Neuritis at Baseline. All values reported with standard deviation.
| All Eyes (n=80) |
ON eyes (n=23, 29%) |
NON eyes | ON vs NON eyesc (p value) |
|
|---|---|---|---|---|
| Mean RNFL thickness, μm | 77.3 (10.3) | 74.0 (11.3) | 78.7 (9.6) | 0.012 |
| Temporal quadrant, μm | 48.6 (11.5) | 46.0 (13.4) | 49.6 (10.6) | 0.214 |
| Superior quadrant, μm | 95.5 (16.3) | 91.8 (19.0) | 97.0 (15.1) | 0.053 |
| Nasal quadrant, μm | 64.5 (10.5) | 63.0 (11.4) | 65.1 (10.2) | 0.385 |
| Inferior quadrant, μm | 100.6 (17.2) | 95.1 (18.2) | 102.8 (16.4) | 0.178 |
| Mean Macular GCIPL thickness, μm | 67.4 (9.0)a | 62.1 (10.2)b | 69.7 (7.4) | <0.001 |
Bold values indicate p < 0.05.
n=74, GCIPL all eyes
n=22, 30% GCIPL ON eyes
Linear Mixed Modeling used to account for age, sex, and disease duration. ON= history of optic neuritis; NON= no history of optic neuritis; μm =microns; GCIPL=ganglion cell plus inner plexiform layer.
Annualized rates of atrophy of RNFL and GCIPL
Annualized mean RNFL atrophy rate was −0.31 (SE 1.56) μm/year, and annualized mean GCIPL atrophy rate was −0.29 (SE 1.37) μm/year (Table 3). No significant differences in atrophy rates were found between ON and NON eyes for RNFL (p=0.97) or GCIPL (p=0.76). Annualized atrophy rates for mean RNFL, mean GCIPL, and individual RNFL quadrant thicknesses were not significantly different from zero over the course of the 2-year study, with the exception of RNFL thickness in the inferior quadrant (p=0.03).
Table 3.
Rate of Change of SD-OCT Measures in SPMS Eyes with and without History of ON.
| All Eyes | ON eyes | NON eyes | Significance of Rate of change vs. zero in All eyes* (p value) | Significance of Rate of Change in ON vs NON eyes* (p value) | |
|---|---|---|---|---|---|
| Mean change in RNFL thickness, μm/year (SD) | −0.31 (1.56) |
−0.29 (4.24) |
−0.32 (2.76) |
0.38 | 0.97 |
| Temporal quadrant | −0.33 (2.54) |
0.66 (6.89) |
−0.74 (4.48) |
0.57 | 0.25 |
| Superior quadrant | 0.46 (3.18) |
−0.47 (8.62) |
0.85 (5.61) |
0.52 | 0.39 |
| Nasal quadrant | 0.08 (2.16) |
−0.01 (5.89) |
0.11 (3.83) |
0.87 | 0.91 |
| Inferior quadrant | −1.41 (2.84) |
−1.13 (7.72) |
−1.53 (5.03) |
0.03 | 0.77 |
| Mean change in Macular GCIPL thickness, μm/year (SD) | −0.29 (1.37) |
−0.15 (3.71) |
−0.35 (2.46) |
0.36 | 0.76 |
Bold values indicate p < 0.05.
Generated using mixed-effects linear regression (accounting for within-subject intereye correlation) for OCT measures. All models are covariate-corrected for age at baseline visit, sex, and disease duration.
ON=optic neuritis; NON=non-optic neuritis; GCIPL=ganglion cell plus inner plexiform layer; SD=standard deviation; μm =microns.
Correlation of RNFL and GCIPL atrophy with whole brain and gray matter atrophy
The mean annualized whole brain atrophy rate of patients included in this analysis was −0.48 (SE 0.57) % per year for the 44 subjects who had at least one viable RNFL measurement. Annualized RNFL and GCIPL atrophy rates did not correlate with whole brain atrophy (p = 0.30 and p = 0.87, respectively), even after correcting for the potential effect modification of ON history.
