Highlights
-
•
The potential systemic toxicity and mutagenicity of Oligopin®, a French Maritime Pine Bark extract, was evaluated.
-
•
Oligopin® was nongenotoxic in both bacterial and human cell assays.
-
•
Oligopin® was not acutely toxic and 90 days of oral administration to rats was well tolerated with a NOAEL of 1000 mg/kg/day.
-
•
The lack of adverse systemic effects in the 90 day study is concordant with findings from human clinical trials.
-
•
The NOAEL from the 90-day study suggests that Oligopin® is less systemically toxic than other FMPBE evaluated in subchronic studies.
Keywords: French Maritime Pine Bark extract (FMPBE) Oligopin, Acute toxicity, Subchronic toxicity, In vitro mutagenicity, Oral, Rat, Human, Safety
Abstract
The potential systemic toxicity of Oligopin®, a French Maritime Pine Bark extract (FMPBE) rich in procyanidolic oligomers, was evaluated in an acute oral limit test and a 90-day repeated dose oral toxicity study with Sprague Dawley rats. The potential mutagenicity was assessed in a bacterial reverse mutation assay and in vitro mammalian chromosome aberration assay with human lymphocytes. The results indicate that Oligopin® was nongenotoxic in both bacterial and human cell assays, was not acutely toxic via oral administration at up to 2000 mg/kg and was well tolerated following 90 days of oral administration to SD rats, with a no observed adverse effect level of 1000 mg/kg/day. The lack of significant adverse systemic effects in the 90 day study is concordant with findings from several human clinical trials. The acute toxicity and mutagenicity data are consistent with data reported by AFSSA in a summary of FMPBE safety, in which a NOAEL of 100 mg/kg/day was established. In contrast, the NOAEL derived from the 90-day study with Oligopin® was 1000 mg/kg/day, suggesting that it is less systemically toxic than other FMPBE previously evaluated in subchronic studies, and comparable to proanthocyanidins extracted from grape seeds, which are widely used as nutritional supplement ingredients.
1. Introduction
Oligopin® is a French Maritime Pine Bark extract (FMPBE) rich in procyanidolic oligomers (OPC), obtained using a selective extraction and purification process.
One of the special features of the product is the lack of use of chlorinated solvents during its preparation. The chemical characterization of Oligopin® has been described in detail by [1]. OPC are often designated as proanthocyanidins, procyanidins, or condensed tannins and are catechin and epicatechin oligomers, containing from 2 to 6 monomeric units. These monomers are linked by covalent bonds.
French Maritime Pine Bark Extract (FMPBE) has been used in food supplements and in drugs for more than 30 years. The raw materials and extraction process used to produce FMPBE are key aspects and may lead to differences in the constituent composition, and consequently to differences in biological activity. In the case of OLIGOPIN®, the raw material is always obtained from the pine tree Pinus pinaster, harvested from the largest cultivated forest in Europe (Landes of Gascony, France). The process starts with a solid-liquid (water) extraction to obtain the total polyphenol content, followed by a liquid-liquid extraction to remove the tannins, and ends with a recrystallisation step to remove monomers and phenolic acids. The combination of the specific raw material and the purification process used for OLIGOPIN® results in a unique extract with a specific and constant composition containing a high proportion of OPC. The composition of OLIGOPIN® is shown below in Table 1.
Table 1.
Oligopin® composition.
| Constituents | Average content (%mass) | Method |
|---|---|---|
| Procyanidolic oligomers : | 67–75 | GPC |
| Dimers: | 15–20 | |
| Trimers : | 15–20 | |
| Tetramers and higher oligomers: | 30–40 | |
| Total polyphenol | >96 | HPLC |
| Catechin | 4-10 | |
| Taxifoliol | 0.5-4 | |
| Taxifoliol glucoside | 3-8 | |
| Ferrulate glucoside | 4-10 | |
| Gallic acid | 0.1-1 | |
| Protocatechic acid | 0.5-3 | |
| Caffeic acid | 0.5-3 | |
| p-coumaric acid | 0.3-2 | |
| Ferulic acid | 1-5 | |
OPC are commonly found in all plants, and are also found in many foods such as fruits, vegetables, chocolate, cider, wine, and tea [2]. Historically, OPC was used to complex proteins [[3], [4], [5]] and in tanning [6].
More recently, studies have been conducted with OPC in the fields of nutrition and preventive medicine [[7], [8], [9], [10], [11], [12]], due to their antioxidant properties [13,14]. OPC exhibit strong antioxidant properties in vitro, and in vivo studies have shown that they inhibit the progression of atherosclerosis [1] and provide hepatoprotective effects against cadmium-induced hepatic inflammation, apoptosis and hepatic mitochondrial toxicity in rats [26]. A study with rats and Syrian hamsters evaluated the effects of long term supplementation with FMPBE on plasma antioxidant activity and atherosclerosis prevention [2]. FMPBE showed no toxicity and no side effects during the study and it did not have any influence on daily intake. It was absorbed and improved the resistance of the heart against free radical oxidation. There was a significant reduction of oxidized lipids at the surface of the arteries (atherosclerotic arteries) in the group supplemented with FMPBE.
This paper describes the results of in vitro genotoxicity, acute and subchronic toxicity studies with Oligopin®. Specifically, an acute oral (gavage) limit test and a 90-day repeated dose oral (gavage) toxicity study were conducted with Sprague Dawley rats to evaluate the systemic toxicity and potential target organ toxicity of Oligopin®. The potential mutagenicity of Oligopin® was assessed in a bacterial reverse mutation (Ames) assay and an in vitro mammalian chromosome aberration assay with human lymphocytes. All of these studies were conducted in accordance with Good Laboratory Practice (GLP) requirements.
2. Materials and methods
2.1. Acute toxicity limit test
The study was conducted with 10 Sprague Dawley OFA rats (5/sex), approximately 6 weeks old and weighing 194–212 g (males) or 167–182 g (females) [15]. Rats were acclimatised for 5 days prior to use in the study and were housed 5/sex in polypropylene cages. They were fasted the day before use in the study. All rats were administered a single oral (gavage) dose of 2000 mg/kg Oligopin® in corn oil at a constant volume of 20 ml/kg. Rats were observed for clinical signs of toxicity 1 h after dosing and daily for the following 14 days. Body weights of all rats was determined immediately prior to dosing and 3, 7 and 14 days thereafter. At the end of the 14 day observation period all rats were sacrificed by sodium phenobarbital injection, followed by gross necropsy to identify any gross macroscopic alterations.
