Key Clinical Message
Whilst the malignant transformation of nasal polyps or secondary development of nasal neoplasia after chronic inflammation is likely to be relatively rare, this potential complication should be considered, and the clinician should be vigilant for evidence of malignant transformation.
Keywords: Dog, malignant transformation, mesenchymal malignancy, nasal polyps, oncology, respiratory
Introduction
Chronic nasal disease is relatively common in dogs 1. Nasal disease more commonly occurs in mesocephalic and dolichocephalic breeds 1, 2 with the most common clinical sign being nasal discharge 2, 3. Common causes of nasal disease in dogs include neoplasia, nonspecific inflammatory rhinitis, and mycotic disease 2, 3, 4, 5. Other potential causes including oral/dental disease, bacterial, polyps, and granulomatous rhinitis have been reported in dogs with chronic nasal disease but are less frequent 3, 5, 6. Idiopathic or inflammatory nasal polyps are rarely reported in the veterinary literature 5, 6, 7. In one study, all dogs identified with nasal polyps were Labrador Retrievers, potentially suggesting a breed predilection 5; however, this has not been widely supported 6.
Inflammation leading to subsequent malignancy is a well‐described phenomenon in people and domestic animals, and it has been speculated that nasal polyps can undergo malignant transformation; however, this has not previously been reported in dogs, and to the best of the authors’ knowledge, malignant transformation within a nasal polyp to sarcoma has not been reported in either the human or veterinary literature previously.
Case Report
A 12‐year‐old female neutered Australian Kelpie cross was initially referred to the University of Melbourne U‐Vet Animal Hospital with an 11‐month history of progressive paroxysmal sneezing, stertorous breathing, occasional reverse sneezing, and intermittent epistaxis. A mass occasionally protruded from the right nostril. Five months prior to referral, the dog underwent nasal investigation at a secondary referral center consisting of advanced imaging (MRI), rhinoscopy, and nasal biopsies (Fig. 1A), which were consistent with nasal polyposis. Treatment was continually prescribed following this diagnosis and variably included prednisone 0.45 mg/kg PO q 12 h, doxycycline 4.5 mg/kg PO q 12 h, piroxicam 0.33 mg/kg PO q 24 h, and azathioprine 1.1 mg/kg PO q 48 h. Despite the diagnosis and treatment, the dog had persistent and worsening clinical signs and was subsequently referred.
Figure 1.
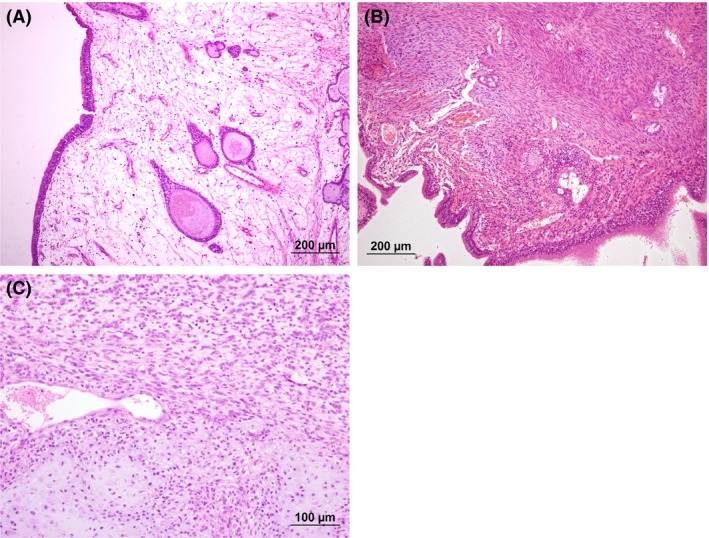
(A) Initial nasal mucosal biopsy (collected on 04 June 2013) displaying markedly edematous stroma containing low numbers of inflammatory cells and cystic mucosal glands consistent with a histopathological diagnosis of nasal polypoid hyperplasia. HE stain. (B) Interim nasal mucosal biopsy (collected on 16 October 2013) displaying area of atypical spindloid mesenchymal cells within the biopsy tissue. HE stain. (C) Third nasal biopsy (collected on 13 August 2015) displaying chondrosarcoma (bottom of field) merging with the surrounding atypical mesenchymal population (top of field). HE stain.