For the same subset of OCT subjects, the annualized rate of deep gray matter atrophy was −0.29 (SE 1.27) % per year while the rate of cortical gray matter atrophy was −0.16 (SE 1.04) % per year. RNFL atrophy correlated modestly with cortical gray matter atrophy (r=0.32; p=0.01) while GCIPL atrophy did not (p=0.19). Significant relationships were not observed between rates of either RNFL or GCIPL thinning with deep gray matter atrophy (p=0.41 and p=0.54, respectively).
Effect of baseline RNFL, GCIPL, and disease duration on OCT atrophy rates
A linear correlation was noted between baseline RNFL thickness and RNFL atrophy rate over two years (r= −0.514, Spearman’s rho= −0.519), such that the higher the baseline mean RNFL value, the more RNFL was lost over the subsequent 2 years (Figure 1). Furthermore, there was a significant difference in atrophy rate between baseline RNFL values clustered above versus below 75 μm (p<0.001, Figure 2). Baseline RNFL thickness did not correlate significantly with longitudinal GCIPL atrophy. Finally, neither baseline GCIPL thickness nor disease duration were significantly associated with subsequent changes in RNFL or GCIPL.
Fig. 1.
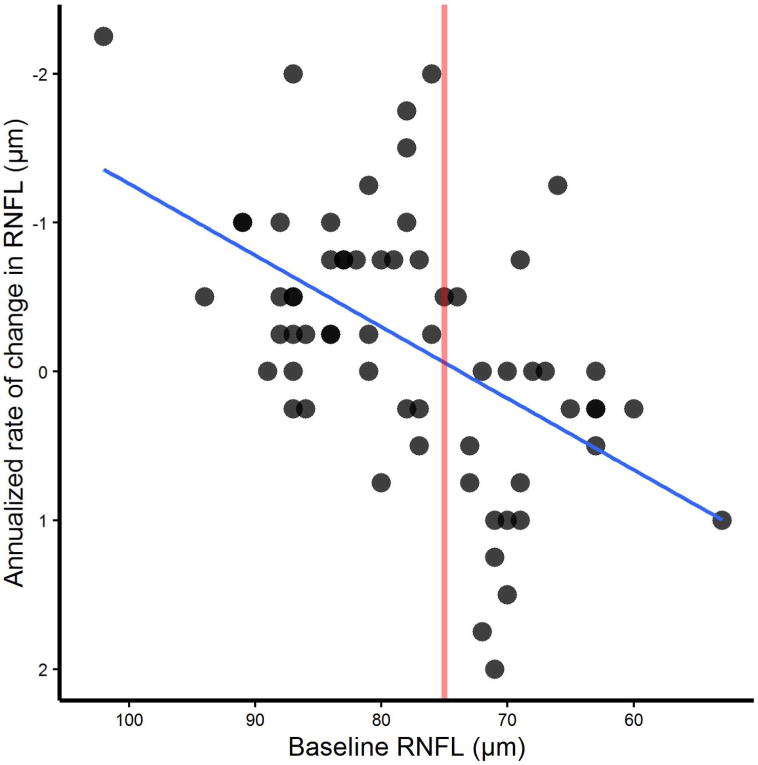
RNFL baseline thickness affects longitudinal RNFL atrophy rate. Baseline mean RNFL SD-OCT value was plotted against amplitude of change in RNFL thickness at two years. The scatterplot shows a linear relationship of starting RNFL thickness to annualized rate of change in μm. Larger mean RNFL thicknesses at baseline showed greater annualized rates of atrophy. Slope =−0.097 (p<0.001), covariate corrected mixed model to account for repeated measures. The solid line shows that at 75 μm, the change appears to go from a net negative to net positive value on average.
Fig.2.