2.2. Repeated dose toxicity
The study was conducted in accordance with OECD Test Guideline 408 [16]. A total of 80 Sprague Dawley SPF rats were divided into four groups, each composed of 10 males and 10 females. The animals were acclimated to the experimental conditions for eight days prior to the start of the study. Rats were randomly assigned to the treatment and control groups. The homogeneity of the groups was checked using Levene’s test after allocation on the basis of body weight. For each treatment group, the mean weight of males and females was calculated to ensure that individual weights of the animals did not deviate from the group mean weight by more than ±20%. The animals were identified individually per cage, by marking with a solution of picric acid with a paintbrush. The location of the marking, which was different for each animal, corresponded to a number. An additional caudal marking represented by a coloured circle made with a marker pen was used to identify the treatment group. Rats were housed five per cage by sex, in polypropylene cages with stainless steel lids. The bedding was renewed regularly, and was composed of wood shavings delivered dust-free and radiation sterilized, supplied by SICSA (94142 Alfortville, France). The cages were placed in an air-conditioned limited-access room, maintained in slight overpressure, with temperature maintained at 22 ± 2 °C and relative humidity of 50 ± 20%, except during washing cycles. Fresh filtered air was renewed at the rate of about 10 cycles per hour. The artificial lighting ensured a sequence of 12 h of light and 12 h of darkness. Oligopin® was dissolved in distilled water that was previously heated at 38 °C and was administered orally by gavage at doses of 1000 mg/kg/day (group 4), 300 mg/kg/day (group 3) and 90 mg/kg/day (group 2) for 91 consecutive days, 7 days per week. Control animals (group 1) received 10 ml/kg/day of the vehicle (distilled water) under the same conditions. The high dose (1000 mg/kg/d) corresponded with the dosage recommended for a limit test in subchronic oral toxicity studies, the mid-dose level (300 mg/kg/d) corresponded with approximately 100-fold of the recommended maximum daily human dosage, while the low dose (90 mg/kg/d) was established in accordance with a geometric progression of approximately 3.3, in order to show any potential relationship between dose and effect. The test item was administered to the animals daily during the morning (between 9:00 and 12:00 a.m.), seven days a week, over a period of 91 consecutive days in a constant dose volume of 10 ml/kg. The volumes of the test solution and vehicle were adjusted weekly for each rat, based on the last weighing. Each dose was orally administered by gavage using a 2.5, 5 or 10 ml syringe fitted with a suitably sized cannula. On days 1, 29 and 85 after preparation of the each test solutions, one sample per dose was collected in plastic disposable syringes (or tubes) and transferred to an analytical chemistry laboratory (Institut du Développement de Produits de Santé Avenue Gay Lussac, 33370 ARTIGUES, France) to verify the concentration of Oligopin® in the preparations administered. The clinical observation period was 92 days. Twice daily (morning and evening) all animals were observed for morbidity and mortality, except on public holidays (once only, in the morning). A general examination for clinical signs was performed twice daily, before treatment then during the hour following gavage. A detailed clinical examination was performed in all animals before the first dose treatment and once per week during the experiment within the hour following gavage. The examinations were made outside the home cage in a standard area and always at the same time of the day. The parameters observed included: changes in skin, fur, eyes, mucous membranes, occurrence of secretions and excretions and autonomic activity (lacrimation, piloerection, pupil size, unusual respiratory pattern…), changes in gait, posture and response to handling as well as the presence of clonic or tonic movements, stereotypes or bizarre behaviour. The sensory reactivity to stimuli of different types (auditory, visual and proprioceptive stimuli), grip strength and motor activity were also assessed. The animals were weighed during the acclimatization period, then on day 1, and once weekly throughout the study. On the day of the necropsy, rats were weighed after fasting. The day of the necropsy (day 92), the animals were weighed after fasting.The quantity of food consumed was assessed weekly per cage over a period of 48 h, by determining the difference between the quantity of food supplied and the quantity of food remaining in feeding bowls. The results were recorded in g of food consumed/48 h/ cage and were expressed in g of food consumed/24 h/100 g of body weight. Before administration of the test preparation (week –1) and during the last week of the study (week 13), an ophthalmological examination was performed with an ophthalmoscope (Heine Optotechnik, Herrshing, Germany) after dilating the pupils of all the animals using an ophthalmic solution (Mydriaticum® 0.5%, Laboratoires Théa, 63017 Clermont-Ferrand cedex 2, France) for 5 min. The observation area included the eye lids, cornea, iris and retina for irritation reactions and other possible lesions. In addition, the pupillary and palpebral reflexes were tested. All the animals were fasted at the end of the study on the evening of day 91 and the following morning, animals were lightly anaesthetized (Clorketam‚ 1 ml/kg i.m.) and blood samples were taken from the retro-orbital sinus. The blood was collected from all animals to perform the following haematological examinations: haematocrit, haemoglobin concentration, erythrocyte count, total and differential leukocyte count, platelet count, erythrocyte indices (MCV, MCH, and MCHC); prothrombin time. After collecting blood for haematological examinations, all the animals were anaesthetized with 6% Sodium pentobarbital (1.16 ml/kg, i.p.) and blood samples were taken from the abdominal aorta. The blood was collected into dry tubes, in order to perform the following biochemical assays: glucose, Creatinine, urea, total bilirubin, total proteins, albumin, total cholesterol, triglycerides, aspartate Aminotransferase (AST/GOT), alanine aminotransferase (ALT/GPT), alkaline phosphatases (AP), Phosphorus, calcium, chloride, sodium, potassium. During the last week of dosing, individual overnight urine samples were collected in all the animals for urinalysis. Each sample was evaluated for urine volume and appearance. The following qualitative analyses were performed using Combur-10 test strips (Roche Diagnostics): specific gravity, pH, leukocytes, nitrite, protein, glucose, ketone bodies, urobilinogen, bilirubin, blood (erythrocytes, haemoglobin).
After collecting blood for clinical biochemistry examinations, the animals were sacrificed by bleeding by abdominal aorta. All the animals used in the test were subjected to a full detailed gross necropsy which included careful examination of the external surfaces of the body, all orifices, and the cranial, thoracic and abdominal cavities and their contents. The liver, kidneys, adrenals, testes, epididymis, thymus, spleen, brain and heart of all the animals (except from those found dead during the test) were trimmed of any adherent tissue and their wet weight taken as soon as possible after dissection. The paired organs were weighed separately. Organ weights were expressed as absolute values and as relative values in comparison with brain weight. The following tissues and organs were sampled and preserved in the most appropriate fixation medium (Bouin's fluid or 10% Formol): all gross lesions, brain (cerebrum, cerebellum and pons), spinal cord, peripheral nerve (sciatic), pituitary, salivary glands, oesophagus, stomach, small and large intestines (including Peyer’s patches), liver, spleen, bone marrow (at sternal level), mesenteric lymph nodes, thymus, heart, aorta, trachea, lungs, thyroid/parathyroids, adrenals, pancreas, kidneys, urinary bladder, gonads, epididymides, accessory sexual organs, (uterus, prostate), vagina, mammary glands, eyes with lachrymal glands, bone (femur), skin and muscle (legs). After embedding in paraffin wax, 4 μm sections were cut and stained with H&E for histopathological examination of the following tissues and organs from all animals of the control and high dose groups: brain, spinal cord, peripheral nerve, pituitary, oesophagus, stomach, small and large intestines, liver, spleen, bone marrow, mesenteric lymph nodes, thymus, heart, trachea, lungs, thyroid/parathyroids, adrenals, pancreas, kidneys, gonads, epididymides, accessory sexual organs and all the macroscopic lesions.
2.3. Bacterial reverse mutation assay
The study was conducted in accordance with OECD Test Guideline 471 [17]. Briefly, four strains of Salmonella typhimurium (TA98, TA1537, TA100, TA1535) and one strain of Escherichia coli (WP2 uvrA) were obtained from Institut Pasteur for use in this assay. A preliminary cytotoxicity test with the test item was conducted at five concentrations to a maximum of 5000 ug/plate with Salmonella typhimurium strain TA100. A metabolic activation system (S-9) prepared from arochlor-1254 induced livers of Sprague Dawley rats (MOLTOX, Boone NC USA) was used for the assays with metabolic activation. For the assays without metabolic activation, 0.1 ml of a bacterial suspension containing 1–5 × 109 bacteria/mL and 0.1 ml of each the test item concentration were successively added to two mL of overlay agar. For the assays with metabolic activation either a plate incorporation method or a pre-incubation method was employed. Plates were incubated at 37 °C for 48 h after which the number of revertant colonies per plate was counted. Three plates were scored per test item concentration for each tester strain. The validity criteria for the test included: that the bacteriostatic activity of the highest test item concentration was less than 75%; the spontaneous reversion rate of the negative control was within the laboratory’s historical control incidence; and mean number of revertants for each strain and the corresponding positive control (with or without metabolic activation) fell within the laboratory’s historical control incidence. The result was considered negative if the number of revertants was less than three-fold the number of spontaneous reversions (for TA 1535 and TA1537) or less than two fold the number of spontaneous reversions (for TA98, TA100 or WP2(uvrA). The result of the test was considered positive if a concentration-related increase in revertant colonies was observed in a least one of the 5 tester strains, with or without metabolic activation. The test items was considered to be mutagenic if the number of revertant colonies was at least two fold greater than the spontaneous rate for Salmonella typhimurium TA98 or TA100 and Escherichia coli (WP2 uvrA) or three fold greater than the spontaneous rate for Salmonella typhimurium TA1537 or TA1535.
2.4. In vitro Mammalian chromosome aberration assay
The study was conducted in accordance with OECD Test Guideline 473 [18]. Peripheral blood from two healthy donors (1/sex, non-smokers) was collected in heparinized tubes under sterile conditions. Human blood (0.35 mL) was mixed in a culture flask with 4.65 ml of complete culture medium (Ham F12 (Gibco) supplemented with 15% (v/v) Fetal Calf Serum (FCS) (Gibco), 0.5% (v/v) antibiotics (penicillin 10 000 U/mL, streptomycin 10 000 μg/mL, amphotericin 25 μg/mL) and 2% (w/v) phytohemagglutinin (Sigma)). Duplicate cultures per concentration were prepared for each blood sample and were incubated at 37 °C in a humidified atmosphere containing 5% (v/v) CO2. A preliminary cytotoxicity study was performed, in order to determine the cytotoxicity of the test item and to determine the mitotic index (MI). The test item was introduced into the cultures and the number of cells in metaphase was determined in 1000 cells, after which the MI was calculated. The tested concentrations (μg/mL) with or without S9-mix were: 5000, 1500, 500, 50, 0. Three concentrations were selected for chromosome analysis, the highest concentration producing some cytotoxicity. The maximum acceptable concentration for the assay was one which suppressed mitotic activity by 50% (EC 50), when compared with the negative or solvent control. For the assay in the absence of metabolic activation three concentrations as defined above were used. The slides which were used for the determination of MI were microscopically examined, with 200 cells observed for the negative control, solvent control and each concentration of the test substance, and 25 cells observed for the positive control. For the chromosome aberration test two experiments were conducted. The duration of treatment and concentrations tested were:
Assay 1: short term treatment (4 h without S9-mix, 3 h with S9-mix)
Assay 2: Without S9-mix: cells are treated continuously during 20 h
With S9-mix (10% S9-mix): cells are treated during 3 h
Positive controls:
Without S9-mix : Mitomycin C [CAS N° 50-07-7] : 0.25 μg/mL
With S9-mix : Cyclophosphamide [CAS N° 6055-19-2] : 50 μg/mL
Four hundred cells in metaphase were analysed per assay and per concentration, without and with metabolic activation. Cytotoxicity at the highest concentration tested did not exceed 50% to 75%. Two assays were run independently: one with lymphocytes from a non-smoking man (M) (assay 1), and the other one with lymphocytes from a non-smoking woman (W) (assay 2). Where the results of assay 1 Produced negative results both with and without metabolic activation, assay 2 was performed as follows:
Cell suspension treated continuously for 20 h without metabolic activation.