Sections of the original nasal biopsies, collected prior to referral, were obtained and reviewed (AWS), with agreement that the initial nasal biopsies were consistent with a histopathological diagnosis of nasal polypoid hyperplasia (Fig. 1A).
At the time of referral, the dog had right‐sided epistaxis during examination with a lack of airflow through the right nostril. There was normal facial symmetry, normal nasal planum pigmentation, no pain elicited on facial palpation, and normal conscious dental and hard palate assessment. The remainder of the physical examination was normal. Hematology, biochemistry, prothrombin, and activated partial thromboplastin time were performed. Increased alkaline phosphatase (766 U/L – reference interval 10–120 U/L), likely due to previous corticosteroid administration, was the only observed abnormality.
The dog was anesthetized for plain and contrast nasal computed tomography (CT; Siemens Emotion 16, Erlangen Germany). Nasal CT findings included a combination of fluid and soft tissue attenuating material occupying the right nasal cavity, right maxillary recess, and right frontal sinus. The material in the right nasal cavity partially obscured the normal turbinate pattern. Contrast enhancement of this material was absent in the right frontal sinus, mild and homogeneous in the right maxillary recess, and mild and heterogeneous in the right nasal cavity (Fig. 2A,B).
Figure 2.
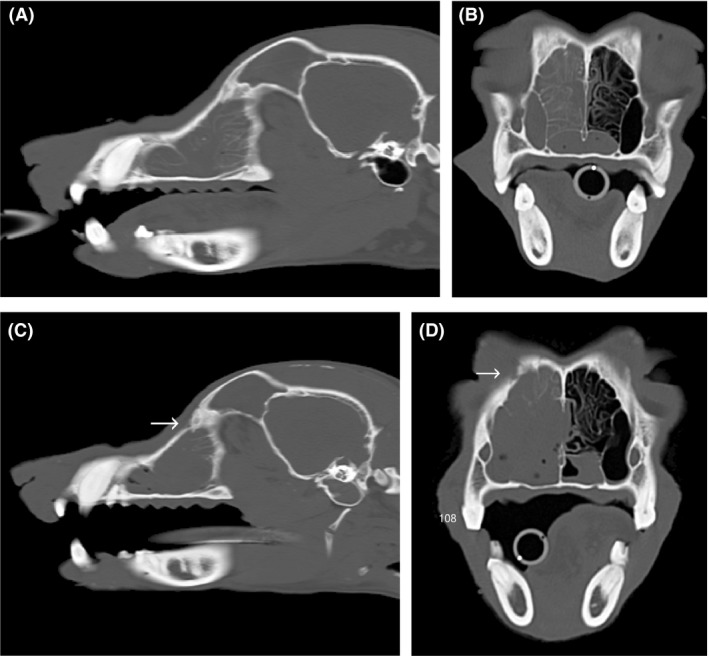
(A, B) Prerhinoscopy computed tomography (CT) (taken on 16 October 2013) taken 4 months after Figure 1A: sagittal 3‐mm (A) and transverse 3‐mm (B) bone windows. The sagittal image is aligned to the right nasal cavity 15 mm lateral to the nasal septum. The transverse images are aligned perpendicular to the hard palate at the level of tooth 108 (right maxillary fourth premolar). Nasal CT findings included a combination of fluid and soft tissue attenuating material occupying the right nasal cavity, right maxillary recess, and right frontal sinus. (C, D) Prerhinoscopy CT (taken on 13 August 2015) (C) sagittal 3‐mm and (D) transverse 3‐mm bone windows. The sagittal image is aligned to the right nasal cavity 15 mm lateral to the nasal septum, and the transverse images are aligned perpendicular to the hard palate at the level of tooth 108 (RUPM4). The arrows on images (C) and (D) indicate right frontal bone destruction that is not present on 10/16/13.