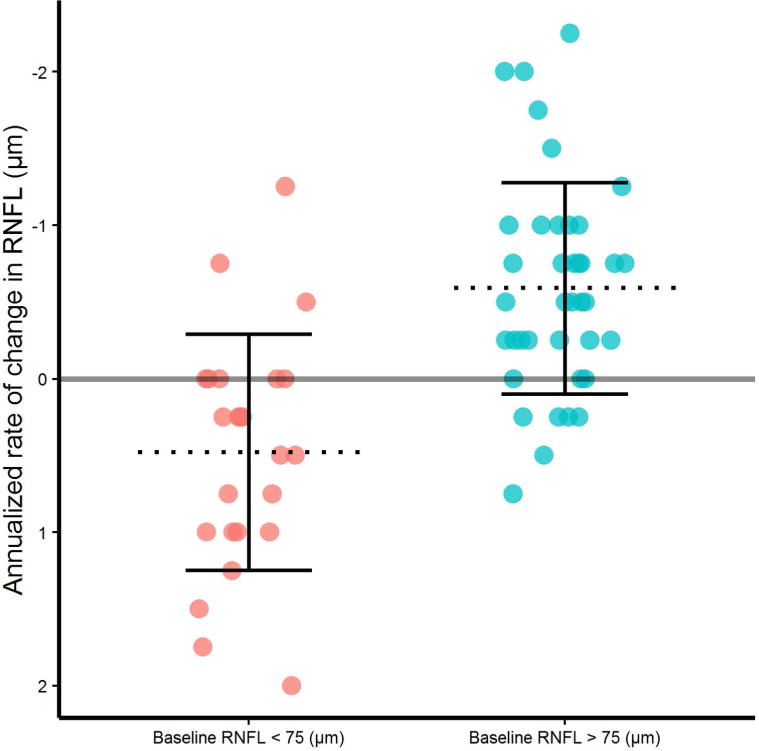
RNFL atrophy rates in SPMS are significantly different above versus below a baseline RNFL thickness of 75 μm. Baseline RNFL values above 75 μm showed significantly more annualized atrophy than those below 75 μm, presented as mean RNFL thickness in μm (SD), p<0.001.
Sample Size Calculation
Due to the low atrophy rates found over the study period, a preliminary sample size calculation was conducted using our results. Assuming an annualized atrophy rate with a similar effect size (−0.31μm/year, SE 0.38 μm, Cohen’s d=0.129), 4513 subjects would be needed per arm of a treatment study to observe a 30% difference in RNFL atrophy in SPMS subjects with 80% power and p-value <0.05, parameters used commonly in clinical trials. Using the change in GCIPL thickness (−0.29 μm/year, SE 1.37 μm, Cohen’s d=0.192), over 4500 subjects per arm would be necessary to observe statistically significant change.
Discussion
This prospective, observational study demonstrated that ON history was associated with lower mean RNFL and GCIPL thicknesses at baseline and again at 2 years in a longstanding and advanced disability SPMS patient cohort. Nevertheless, history of ON did not affect the relatively small rates of annualized RNFL and GCIPL atrophy as measured by SD-OCT. Neither RNFL nor GCIPL atrophy correlated with whole brain or gray matter atrophy in this cohort. Higher baseline RNFL thickness was associated with higher RNFL atrophy rate (observable change for the worse), whereas higher baseline GCIPL thickness and longer disease duration bore no significant longitudinal effect on atrophy rates.
This study is unique because it provides prospective longitudinal data on an underrepresented cohort of older MS patients (mean age 59 years), with an extended average disease duration of 30 years. Our long-duration SPMS cohort also showed evidence of more severe baseline RNFL and GCIPL atrophy than other SPMS studies, with a lower starting mean RNFL thickness of 77 μm (versus 83–88 μm) (6, 10, 18) and mean GCIPL thickness of 67 μm (versus 78 μm) (6).