Cell suspension treated for 3 h with metabolic activation (S9-mix : 10% (v/v)).
3. Results
3.1. Acute toxicity limit test
No rats died during the 14 day period following gavage dosing with 2000 mg/kg Oligopin® in corn oil at a constant volume of 20 ml/kg. No clinical signs were observed in male or female rats 1 or 5 h after gavage dose administration, nor was there any evidence of a change in the general status or comportment of the rats during the daily cage side observations throughout the 14 day observation period. Between dosing and study day 15, male and female rats gained on average 161.4 g and 61.4 g respectively, which was considered to be normal body weight evolution for the Sprague Dawley OFA strain. Gross necropsy revealed no macroscopic effects suggestive of systemic toxicity. Under the conditions of this study, the oral (gavage) LD50 of Oligopin® in male and female rats was greater than 2000 mg/kg.
3.2. Repeated dose toxicity
The Oligopin® concentrations of the solutions administered to the animals in the three dose groups was checked on three occasions (days 1, 29 and 85). As shown in the following Table 2, the concentration of Oligopin® in the dosing solutions administered was aligned with the nominal concentrations, and all differences were within the acceptable limits.
Table 2.
Concentration of Oligopin® in the dosing solutions.
| Sampling dates | Variation (%) Measured [OLIGOPIN®] / theoretical [OLIGOPIN®] (mg/ml) x 100 |
||
|---|---|---|---|
| D1 | D29 | D85 | |
| Group 2 | −3.3 % | +2.7 % | +4.9 % |
| Group 3 | −4.8 % | −1.3 % | +0.2 % |
| Group 4 | −5.8 % | +8.5 % | −8.4 % |
No mortality occurred during the study and no clinical signs considered to be of toxicological significance were observed. No significant differences were found between mean body weight of male and female rats receiving Oligopin® and that of male and female rats from the control group. Mean body weight gain after the 91- day dosing period among rats in groups 2, 3 and 4 was similar to that of control animals (see Table 3).
Table 3.
Body weight (G) - mean value.
| Sex: Male | ||||
|---|---|---|---|---|
| Distilled water | 90 mg/ kg/ day | OLIGOPIN | 1000mg/ kg/ day | |
| 10 ml/ kg/ day | 300 mg/ kg/ day | |||
| Group 1 | Group 2 | Group 3 | Group 4 | |
| D1 Mean | 263.2 | 267.9 | 266.7 | 266.7 |
| SD | 12.6 | 15.5 | 13.3 | 13.3 |
| D8 Mean | 310.5 | 315.6 | 313.5 | 313.2 |
| SD | 19.3 | 21.7 | 16.3 | 13.9 |
| D15 Mean | 349 | 358.4 | 350.8 | 347.8 |
| SD | 25.9 | 24.7 | 22.7 | 16.7 |
| D22 Mean | 381.8 | 387.8 | 379 | 383.2 |
| SD | 31.6 | 24.8 | 29.3 | 29.8 |
| D29 Mean | 404 | 413.1 | 402.4 | 399.2 |
| SD | 39 | 32.8 | 32.5 | 27 |
| D36 Mean | 431 | 438.3 | 424.8 | 417.1 |
| SD | 43.6 | 36.6 | 38 | 27.9 |
| D43 Mean | 449.3 | 459.6 | 441.8 | 435 |
| SD | 47.7 | 38.1 | 42.6 | 30.1 |
| D50 Mean | 467.3 | 480.3 | 457.1 | 448.6 |
| SD | 48.4 | 43 | 44.4 | 29.7 |
| D57 Mean | 481.7 | 493 | 470.7 | 465.8 |
| SD | 51.2 | 42.6 | 44.9 | 27.8 |
| D64 Mean | 484.5 | 493.5 | 476.5 | 468.7 |
| SD | 51.2 | 44.2 | 48.5 | 27.7 |
| D71 Mean | 501.7 | 513.3 | 492.7 | 484.6 |
| SD | 52.1 | 47.6 | 48.1 | 31.9 |
| D78 Mean | 505.5 | 521.8 | 499.1 | 489.1 |
| SD | 54.5 | 49.9 | 48.7 | 31.7 |
| D85 Mean | 517.9 | 533.5 | 510 | 501.4 |
| SD | 55.3 | 54.1 | 53.2 | 33 |
| D91 Mean | 520.3 | 531.8 | 510.9 | 501.8 |
| SD | 59.7 | 53.1 | 54.2 | 34.6 |
| D91-D1 Mean | 257.1 | 263.9 | 244.2 | 235.1 |
| SD | 53.4 | 48.3 | 47.5 | 32.9 |
| Sex: Female | ||||
|---|---|---|---|---|
| Distilled water | 90 mg/ kg/ day | OLIGOPIN | 1000mg/ kg/ day | |
| 10 ml/ kg/ day | 300 mg/ kg/ day | |||
| Group 1 | Group 2 | Group 3 | Group 4 | |
| D1 Mean | 214.6 | 214.5 | 219.8 | 219.3 |
| SD | 11.9 | 9 | 11.4 | 11 |
| D8 Mean | 235.7 | 232.9 | 237.9 | 238.5 |
| SD | 12 | 13.7 | 11.7 | 12.4 |
| D15 Mean | 254.7 | 248.4 | 256.1 | 253.2 |
| SD | 16.7 | 16.1 | 15.3 | 16 |
| D22 Mean | 264.6 | 260.3 | 271.7 | 267.9 |
| SD | 17.8 | 20 | 19.3 | 17.4 |
| D29 Mean | 274.4 | 270.7 | 278.7 | 276.2 |
| SD | 20.3 | 22.7 | 21.6 | 14.9 |
| D36 Mean | 279.7 | 275.3 | 285.3 | 282.5 |
| SD | 19.4 | 25.3 | 21.8 | 13.9 |
| D43 Mean | 284 | 277.9 | 297.5 | 286.5 |
| SD | 18.8 | 26.2 | 26.4 | 15.5 |
| D50 Mean | 292.4 | 285 | 300.9 | 294 |
| SD | 19.7 | 26.9 | 26 | 18.7 |
| D57 Mean | 297.2 | 289.6 | 305.6 | 296.9 |
| SD | 23 | 27.1 | 30.1 | 17.8 |
| D64 Mean | 293.3 | 289.1 | 304.2 | 296.9 |
| SD | 19.6 | 28.2 | 27.3 | 15.8 |
| D71 Mean | 304.2 | 298.5 | 311.8 | 303.6 |
| SD | 23.4 | 29.2 | 28.1 | 15.3 |
| D78 Mean | 304.5 | 299.4 | 316 | 309.9 |
| SD | 22.7 | 30.2 | 26.4 | 18.8 |
| D85 Mean | 308.6 | 304.1 | 313.9 | 311.8 |
| SD | 26.2 | 32.7 | 26.4 | 20.1 |
| D91 Mean | 311.7 | 301.9 | 319.2 | 311.2 |
| SD | 26.2 | 33.4 | 24.4 | 22.9 |
| D91-D1 Mean | 97.2 | 87.4 | 99.4 | 91.9 |
| SD | 17 | 26.5 | 14.7 | 18.1 |
*: p < 0.05, **: p < 0.01, ***: p < 0.001 vs Group 1 (Dunnett’s “t” test or Mann Whitney U test).