Immediately following CT, rhinoscopic evaluation confirmed the presence of soft, friable masses blocking both choanae on retrograde examination (Fig. 3A). Antegrade rhinoscopy showed several large friable masses in the right nasal cavity (Fig. 3B). Biopsies were obtained from inspected lesions and both nasal cavities and the nasopharynx. Vigorous nasal flushing and debridement using endoscopic forceps also dislodged material and resulted in patency of the right nostril.
Figure 3.
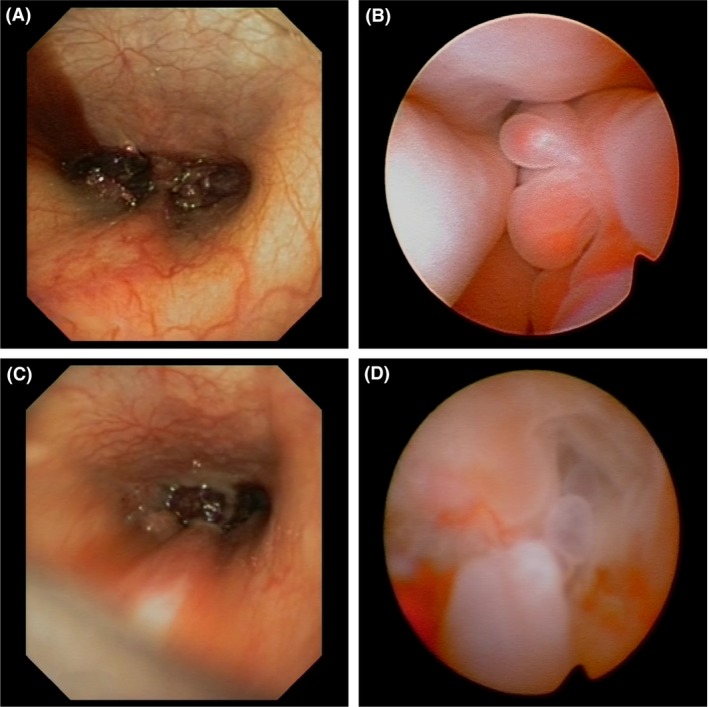
(A, B) Rhinoscopy performed on 16 October 2013. (A) Retrograde rhinoscopy demonstrated bilateral masses occluding both choanae, and (B) Antegrade rhinoscopy of the right nasal passage identified multiple pale‐pink friable masses. (C, D) Rhinoscopy performed on 13 August 2015. (C) Retrograde rhinoscopy demonstrated persistent bilateral masses occluding both choanae with distortion of the nasopharynx. (D) Antegrade rhinoscopy of the right nasal passage identified multiple irregular pale‐pink friable masses and loss of turbinate structure.
Samples were submitted for histopathology and microbiological culture, with negative bacterial and fungal cultures. Histopathological examination identified edematous submucosal proliferation similar to the initial biopsies, but with a more severe inflammatory infiltrate of neutrophils, eosinophils, plasma cells, and lymphocytes, as well as multiple discrete foci of atypical mesenchymal cells throughout the biopsy tissue from both nasal passages (Fig. 1B). The atypical mesenchymal cells were spindloid to fusiform with minimal cytoplasm and were arranged in loose streams associated with slightly mucinous stroma. The cells within these foci were uniform, and no mitoses were noted. The histopathological diagnosis for these biopsy specimens was bilateral inflammatory polypoid hyperplasia with mesenchymal atypia.