Our study results agree with prior reports that a history of ON is a significant variable which should be included in clinical trial data collection (4, 10–12), since we found significant cross-sectional baseline and 2-year differences in RNFL and GCIPL averages between ON and NON groups. The lack of significant differences in RNFL quadrants between groups may be due to the relatively low sample size for these subset comparisons, given that almost all values were lower in the ON group. Importantly, atrophy rates did not differ longitudinally between ON and NON eyes, which is promising for reducing sample sizes in future longitudinal SD-OCT studies in SPMS by pooling ON and NON eyes.
Our longitudinal atrophy rates for RNFL and GCIPL are comparable to those found in the few longitudinal studies that also included progressive MS populations across SD-OCT platforms (6, 19). Moreover, this study suggests that the driver for the low OCT atrophy rates in our population is the lower starting RNFL thickness rather than effects of disease duration. Additionally, a mean RNFL cutoff of 75 μm has previously been associated with poorer visual recovery after acute ON, albeit measured by time-domain platform (TD-OCT) (4). In our cohort, this number also appears to be a threshold for detecting future change in RNFL on SD-OCT, although clinical correlation with low-contrast letter acuity and visual fields was not part of our study design.
Longitudinal studies including progressive MS populations also propose utility of OCT as a neuroprotective outcome measure based on significant correlations with brain atrophy (6, 19). Our numerically comparable OCT atrophy rates did not correlate with whole brain atrophy, although RNFL alone correlated modestly with cortical gray matter. Differences in MRI and statistical techniques from other studies may be at play. It is also possible that RNFL and cortical gray matter atrophy have closer underlying pathophysiological mechanisms. Nevertheless, the lack of correlation with whole brain atrophy is discouraging for use of OCT simply as a proxy for brain atrophy in longstanding SPMS.
The slow atrophy rates and resulting large sample size calculations based on our findings calls into question the utility of OCT as a biomarker of disease progression at all in advanced SPMS clinical trials. Prior work supporting the use of OCT as a biomarker in neuroprotective trials of acute ON calculated sample sizes in the range of 100 patients per arm over study period of 6 months on TD-OCT (28). Talman et al. used a predominantly relapsing MS population and time-domain OCT platform to calculate sample size at approximated 400–600 patients per arm studied over 2–3 years, using 80–90% power and p<0.05 (17). Our preliminary sample size calculations, based on data from 47 patients, suggest that longitudinal studies on OCT specifically in this population will need to be longer and larger as severe baseline atrophy approaches a nadir.
A plateau (or rather, “floor”) effect of atrophy of RNFL and GCIPL in later stages of disease activity has been previously proposed in MS, and it may be one reason for the lack of correlation with brain atrophy and slow atrophy rates in general (19). In other fields of ophthalmology, the nadir of RNFL thickness has been reported in the range of 45 μm (Stratus OCT) to 57 μm (Cirrus OCT). It represents residual glial cells and retinal architecture after ganglion cell axon atrophy is complete, with over 50% of visual function still present at this thickness (29, 30). Future prospective studies looking at the natural history of OCT in this advanced disease population are needed with functional visual measures and MRI correlation to validate the utility of OCT in advanced SPMS, since RNFL, and especially GCIPL, appear promising in earlier inflammatory disease (7).
Statistical comparison of our treatment and placebo groups in the parent study revealed no significant effect of lipoic acid cross-sectional or longitudinal OCT values in our cohort and allowed us to pool the two groups, which was unexpected, given lipoic acid’s effect on rates of percent change brain volume (20). This may simply be due to a lack of power in the current study as the parent study was powered for detecting brain atrophy (31). Another explanation could be that there is a differential effect of lipoic acid in the brain versus the retina; a trial of lipoic acid in acute ON also found no treatment effect on RNFL thickness (32).
Other limitations of this study include history of ON and ocular disease, which was obtained via patient report as well as chart review and subject to recall error. These factors may have introduced confounders, thus scans with findings suspicious for other ocular pathologies were eliminated during quality control after collection. In addition, attenuated atrophy rates (and indeed some observed thicker RNFL values) found in our study and others raise the question of whether they represent true changes or simply test-retest variability (7, 33). Use of SD-OCT and strict quality control review helped address these concerns, since there is a high degree of reproducibility across testers and repeated measures versus TD-OCT platforms (34, 35).