Mean daily food consumption for male and female rats (in g/100 g of body weight/24 h) receiving Oligopin® was comparable to control values (see Table 4). Ophthalmologic examinations revealed no treatment-related ocular abnormalities. The haematological data revealed a statistically-significant increase (+7.5%) in mean corpuscular haemoglobin (MCH) for males receiving 1000 mg/kg/day of Oligopin® (p < 0.01) and in females receiving 90 mg/kg/day (p < 0.01) or 1000 mg/kg/day (p < 0.001) of Oligopin® (see Table 5). In females of the middle dose group, the slight increase in MCH was non-significant. However, the increase in MCH was considered to be non-adverse, because all other red blood cell parameters were within the normal ranges, histopathological examination of bone marrow from the high dose group revealed no alterations, in females changes in MCH did not occur in a dose-related manner, and treated animals did not exhibit any clinical signs of toxicity. No other statistically-significant changes were observed in the haematology data. There were no treatment-related or statistically significant adverse effects on blood chemistry. However, statistically increased calcium was observed in males dosed with 90 mg/kg/day (+7%, p < 0.05) or 300 mg/kg/day and 1000 mg/kg/day (+9%, p < 0.01) (see Table 6). This isolated change was considered to be of no toxicological or physiological significance, since the magnitude of increase was small and not dose-related, other parameters were within normal ranges and a similar effect was not observed in females. The significant decrease in phosphorus only seen in males receiving the intermediate dose was considered to be unrelated to treatment and non-adverse.
Table 4.
Food consumption - mean values (g/24 h / 100 g of body weight).
| Sex: Male | ||||
|---|---|---|---|---|
| Distilled water | 90 mg/kg/day | OLIGOPIN | 1000 mg/kg/day | |
| 10 ml/kg/day | 300 mg/kg/day | |||
| Group 1 | Group 2 | Group 3 | Group 4 | |
| Week 1 | 9.4 | 9.8 | 9.7 | 9.8 |
| Week 2 | 8.3 | 8.3 | 8.6 | 8.5 |
| Week 3 | 7.9 | 7.7 | 7.3 | 7.6 |
| Week 4 | 7.1 | 7.1 | 7 | 7.1 |
| Week 5 | 6.2 | 6.4 | 5.9 | 6.5 |
| Week 6 | 6.5 | 5.9 | 5.3 | 5.7 |
| Week 7 | 5.9 | 5.8 | 5.8 | 5.9 |
| Week 8 | 6.1 | 5.5 | 5.7 | 6 |
| Week 9 | 5.1 | 5 | 5.3 | 5.5 |
| Week 10 | 5.3 | 4.9 | 5 | 5.7 |
| Week 11 | 5.2 | 5 | 5.2 | 5.6 |
| Week 12 | 5 | 4.9 | 4.9 | 5.2 |
| Week 13 | 5.3 | 4.8 | 5.2 | 51 |
| Sex: Female | ||||
|---|---|---|---|---|
| Distilled water | 90 mg/kg/day | OLIGOPIN | 1000 mg/kg/day | |
| 10 ml/kg/day | 300 mg/kg/day | |||
| Group 1 | Group 2 | Group 3 | Group 4 | |
| Week 1 | 8.6 | 8.8 | 9.1 | 8.4 |
| Week 2 | 8.9 | 8.6 | 9.2 | 9.1 |
| Week 3 | 7.5 | 8 | 8.3 | 8.4 |
| Week 4 | 7 | 7.1 | 7.9 | 7 |
| Week 5 | 7.7 | 6.6 | 7 | 7 |
| Week 6 | 6.3 | 6.8 | 6.9 | 7.3 |
| Week 7 | 6.7 | 6.7 | 6.9 | 6.7 |
| Week 8 | 7 | 6.9 | 6.7 | 6.4 |
| Week 9 | 5.9 | 6 | 6.4 | 6 |
| Week 10 | 6.5 | 6.6 | 6 | 6.7 |
| Week 11 | 6.1 | 6.5 | 6.5 | 6.9 |
| Week 12 | 6.1 | 6 | 5.9 | 6.4 |
| Week 13 | 6.3 | 5.6 | 5.8 | 6 |
Table 5.
Haematology - mean values.
| Eryth | Hb | Ht | MCV | MCH | MCHC | Leuko | Platelets | Prothro, T (s) | |
|---|---|---|---|---|---|---|---|---|---|
| T/l | g/dl | % | fl | pg | g/dl | G/l | G/l | ||
| Sex: Male | |||||||||
| GROUP 1 | 10 ml/ kg distilled water | ||||||||
| Mean | 8.53 | 16 | 40.1 | 52 | 18.7 | 39.8 | 7.8 | 680 | 16.4 |
| SD | 0.65 | 1.4 | 2.4 | 2 | 0.8 | 2.2 | 2.2 | 184 | 0.6 |
| GROUP 2 | 90 mg/ kg/ day, OLIGOPIN | ||||||||
| Mean | 8.61 | 16.5 | 40.6 | 52 | 19.2 | 40.8 | 6.7 | 632 | 16.2 |
| SD | 0.42 | 0.6 | 2.9 | 2 | 0.8 | 2.4 | 1,6 | 268 | 0.5 |
| GROUP 3 | 300 mg/ kg/ day, OLIGOPIN | ||||||||
| Mean | 8.34 | 16.7 | 40.1 | 53 | 20 | 41.7 | 8.5 | 660 | 16.3 |
| SD | 0.14 | 0.7 | 1.3 | 2 | 0.8 | 1.9 | 2.6 | 208 | 0.4 |
| GROUP 4 | 1000 mg/ kg/ day, OLIGOPIN | ||||||||
| Mean | 8.34 | 16.8 | 40.1 | 54 | 20.1 | 41.9 | 7.9 | 681 | 16.4 |
| SD | 0.43 | 0.8 | 2.1 | 2 | 0.9 ** | 1.8 | 1.8 | 264 | 0.6 |
| Sex: Female | |||||||||
| GROUP 1 | 10 ml/ kg distilled water | ||||||||
| Mean | 7.73 | 15 | 38.6 | 55 | 19.4 | 38.5 | 5.1 | 942 | 16.1 |
| SD | 0.39 | 0.9 | 1.7 | 1 | 0.8 | 1.9 | 1.2 | 176 | 0.4 |
| GROUP 2 | 90 mg/ kg/ day, OLIGOPIN | ||||||||
| Mean | 7.55 | 15.9 | 39.1 | 57 | 21.2 | 41.1 | 5.3 | 1041 | 16 |
| SD | 0.92 | 1.5 | 5.5 | 2 | 2.5 | 5.5 | 1.8 | 173 | 0.6 |
| GROUP 3 | 300 mg/ kg/ day, OLIGOPIN | ||||||||
| Mean | 7.79 | 15.7 | 39.8 | 57 | 20.1 | 39.4 | 5.2 | 876 | 16.2 |
| SD | 0.49 | 0.5 | 2.1 | 2 | 0.9 | 1.7 | 0.8 | 255 | 0.6 |
| GROUP 4 | 1000 mg/ kg/ day, OLIGOPIN | ||||||||
| Mean | 7.43 | 15.5 | 38.2 | 57 | 20.9 | 40.7 | 5.8 | 1031 | 15.7 |
| SD | 0.37 | 0.9 | 2.9 | 2 | 0.5 *** | 1.8 | 1.7 | 133 | 0.4 |
| N | E | B | L | M | Total | N | E | B | L | M | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | G/l | G/l | G/l | G/l | G/l | G/l | |
| Sex: Male | |||||||||||
| GROUP 1 | 10 ml/ kg distilled water | ||||||||||
| Mean | 8 | 1 | 0 | 91 | 0 | 7.8 | 0.61 | 0.07 | 0 | 7.1 | 0.02 |
| SD | 2 | 1 | 0 | 3 | 0 | 2.2 | 0.32 | 0.09 | 0 | 7.1 | 0.04 |
| GROUP 2 | 90 mg/ kg/ day OLIGOPIN | ||||||||||
| Mean | 11 | 1 | 0 | 88 | 0 | 6.7 | 0.72 | 0.04 | 0 | 5.91 | 0.01 |
| SD | 5 | 1 | 0 | 5 | 0 | 1.6 | 0.38 | 0.07 | 0 | 1.56 | 0.03 |
| GROUP 3 | 300 mg/ kg/ day OLIGOPIN | ||||||||||
| Mean | 8 | 1 | 0 | 91 | 0 | 8.5 | 0.68 | 0.06 | 0 | 7.79 | 0.01 |
| SD | 5 | 1 | 0 | 4 | 0 | 2.6 | 0.37 | 0.08 | 0 | 2.46 | 0.02 |
| GROUP 4 | 1000 mg/ kg/ day OLIGOPIN | ||||||||||
| Mean | 13 | 1 | 0 | 87 | 0 | 7.9 | 1.04 | 0.04 | 0 | 6.8 | 0.01 |
| SD | 8 | 1 | 0 | 8 | 0 | 1.8 | 0.9 | 0.07 | 0 | 1.47 | 0.02 |
| Sex: Female | |||||||||||
| GROUP 1 | 10 ml/ kg distilled water | ||||||||||
| Mean | 9 | 1 | 0 | 90 | 0 | 5.1 | 0.48 | 0.03 | 0 | 4.57 | 0.01 |
| SD | 5 | 1 | 0 | 5 | 0 | 1.2 | 0.31 | 0.04 | 0 | 1.1 | 0.03 |
| GROUP 2 | 90 mg/ kg/ day OLIGOPIN | ||||||||||
| Mean | 10 | 1 | 0 | 90 | 0 | 5.3 | 0.49 | 0.05 | 0 | 4.73 | 0 |
| SD | 4 | 1 | 0 | 4 | 0 | 1.8 | 0.21 | 0.05 | 0 | 1.65 | 0 |
| GROUP 3 | 300 mg/ kg/ day OLIGOPIN | ||||||||||
| Mean | 9 | 1 | 0 | 89 | 0 | 5.2 | 0.49 | 0.07 | 0 | 4.61 | 0 |
| SD | 3 | 2 | 0 | 3 | 0 | 0.8 | 0.17 | 0.09 | 0 | 0.67 | 0 |
| GROUP 4 | 1000 mg/ kg/ day OLIGOPIN | ||||||||||
| Mean | 13 | 1 | 0 | 87 | 0 | 5.8 | 0.66 | 0.05 | 0 | 5.08 | 0 |
| SD | 7 | 1 | 0 | 7 | 0 | 1.7 | 0.28 | 0.04 | 0 | 1.82 | 0 |
*p < 0.05, **: p < 0.01, ***: p < 0.001 vs Group 1 (Dunnett’s “t” test or Mann Whitney U test).