The dog was treated with doxycycline (prescription for human pharmacy: trade name/brand unknown) 100 mg, 4 mg/kg PO q 12 h until bacterial culture results returned. Based upon the histopathological diagnosis, inhaled fluticasone (Flixotide®; GlaxoSmithKline, Victoria, Australia) 250 μg, q 12 h was prescribed for this dog; she initially responded well to treatment with a marked reduction in clinical signs as only infrequent reverse sneezing occurred. After a period of 3 months, episodes of stertorous breathing, reverse sneezing, and epistaxis subsequently recurred. The periodic relapses were treated with an antimicrobial (doxycycline 100 mg, 4 mg/kg PO q 12 h for 1 month) and short courses of systemic corticosteroids (prednisone [Pred‐X 20 mg®; APEX Laboratories, New South Wales, Australia] 10 mg, 0.45 mg/kg PO q 24 h on a tapering schedule for 5 days before being reduced to 5 mg PO q 48 h before discontinuation), in addition to inhaled corticosteroids and an oral mucolytic (bromhexine [Bisolvon®; Boehringer Ingelheim, New South Wales, Australia] 8 mg, 0.3 mg/kg PO q 8 h for 5 weeks). This treatment was temporarily successful, but three additional endoscopic debulking procedures were performed to control clinical signs over the following 18 months. Debulking was achieved by grasping the polyps with forceps and retracting, as well using a soft urinary catheter and flushing sterile saline vigorously to help dislodge the polyps.
Despite combination medical therapy and debulking procedures, the dog continued to show clinical signs with stertorous breathing and intermittent epistaxis that occurred in progressively shortening intervals. Further nasal investigation was subsequently performed (33 months after initial clinical signs and 26 months after the initial nasal biopsies). Nasal CT again identified similar findings; however, new findings included right nasal turbinate destruction and focal bone destruction of the right maxilla, nasal septum and right wing of the vomer, with extension of soft tissue material into the left nasal cavity and right nasopharynx (Fig. 2C,D).
Rhinoscopy confirmed an extensive obstructive right nasal mass, turbinate destruction, increased mucus, and prominent mucosal lymphoid follicles (Fig. 3C,D). Biopsy specimens obtained from the right nasal passage identified polypoid changes with foci of atypical mesenchyme similar to the previous biopsy, but in some areas, these mesenchymal cells displayed anisokaryosis with prominent nucleoli and a high mitotic rate with occasional bizarre mitoses. In multiple areas, these cells were arranged in lacunae within chondroid stroma (Fig. 1C), consistent with chondrosarcoma. Low pH Alcian blue staining confirmed the presence of sulfonated proteoglycans within the stroma of these areas, consistent with cartilage; this staining was not observed in areas of mesenchymal atypia from the previous biopsy. A diagnosis of nasal chondrosarcoma was made. The dog was then treated with external beam radiation with 19 cycles of 2.85 Gy daily‐fractionated radiation therapy (Monday through Friday) to 54.15 Gy to 95% isodose line on a 6MV linear accelerator radiotherapy unit. Radiation therapy was effective in controlling clinical signs with absence of clinical signs for 10 months. Following this time, the dog developed nonspecific clinical signs of reduced appetite and lethargy with gastrointestinal signs, right‐sided glaucoma and subsequently developed a recurrence of nasal signs with stertorous breathing, reverse sneezing, sneezing, gagging, and a mild bilateral seromucoid discharge. The presumptive diagnosis was local disease progression, and repeated nasal investigation was performed with nasal CT, rhinoscopy, and nasal biopsies. This confirmed the diagnosis of chondrosarcoma and local disease progression. The owners did not wish to repeat radiation therapy or pursue systemic chemotherapy due to the side effects and poor prognosis, respectively. The dog was managed for a further 4 months with inhaled fluticasone, and oral analgesia/nonsteroidal anti‐inflammatory medication before being euthanized.