In conclusion, this work comprises one of the largest prospective longitudinal SD-OCT studies investigating the natural history of RNFL and GCIPL atrophy in an underrepresented advanced SPMS cohort that is becoming more common, both in clinical practice and clinical trials. While ON history should be accounted for in cross-sectional analyses, our results suggest that eyes with and without ON history can be combined in longitudinal SPMS analyses. Based on our observations, low OCT atrophy rates and their lack of correlations with whole brain atrophy suggests that RNFL and GCIPL thicknesses need further validation for use as outcome measures in SPMS clinical trials.
Supplementary Material
Acknowledgments
The authors would like to thank ophthalmic photographer David Duff and clinical research coordinator Elizabeth Heriza for their contributions to the collection and organization of data in this study.
References
- 1.Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5(5):256–66. doi: 10.1038/nrneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- 2.Bock M, Brandt AU, Dorr J, Kraft H, Weinges-Evers N, Gaede G, et al. Patterns of retinal nerve fiber layer loss in multiple sclerosis patients with or without optic neuritis and glaucoma patients. Clin Neurol Neurosurg. 2010;112(8):647–52. doi: 10.1016/j.clineuro.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40(11):2520–7. [PubMed] [Google Scholar]
- 4.Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59(6):963–9. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Lapiscina EH, Arnow S, Wilson JA, Saidha S, Preiningerova JL, Oberwahrenbrock T, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15(6):574–84. doi: 10.1016/S1474-4422(16)00068-5. [DOI] [PubMed] [Google Scholar]
- 6.Saidha S, Al-Louzi O, Ratchford JN, Bhargava P, Oh J, Newsome SD, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: A four-year study. Ann Neurol. 2015;78(5):801–13. doi: 10.1002/ana.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratchford JN, Saidha S, Sotirchos ES, Oh JA, Seigo MA, Eckstein C, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013;80(1):47–54. doi: 10.1212/WNL.0b013e31827b1a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69(16):1603–9. doi: 10.1212/01.wnl.0000295995.46586.ae. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann H, Freing A, Kaufhold F, Gaede G, Bohn E, Bock M, et al. Optic neuritis interferes with optical coherence tomography and magnetic resonance imaging correlations. Mult Scler. 2013;19(4):443–50. doi: 10.1177/1352458512457844. [DOI] [PubMed] [Google Scholar]
- 10.Oberwahrenbrock T, Schippling S, Ringelstein M, Kaufhold F, Zimmermann H, Keser N, et al. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int. 2012;2012:530305. doi: 10.1155/2012/530305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costello F, Hodge W, Pan YI, Freedman M, DeMeulemeester C. Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. J Neurol Sci. 2009;281(1–2):74–9. doi: 10.1016/j.jns.2009.02.354. [DOI] [PubMed] [Google Scholar]
- 12.Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135(Pt 2):521–33. doi: 10.1093/brain/awr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winges KM, Werner JS, Harvey DJ, Cello KE, Durbin MK, Balcer LJ, et al. Baseline retinal nerve fiber layer thickness and macular volume quantified by OCT in the North American phase 3 fingolimod trial for relapsing-remitting multiple sclerosis. J Neuroophthalmol. 2013;33(4):322–9. doi: 10.1097/WNO.0b013e31829c51f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9(9):921–32. doi: 10.1016/S1474-4422(10)70168-X. [DOI] [PubMed] [Google Scholar]
- 15.Narayanan D, Cheng H, Bonem KN, Saenz R, Tang RA, Frishman LJ. Tracking changes over time in retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in multiple sclerosis. Mult Scler. 2014;20(10):1331–41. doi: 10.1177/1352458514523498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikuta F, Zimmerman HM. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology. 1976;26(6 PT 2):26–8. doi: 10.1212/wnl.26.6_part_2.26. [DOI] [PubMed] [Google Scholar]