Table 6.
Biochemistry - mean values.
| Gluc | Crea | Urea | Bill,Tot | Prot | Cholesterol | Alb | |
|---|---|---|---|---|---|---|---|
| mmol/l | % | mmol/l | % | g/l | mmol/l | g/l | |
| Sex: Male | |||||||
| GROUP 1 | 10 ml/kg distilled water | ||||||
| Mean | 6.53 | 62.5 | 8.38 | 3.3 | 51.3 | 1.56 | 36.1 |
| SD | 1.38 | 17.3 | 1.39 | 0.5 | 3.4 | 0.32 | 2.2 |
| GROUP 2 | 90 mg/ kg/ day OLIGOPIN | ||||||
| Mean | 5.98 | 56.9 | 7.81 | 3.5 | 51.7 | 1.54 | 36.3 |
| SD | 1 | 11.6 | 1.68 | 0.5 | 2.2 | 0.21 | 1.2 |
| GROUP 3 | 300 mg/ kg/ day OLIGOPIN | ||||||
| Mean | 5.65 | 53.5 | 7.28 | 3.3 | 51.5 | 1.59 | 35.5 |
| SD | 1.13 | 8.7 | 1.13 | 0.5 | 7.7 | 0.29 | 4.9 |
| GROUP 4 | 1000 mg/ kg/ day OLIGOPIN | ||||||
| Mean | 5.8 | 64.2 | 6.95 | 3.2 | 53.3 | 1.71 | 37.2 |
| SD | 1.66 | 12.9 | 1.74 | 0.4 | 3.6 | 0.19 | 2.4 |
| Sex : Female | |||||||
| GROUP 1 | 10 ml/ kg distilled water | ||||||
| Mean | 5.74 | 66.8 | 8.65 | 3.9 | 53.1 | 1.82 | 38.9 |
| SD | 1.37 | 17.5 | 1.69 | 0.3 | 4.6 | 0.3 | 3.3 |
| GROUP 2 | 90 mg/ kg/ day OLIGOPIN | ||||||
| Mean | 5.52 | 62.4 | 7.9 | 4.1 | 55.5 | 2.04 | 39.8 |
| SD | 1.13 | 6.2 | 1.27 | 0.3 | 3.5 | 0.39 | 2.6 |
| GROUP 3 | 300 mg/ kg/ day OLIGOPIN | ||||||
| Mean | 5.58 | 65.7 | 9.08 | 4 | 53.5 | 1.7 | 37.8 |
| SD | 1.03 | 11.9 | 1.63 | 0.5 | 2.9 | 0.38 | 2 |
| GROUP 4 | 1000 mg/ kg/ day OLIGOPIN | ||||||
| Mean | 5.16 | 68.8 | 7.62 | 3.7 | 53.6 | 1.93 | 38.4 |
| SD | 0.58 | 6.3 | 1.79 | 0.5 | 3.4 | 0.62 | 2.7 |
| AST | ALT | A,P, | Calcium | Phosphorus | Chloride | Sodium | Potassium | |
|---|---|---|---|---|---|---|---|---|
| IU/l | IU/l | IU/l | mmol/l | mmol/l | mmol/l | mmol/l | mmol/l | |
| Sex: Male | ||||||||
| GROUP 1 | 10 ml/kg distilled water | |||||||
| Mean | 237 | 58 | 88 | 2.11 | 2.13 | 101 | 137 | 3.9 |
| SD | 97 | 24 | 20 | 0.23 | 0.22 | 5 | 10 | 0.8 |
| GROUP 2 | 90 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 230 | 50 | 86 | 2.26 | 1.92 | 105 | 143 | 4.4 |
| SD | 105 | 21 | 17 | 0.08 * | 0.17 | 1 | 2 | 0.3 |
| GROUP 3 | 300 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 211 | 45 | 91 | 2.3 | 1.8 | 109 | 140 | 4.5 |
| SD | 51 | 9 | 20 | 0.05 ** | 0.37 * | 11 | 14 | 0.5 |
| GROUP 4 | 1000 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 229 | 48 | 89 | 2.31 | 2.02 | 103 | 144 | 4.2 |
| SD | 148 | 26 | 22 | 0.09 ** | 0.21 | 1 | 2 | 0.2 |
| Sex : Female | ||||||||
| GROUP 1 | 10 ml/ kg distilled water | |||||||
| Mean | 268 | 49 | 69 | 2.39 | 1.93 | 106 | 143 | 4.3 |
| SD | 111 | 19 | 13 | 0.04 | 0.38 | 1 | 2 | 0.5 |
| GROUP 2 | 90 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 246 | 42 | 64 | 2.37 | 1.74 | 106 | 143 | 4 |
| SD | 85 | 10 | 22 | 0.09 | 0.3 | 2 | 2 | 0 |
| GROUP 3 | 300 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 254 | 46 | 67 | 2.33 | 1.78 | 106 | 143 | 4.1 |
| SD | 104 | 14 | 18 | 0.09 | 0.21 | 1 | 2 | 0.3 |
| GROUP 4 | 1000 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 221 | 42 | 61 | 2.38 | 1.81 | 105 | 143 | 4.2 |
| SD | 82 | 15 | 11 | 0.09 | 0.27 | 2 | 2 | 0.4 |
*: p < 0.05, **: p < 0.01, ***: p < 0.001 vs Group 1 (Dunett’s “t” test or Mann Whitney U test).
Compared to control values, there were no statistically significant differences in the volume, specific gravity and pH of urine voided by males and females dosed with Oligopin®. In treated males, haemoglobin pigments and ketone bodies were detected more frequently in urine samples than in control animals. However, these changes were not considered to be biologically significant, since no similar effect was observed in treated females, and kidney weights and histological examination revealed no evidence of renal damage. Macroscopic examination of the spleen performed on animals sacrificed after 91 days of dosing revealed a dark pigmentation (nearly black) in the spleen of one male and six females in the 1000 mg/kg/day dose group. All other gross observations recorded in control or treated groups were incidental and spontaneous in nature and had no relation to treatment with Oligopin®.
Absolute spleen weights and the spleen to brain weight ratio were significantly increased in females receiving 300 mg/kg/day (+18.7%, p < 0.05) or 1000 mg/kg/day (+21.6%, p < 0.01) compared to controls (see Table 7, Table 8). Although the increase seemed to be dose-related, this change was considered to have no toxicological significance, since the spleens of the animals from the high dose group did not exhibit any histopathological lesions and no similar effect was observed in males. Treatment related findings consisting of depositions of brown pigment located diffusely throughout the red pulp, mainly within sinusoidal macrophages, were seen in the spleen of females dosed with 1000 mg/kg/day Oligopin®. These depositions were marked in the spleens of 5 females and moderate in the one male and one female. In order to identify and evaluate the incidence and distribution of this brown pigment, spleen sections from all females from the 1000 mg/kg/day and 300 mg/kg/day groups and one high dose male were stained with Perl’s stain. The results showed the brown pigment stained positively for iron with Perl’s stain. The incidence and distribution of iron pigment in the Perl’s stained sections of spleen is shown in the Table 9 below.
Table 7.