- 17.Talman LS, Bisker ER, Sackel DJ, Long DA, Jr, Galetta KM, Ratchford JN, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749–60. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;131(Pt 1):277–87. doi: 10.1093/brain/awm285. [DOI] [PubMed] [Google Scholar]
- 19.Balk LJ, Cruz-Herranz A, Albrecht P, Arnow S, Gelfand JM, Tewarie P, et al. Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol. 2016;263(7):1323–31. doi: 10.1007/s00415-016-8127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spain R, Powers K, Murchison C, Heriza E, Winges K, Yadav V, et al. Lipoic acid in secondary progressive MS: A randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e374. doi: 10.1212/NXI.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 22.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999;5(4):244–50. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 23.Keltner JL, Cello KE, Balcer LJ, Calabresi PA, Markowitz CE, Werner JS. Stratus OCT Quality Control in Two Multi-Centre Multiple Sclerosis Clinical Trials. Neuro-Ophthalmology. 2011;35(2):57–64. doi: 10.3109/01658107.2011.557760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schippling S, Balk LJ, Costello F, Albrecht P, Balcer L, Calabresi PA, Frederiksen JL, Frohman E, Green AJ, Klistorner A, Outteryck O, Paul F, Plant GT, Traber G, Vermersch P, Villoslada P, Wolf S, Petzold A. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler Journal. 2015;21(2):8. doi: 10.1177/1352458514538110. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 26.Team RC. R: A language and environment for statistical computing. 2016 [Google Scholar]
- 27.Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Statistical Software. 2015;67(1):1–48. [Google Scholar]
- 28.Henderson AP, Altmann DR, Trip AS, Kallis C, Jones SJ, Schlottmann PG, et al. A serial study of retinal changes following optic neuritis with sample size estimates for acute neuroprotection trials. Brain. 2010;133(9):2592–602. doi: 10.1093/brain/awq146. [DOI] [PubMed] [Google Scholar]
- 29.Mwanza JC, Kim HY, Budenz DL, Warren JL, Margolis M, Lawrence SD, et al. Residual and Dynamic Range of Retinal Nerve Fiber Layer Thickness in Glaucoma: Comparison of Three OCT Platforms. Invest Ophthalmol Vis Sci. 2015;56(11):6344–51. doi: 10.1167/iovs.15-17248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26(6):688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altmann DR, Jasperse B, Barkhof F, Beckmann K, Filippi M, Kappos LD, et al. Sample sizes for brain atrophy outcomes in trials for secondary progressive multiple sclerosis. Neurology. 2009;72(7):595–601. doi: 10.1212/01.wnl.0000335765.55346.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadav V, Mass M, Marracci G, Wanchu R, Wyman A, Hills W, et al. Oral lipoic acid as a treatment for acute optic neuritis: a blinded, placebo-controlled randomized trial. ECTRIMS Abstract: P674. 2016:146514. doi: 10.1177/2055217319850193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serbecic N, Beutelspacher SC, Geitzenauer W, Kircher K, Lassmann H, Reitner A, et al. RNFL thickness in MS-associated acute optic neuritis using SD-OCT: critical interpretation and limitations. Acta Ophthalmol. 2011;89(5):e451–60. doi: 10.1111/j.1755-3768.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- 34.Leung CK, Cheung CY, Weinreb RN, Qiu Q, Liu S, Li H, et al. Retinal Nerve Fiber Layer Imaging with Spectral-Domain Optical Coherence Tomography. Ophthalmology. 2009;116:1257–63. doi: 10.1016/j.ophtha.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Syc SB, Warner CV, Hiremath GS, Farrell SK, Ratchford JN, Conger A, et al. Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler. 2010;16(7):829–39. doi: 10.1177/1352458510371640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