Absolute organ weight - mean values.
| Brain (g) | Liver (g) | Spleen (g) | Thymus (g) | Heart (g) | Adrenal (mg) | Kidney(g) | Testis (g) | Epididymis (g) | |
|---|---|---|---|---|---|---|---|---|---|
| Sex: Male | |||||||||
| GROUP 1 | 10 ml/ kg distilled water | ||||||||
| Mean | 2.059 | 12.244 | 0.668 | 0.356 | 1.457 | 32.8 | 1.469 | 1.717 | 0.732 |
| SD | 0.102 | 1.918 | 0.101 | 0.092 | 0.11 | 5.6 | 0.204 | 0.118 | 0.06 |
| GROUP 2 | 90 mg/ kg/ day OLIGOPIN | ||||||||
| Mean | 2.189 | 12.184 | 0.661 | 0.372 | 1.485 | 30.3 | 1.521 | 1.823 | 0.691 |
| SD | 0.069** | 1.521 | 0.074 | 0.093 | 0.139 | 4.4 | 0.17 | 0.12 | 0.039 |
| GROUP 3 | 300 mg/ kg/ day OLIGOPIN | ||||||||
| Mean | 2.154 | 12.138 | 0.66 | 0.328 | 1.38 | 31.6 | 1.502 | 1.708 | 0.71 |
| SD | 0.097 | 1.772 | 0.108 | 0.059 | 0.122 | 4.8 | 0.143 | 0.125 | 0.051 |
| GROUP 4 | 1000 mg/ kg/ day OLIGOPIN | ||||||||
| Mean | 2.121 | 11.483 | 0.676 | 0.327 | 1.355 | 33.1 | 1.505 | 1.782 | 0.711 |
| SD | 0.091 | 1.386 | 0.107 | 0.064 | 0.12 | 5.3 | 0.096 | 0.193 | 0.076 |
| Brain | Liver | Spleen | Thymus | Heart | Adrenal | Kidney | Ovary | Uterus | |
| (g) | (g) | (g) | (g) | (g) | (mg) | (g) | (g) | (g) | |
| Sex: Female | |||||||||
| GROUP 1 | 10 ml/ kg distilled water | ||||||||
| Mean | 1.978 | 6.914 | 0.416 | 0.304 | 0.974 | 35.8 | 0.806 | 68.4 | 0.795 |
| SD | 0.065 | 0.696 | 0.05 | 0.068 | 0.068 | 8.5 | 0.063 | 9.4 | 0.332 |
| GROUP 2 | 90 mg/ kg/ day OLIGOPIN | ||||||||
| Mean | 1.998 | 6.913 | 0.469 | 0.271 | 0.979 | 34.4 | 0.79 | 62.4 | 0.689 |
| SD | 0.107 | 0.974 | 0.061 | 0.088 | 0.13 | 5.4 | 0.089 | 14.5 | 0.163 |
| GROUP 3 | 300 mg/ kg/ day OLIGOPIN | ||||||||
| Mean | 1.982 | 6.961 | 0.494 | 0.305 | 1.008 | 37.1 | 0.817 | 61.5 | 0.782 |
| SD | 0,13 | 0.505 | 0.048* | 0.055 | 0.072 | 6.1 | 0.047 | 11.3 | 0.259 |
| GROUP 4 | 1000 mg/ kg/ day | ||||||||
| Mean | 1.998 | 7.424 | 0.506 | 0.243 | 0.983 | 38 | 0.797 | 67.4 | 0.667 |
| SD | 0.069 | 1.102 | 0.063 ** | 0.068 | 0.125 | 3.7 | 0.063 | 13.1 | 0.174 |
*: p < 0.05, **: p < 0.01, ***: p < 0.001 vs Group 1 (Dunett’s “t” test or Mann Whitney U test).
Table 8.
Absolute organ weight (Brain weight ratio ×100) - mean values.
| Liver | pleen | Thymus | Heart | Adrenal | Kidney | Testis | Epididymis | |
|---|---|---|---|---|---|---|---|---|
| Sex: Male | ||||||||
| GROUP 1 | 10 ml/ kg distilled water | |||||||
| Mean | 594.82 | 32.47 | 17.31 | 70.81 | 1.59 | 71.29 | 83.52 | 35.64 |
| SD | 88.77 | 4.98 | 4.39 | 4.82 | 0.26 | 8.7 | 6.46 | 3.31 |
| GROUP 2 | 90 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 556.48 | 30.22 | 16.96 | 67.9 | 1.39 | 69.55 | 83.29 | 31.57 |
| SD | 66.17 | 3.35 | 4.09 | 6.81 | 0.21 | 8.34 | 5.63 | 2.09 * |
| GROUP 3 | 300 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 563.15 | 30.67 | 15.23 | 64.11 | 1.47 | 69.75 | 79.33 | 33.03 |
| SD | 76.8 | 5.16 | 2.61 | 5.75* | 0.22 | 6.19 | 5.3 | 2.92 |
| GROUP 4 | 1000 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 540.69 | 31.85 | 15.42 | 63.88 | 1.56 | 71.07 | 84.18 | 33.55 |
| SD | 53.36 | 4.69 | 2.83 | 5.18* | 0.22 | 5.23 | 9.77 | 3.77 |
| Liver | Spleen | Thymus | Heart | Adrenal | Kidney | Ovary | Uterus | |
| Sex: Female | ||||||||
| GROUP 1 | 10 ml/ kg distilled water | |||||||
| Mean | 349.4 | 21.03 | 15.38 | 49.28 | 1.8 | 40.78 | 3.46 | 40.28 |
| SD | 31.83 | 2.22 | 3.59 | 3.4 | 0.38 | 3.1 | 0.47 | 17.14 |
| GROUP 2 | 90 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 344.97 | 23.4 | 13.48 | 48.9 | 1.72 | 39.5 | 3.12 | 34.48 |
| SD | 37.44 | 2.26 | 4.2 | 5.29 | 0.24 | 3.43 | 0.7 | 7.84 |
| GROUP 3 | 300 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 352.23 | 25.03 | 15.41 | 50.92 | 1.88 | 41.3 | 3.12 | 39.87 |
| SD | 28,5 | 2.84** | 2.64 | 2.96 | 0.3 | 2.25 | 0.6 | 14.17 |
| GROUP 4 | 1000 mg/ kg/ day OLIGOPIN | |||||||
| Mean | 371.83 | 25.33 | 12.12 | 49.18 | 1.9 | 39.89 | 3.38 | 33.28 |
| SD | 56.83 | 2.86** | 3.21 | 6.2 | 0.2 | 3.03 | 0.65 | 7.97 |
*: p < 0.05, **: p < 0.01, ***: p < 0.001 vs Group 1 (Dunett’s “t” test or Mann Whitney U test).
Table 9.
Incidence and distribution of iron pigment in the Perl’s stained sections of spleen.
| Females |
Males | |||
|---|---|---|---|---|
| Dose level (mg/kg/day) | 0 | 300 | 1000 | 1000 |
| Iron pigment in macrophage (red pulp) | ||||
| Minimal | 2 | 10 | 2 | 0 |
| Moderate | 0 | 0 | 5 | 0 |
| Marked | 0 | 0 | 3 | 1 |
| Total Number of examined spleens | 2 | 10 | 10 | 1 |
This table shows that the deposition of iron pigment within macrophages predominantly in red pulp of the spleen was increased in animals dosed with 1000 mg/kg/day Oligopin®. In females receiving 300 mg/kg/day OLIGOPIN, deposition of iron pigment appeared to be similar to the controls. As the deposition of iron was in the cells of the mononuclear phagocytic system and in the absence of treatment-related cellular damage of the spleen, the pigment deposition was not considered to be toxicologically significant. Pigment is commonly seen in the spleen of the aged rat. The ultrastructural appearance of this pigment within siderosomes in spleen macrophages suggests that erythrocyte degradation may be responsible for pigment production [19]. All other changes in liver, lungs, heart, oesophagus or uterus noted in rats from control and treated groups with the same incidence in both sexes or only in one animal were considered as incidental and not treatment-related.
3.3. Bacterial reverse mutation assay
The bacteriostatic activity of Oligopin® was evaluated in two independent trials with Salmonella typhimurium strain TA100 at concentrations of 50, 150, 500, 1500 or 5000 ug/plate (triplicate plates) and compared with and a negative and ethanol control. The percentage survival vs. negative control was 25.5% at 5000 ug/plate, indicating a bacteriostatic activity of the highest concentration (74.5%) was within the acceptance criterion (75%). The concentrations used in the mutation assay were 0, 50, 150, 500, 1500 or 5000 ug/plate. Exposure to 0, 50, 150, 500, 1500 or 5000 ug/plate of Oligopin® in the presence or absence of metabolic activation produced no evidence of an increase in the number of revertant colonies with Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 and Escherichia coli (WP2 uvrA). In the absence of metabolic activation, 5000 ug/plate produced a significant decrease in the number of spontaneous reversions which confirmed the bacteriostatic activity at this concentration.
3.4. In vitro Mammalian chromosome aberration assay
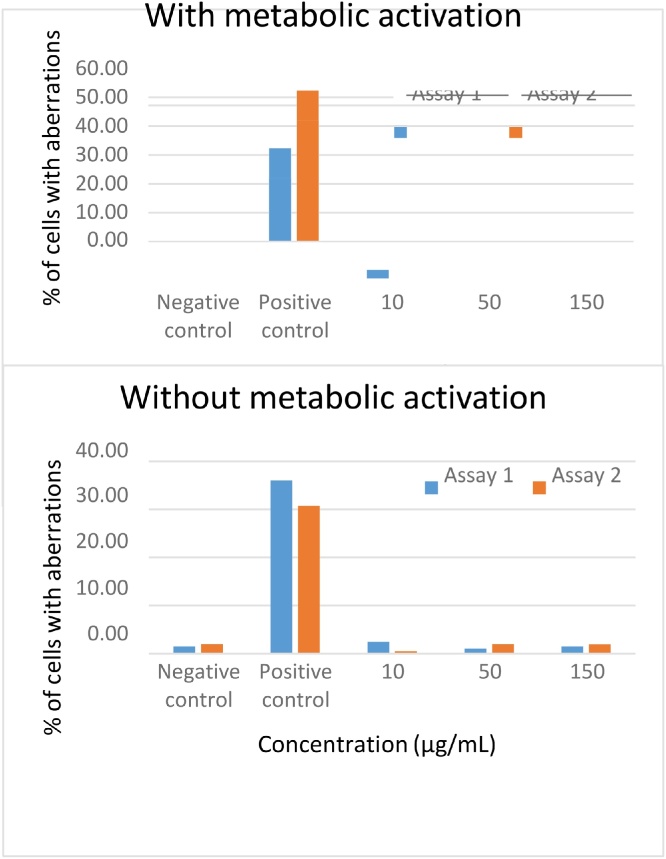
In a preliminary cytotoxicity study with human lymphocytes, markedcytotoxicity was observed at 5000, 1500, and 500 μg/ml of Oligopin®. A significant reduction (46.2%) of the mitotic index was observed at 150 μg/mL, which was considered the maximum acceptable concentration for the study. In the presence of metabolic activation (S9), a slight reduction of the mitotic index (25.4 and 21.7%) was observed at 10 and 150 μg/mL. Based on the preliminary cytotoxicity study, three analysable Oligopin® concentrations (10, 50 and 150 μg/mL) were chosen for the assessment of chromosome aberrations in the absence or presence of metabolic activation in Assay 1 (4 h without S9-mix; 3 h with 5% (v/v) S9- mix) and Assay 2 (20 h without S9-mix; 3 h with 10% (v/v) S9-mix). The results are shown in the Fig. 1 below:
Fig. 1.
Chromosome aberrations in the absence or presence of metabolic activation.
In the solvent (negative) control group, the percentage of cells with aberrations was between 0.5% and 2%, whether in the absence or presence of metabolic activation. In the positive control group without metabolic activation, Mitomycin C produced a significant increase (P < 0.001) in the percentage of cells with aberrations (30.8–36%), while in the positive control group with metabolic activation, cyclophosphamide also induced a significant increase (P < 0.001) in the percentage of cells with aberrations (32–52%). The values obtained for the negative and positive controls confirmed the sensitivity and specificity of the assays performed with Oligopin®. Four or 20 h of exposure of human lymphocytes to 10, 50, or 150 μg/ml Oligopin® without metabolic activation produced no statistically significant increases in the frequency of structural chromosome aberrations at any tested concentration. The percentage of cells with aberrations ranged from 0.5% to 2.5%, which was comparable to the incidence in the negative control group. The exposure of human lymphocytes for three hours to 10, 50, or 150 μg/ml Oligopin® in the presence of 5% (v/v) or 10% (v/v) S9 produced no statistically significant increases in the frequency of structural chromosome aberrations at any of the tested concentrations. The percentage of cells with aberrations was from 1.5% to 3%. Based on these results it was concluded that Oligopin® is not clastogenic at the concentrations studied (10 to 150 μg/mL) in the presence or absence of metabolic activation.
4. Discussion
The potential genotoxicity and systemic toxicity of Oligopin®, a French Maritime Pine Bark extract (FMPBE) rich in procyanidolic oligomers (OPC), was examined in a bacterial reverse mutation assay, a chromosomal aberration assay with human lymphocytes, as well as acute and 90-day repeated dose oral toxicity studies with Sprague Dawley rats. All of these studies were conducted in accordance with GLP requirements. Oligopin® showed no evidence of mutagenic effects in an Ames assay in the presence or absence of metabolic activation in Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 or Escherichia coli (WP2 uvrA). No clastogenic effects were observed in cultured human lymphocytes at the concentrations studied (10–150 μg/mL), with or without exogenous metabolic activation. Thus, Oligopin® was non genotoxic in both bacterial and human cells under the conditions of the assays performed. A single oral (gavage) dose of 2000 mg/kg Oligopin® to male and female Sprague Dawley OFA strain rats produced no mortality, clinical signs, changes in general status or behaviour during the 14 days observation period following dosing. The rats had normal weight gain and gross necropsy revealed no macroscopic effects suggestive of systemic toxicity. Daily oral (gavage) administration of Oligopin® to male and female Sprague Dawley rats for 91 consecutive days at 90, 300 or 1000 mg/kg/day induced no treatment-related adverse changes in clinical signs, body weight, food consumption, ophthalmology, urinalysis or clinical chemistry. An increase in mean corpuscular haemoglobin, considered to be non-adverse, was noted in both sexes at the 1000 mg/kg/day dose. This was associated, in females only, with a significant increase in absolute and relative spleen weight and marked deposition of brown pigment throughout the red pulp of the spleen within macrophages. However, since the spleen did not exhibit any histomorphologic lesions, these changes were not considered to be toxicologically significant. Based on the results of this study, the 1000 mg/kg/day Oligopin® dose level was considered to be the No Observed Adverse Effect Level (NOAEL) for repeated oral exposure. The results of the toxicity studies reported in this paper with Oligopin® are consistent with the data reported by AFSSA [20] in a summary of the safety of FMPBE as a dietary supplement ingredient. The AFSSA summary noted oral LD50 values in the rat, mouse and guinea pig of 1000–4800 mg/kg and the absence of mutagenic or genotoxic effects in an Ames assay, and a human lymphocyte chromosome aberration assay. Five subchronic toxicity studies in several species with exposure durations of between 3–6 months were performed with FMPBE [20]. These studies permitted AFSSA to determine a NOAEL of 100 mg/kg/day in the rat, which was based on reduced food and water consumption, reduced body weight gain, increased blood glucose in females, and changes in the weight of certain organs at higher doses. In contrast, the NOAEL derived from the 90-day oral (gavage) study with Oligopin® in Sprague Dawley rats was 1000 mg/kg/day, suggesting that it is less systemically toxic than other FMPBE that were previously evaluated in subchronic toxicity studies. Although Oligopin® was not evaluated for reproductive or developmental toxicity or in vivo genotoxicity, all of these toxicological endpoints were evaluated with FMPBE, as reported by AFSSA [20]. No adverse effects on reproductive functions were demonstrated at the doses tested in the mouse and rabbit (3.6 to 8.5 mg/kg/day) or in rats at up to 750 mg/kg/day. FMPBE was not a skin or eye irritant in studies with rabbits, it did not produce skin sensitisation in male albino guinea pigs, and was not clastogenic in an in vivo mouse micronucleus study. Given the similarity of Oligopin® to other FMPBE, it is considered reasonable to assume that Oligopin® would have similar properties for each of these toxicological endpoints. It is also of interest to compare the toxicity of Oligopin® with that of proanthocyanidins extracted from grape seeds [21], and Clovinol, a standardized polyphenolic extract of clove buds [25], which are also used as nutritional supplements.
The toxicity of a proanthocyanidin-rich extract from grape seeds was examined for acute and subchronic oral toxicity using Fischer 344 rats and for mutagenic potential by the reverse mutation test using Salmonella typhimurium, the chromosomal aberration test using CHL cells, and the micronucleus test using ddY mice [21]. In those studies, no evidence of mutagenicity or of acute oral toxicity at dosages of 2 and 4 g/kg was observed. Oral dietary exposure for 90 days at levels of 0.02, 0.2 and 2% (w/w) to rats did not induce any noticeable signs of toxicity, and the NOAEL of grape seed extract in this study was 2% in the diet, equal to approximately 1410 mg/kg/day in males and 1501 mg/kg/day in females.
The acute and subchronic oral toxicity of Clovinol was assessed in Wistar rats, along with its mutagenic potential in the reverse mutation assay using Salmonella typhimurium strains TA-98, TA-100 and TA-102 with or without metabolic activation [25]. Administration of Clovinol did not result in any toxicologically significant changes in clinical or behavioural observations, ophthalmic examinations, body weights, organ weights, feed consumption, urinalysis, haematology or clinical biochemistry parameters compared to the control animals, and terminal necropsy did not reveal any treatment-related histopathological changes. The NOAEL in the 90 day subchronic study was 1000 mg/kg/day. Clovinol was non-mutagenic when tested with or without metabolic activation in Salmonella typhimurium strains TA-98, TA-100 and TA-102.
The human safety of Oligopin® has been evaluated in several clinical trials. In one study, 100 mg of Oligopin® was administered orally for 56 days to thirty-four female human subjects with a mean age of 60 ± 1 years [22]. Oral exposure to Oligopin® was well tolerated, with no declared adverse effects after 56 days of daily intake. In another study [23], 150 mg/day of Oligopin® (75 mg twice daily) was administered orally for 5 weeks to 24 participants (17 men and 7 women). There were no statistically significant differences between the placebo control and Oligopin®-treated subjects with respect to the adverse events reported and Oligopin® was well tolerated. In a clinical trial reported by Chen et al. [24] a dose of 25 mg/day of Oligopin® (appro x1 mg/kg/d) for 10 weeks to 8 yr old subjects had no effect on blood biochemical parameters, including liver function, kidney function, nutritional status, lipid profile, haematology and iron status. Thus, the lack of significant adverse systemic effects in the 90 day oral (gavage) study in rats is concordant with the findings in these human clinical trials.
5. Conclusion
The toxicity studies conducted with Oligopin® indicate that it was non-genotoxic in vitro in both bacterial and human cell assays, was not acutely toxic via oral (gavage) administration at up to 2000 mg/kg and was well tolerated following repeated oral (gavage) administration to Sprague Dawley rats, with a NOAEL of 1000 mg/kg/day. These results suggest that Oligopin® may be less systemically toxic than other FMPBE, and comparable to that of proanthocyanidins extracted from grape seeds, which are widely used as nutritional supplement ingredients.
References
- 1.Assouad J.-L., Piriou Y. Procyanidins from French Maritime Pine Bark Extraction and biological properties. NUTRAfoods. 2007;6(3) [Google Scholar]
- 2.Busserolles In vivo antioxidant activity of procyanidin-rich extracts from grape seed and Pine (Pinus maritima) bark in rats. Int. J. Vitam. Nutr. Res. 2006;2018(1):22–27. doi: 10.1024/0300-9831.76.1.22. [DOI] [PubMed] [Google Scholar]
- 3.Masquelier J. Pycnogenols: recent advances in the therapeutical activity of procyanidins. Planta Med. J. Med. Plant Res. 1980:243–256. [Google Scholar]
- 4.Masquelier J., Michaud J., Laparra J., Dumon M.C. Flavonoides et pycnogenols. Int. J. Vitam. Nutr. Res. 1979;49:307–311. [PubMed] [Google Scholar]
- 5.Masquelier J., Michaud J., Laparra J., Dumon M.C. Pycnogenols – un nouvel essor thérapeutique des dérivés catéchiques. Bull. Soc. Pharm. Bordeaux. 1979;118:95–108. [Google Scholar]
- 6.Tixier J.M., Godeau G., Robert A.M., Hornebeck W. Evidence by in vivo and in vitro studies that binding of pycnogenols to elastin affects its rate of degradation by elastases. Biochem. Pharmacol. 1984;33(24):3933–3939. doi: 10.1016/0006-2952(84)90004-2. [DOI] [PubMed] [Google Scholar]
- 7.De Bruyne T., Pieters L., Witvrouw M., De Clercq E., Vanden Berghe D., Vlientinck A.J. Biological evaluation of proanthocyanidin dimers and related polyphenols. J. Nat. Prod. 1999;62:954–958. doi: 10.1021/np980481o. [DOI] [PubMed] [Google Scholar]
- 8.Beecher G.R. Proanthocyanidins: biological activities associated with human health. Pharm. Biol. 2004;42(sup):2–20. [Google Scholar]
- 9.Cos P., De Bruyne T., Hermans N., Apers S., Vanden Berghe D., Vlietinck A.J. Proanthocyanidins in health care: current and new trends. Curr. Med. Chem. 2003;1345–1359(10):1345–1359. doi: 10.2174/0929867043365288. [DOI] [PubMed] [Google Scholar]
- 10.Gali H.U., Perchellet E.M., Gao X.M., Karchesy J.J., Perchellet J.P. Comparison of the inhibitory effects of monomeric, dimeric, and trimeric procyanidins on the biochemical markers of skin tumor promotion in mouse epidermis in vivo. Planta Med. 1994;60(3):235–239. doi: 10.1055/s-2006-959466. [DOI] [PubMed] [Google Scholar]
- 11.Sano A., Yamakoshi J., Tokutake S., Tobe K., Kubota Y., Kiruchi M. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci. Biotechnol. Biochem. 2003;67(5):1140–1143. doi: 10.1271/bbb.67.1140. [DOI] [PubMed] [Google Scholar]
- 12.Weber H.A., Hodges A.E., Guthrie J.R., O’Brien B.M., Robaugh D., Clark A.P. Comparison of proanthocyanidins in commercial antioxidants: grape seed and pine bark extracts. J. Agric. Food Chem. 2007;55:148. doi: 10.1021/jf063150n. [DOI] [PubMed] [Google Scholar]
- 13.Battino M., Ferreiro M.S., Armeni T., Politi A., Bompadre S., Massoli A., Bullon P. In vitro antioxidant activities of antioxidant-enriched toothpastes. Free Rad. Res. 2005;39(3):343–350. doi: 10.1080/10715760400023853. [DOI] [PubMed] [Google Scholar]
- 14.Finley J.W. Phenolic antioxidants and prevention of chronic inflammation. Food Technol. Feature. 2004;58(11):42–46. [Google Scholar]
- 15.OECD . OECD; Paris: 1987. Test No. 401: Acute Oral Toxicity. 24 February https://ntp.niehs.nih.gov/iccvam/docs/acutetox_docs/udpproc/udpfin01/append/appi.pdf. [Google Scholar]
- 16.OECD . OECD; Paris: 1998. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents. (Accessed 21 September 1998) [Google Scholar]
- 17.OECD . OECD; Paris: 1997. Test No. 471: Bacterial Reverse Mutation. (Accessed 21 July 2018) [Google Scholar]
- 18.OECD . OECD; Paris: 2014. Test No. 473: In Vitro Mammalian Chromosome Aberration Test. (Accessed 26 September 2014) [Google Scholar]
- 19.Greaves P., Faccini J.M. Elsevier; Amsterdam, The Netherlands: 1984. Rat Histopathology: A Glossary for Use in Toxicity and Carcinogenicity Studies. [Google Scholar]
- 20.AFSSA (2005) Avis de l’Agence française de sécurité sanitaire des aliments relatif à l’emploi d’un extrait d’écorce de pin maritime français dans les compléments alimentaires. AFSSA – Saisine n° 2004-SA-0280, Maisons-Alfort, 1 avril 2005, Martin HIRSCH.
- 21.Yamakoshi J., Saito M., Kataoka S., Kikuchi M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002;40:599–607. doi: 10.1016/s0278-6915(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 22.Piriou Y., Sirvent A., Natalizio A., Girardory F. Skin-lightening and anti- ageing effect of a food supplement containing Pinus pinaster extract. Nutrafoods. 2014 [Google Scholar]
- 23.Valls R.-M., Llauradó E., Fernández-Castillejo S., Puiggrós F., Solà R., Arola L., Pedret A. Effects of low molecular weight procyanidin rich extract from french maritime pine bark on cardiovascular disease risk factors in stage-1 hypertensive subjects: randomized, double-blind, crossover, placebo-controlled intervention trial. Phytomedicine. 2016;23(12):1451–1461. doi: 10.1016/j.phymed.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y.R., Su Y.J., Piriou Y., Wang S.C. Effects of polyphenolic extract from pine bark on the improvement of attention deficit/hyperactivity disorder in children and adolescent. Int. J. Clin. Nutr. Diet. 2017;3:116–119. [Google Scholar]
- 25.Vijayasteltar L., Nair G., Maliakel B., Kuttan R., Krishnakumar I.M. Safety assessment of a standardized polyphenolic extract of clove buds: subchronic toxicity and mutagenicity studies. Toxicol. Rep. 2016;3:439–449. doi: 10.1016/j.toxrep.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miltonprabu S., Nazimabashir, Manoharanet V. Hepatoprotective effect of grape seed proanthocyanidins on Cadmium-induced hepatic injury in rats: possible involvement of mitochondrial dysfunction, inflammation and apoptosis. Toxicol. Rep. 2016;3:63–77. doi: 10.1016/j.toxrep.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]