Discussion
The underlying etiology of nasal polyp formation in dogs – as in humans – remains unclear, although chronic inflammation may predispose to the development of nasal polyps 8, 9. The optimal treatment for inflammatory nasal polyps in dogs also remains unknown, similar to the situation in human medicine, with both the potential for medical and surgical treatment options; however, regardless of the type of therapy, recurrence is considered to be common 8, 9, 10, 11. In human medicine, the generally accepted medical practice is to attempt medical therapy as the primary treatment modality for 3 months with surgical intervention then only attempted in refractory cases 8. A small case series of five dogs with nasal polyps reported clinical resolution of nasal disease in all cases with surgical management (rhinotomies), but the polyps recurred in two of five (40%) of the dogs 6. As there is no consensus regarding the requirement for surgery in people, and given the relatively high rate of recurrence of clinical signs in dogs with nasal polyps that underwent rhinotomy in addition to the high morbidity associated with these procedures, invasive surgery cannot currently be recommended for dogs with nasal polyps. In the human literature, topical corticosteroids are effective in reducing the symptoms, size of the nasal polyps, and recurrence as well as having minimal systemic side effects 11. Based on these considerations, medical therapy was attempted first in the case, but was ineffective in controlling clinical signs. Institution of endoscopic debulking procedures in addition to the medical therapy helped to achieve temporary clinical remission, but clinical signs of nasal disease repeatedly recurred after debulking. The time between debulking and recurrence of clinical signs progressively reduced, ultimately leading to repeat nasal investigation given the concerns over potential malignant transformation.
As previously mentioned, inflammation leading to subsequent malignancy is a well‐described phenomenon in people and domestic animals. The inflammation may have been primarily from the polyps or potentially exacerbated by the debridement procedures; however, we feel the latter is unlikely due to only four procedures over 18 months being performed. The potential for malignant transformation of nasal polyps has been demonstrated by the development of precancerous changes in people, with carcinoma in situ developing in a subset of these patients 12. Carcinoma in situ is considered to be a “precancerous change” given its morphology is consistent with malignancy; however, it does not yet show malignant behavior of invasion or metastasis and whilst this only occurs in <2% of people with nasal polyps 12, 13, there is a link between carcinoma in situ and microinvasion, which demonstrates the potential for malignant transformation of nasal polyps to invasive carcinoma 13. Whilst malignant transformation within a nasal polyp to an epithelial tumor has been reported in people 13, to the best of the authors’ knowledge, malignant transformation within a nasal polyp to sarcoma has not been reported in either the human or veterinary literature previously. The exact pathophysiological mechanism by which inflammation leads to neoplasia remains unknown; however, it is recognized that inflammation has the potential to lead to mesenchymal malignant transformation, with one of the most notable occurrences in veterinary medicine being feline injection‐site sarcomas (FISS) 14.
Pseudosarcomatous changes in nasal polyps have been described rarely in people, characterized by proliferation of atypical mesenchymal cells with a low mitotic index 15; the interim nasal biopsies in this case were consistent with this described phenomenon. In contrast, the frequent mitoses and formation of abnormal chondroid stroma observed in the final biopsy specimens from this case are inconsistent with descriptions of pseudosarcomatous change and indicate that this is a true mesenchymal neoplasm rather than pseudosarcomatous change.
We believe this case demonstrates malignant mesenchymal transformation rather than a missed initial diagnosis of chondrosarcoma or two independent processes – nasal polyposis and neoplasia – occurring concurrently. All biopsies from different time points being independently reviewed by one of the authors (AWS) and additionally were also blindly reviewed by a board‐certified pathologist. Firstly, the original biopsies were consistent with a diagnosis of nasal polyposis. The second nasal biopsies performed (at the first presentation to U‐Vet) showed bilateral inflammatory polypoid hyperplasia and mesenchymal atypia, and the third biopsy was consistent with chondrosarcoma. There was 100% consensus that the stromal tissue in the second biopsy was atypical but non‐neoplastic, whilst the final biopsy specimens displayed features consistent with neoplastic transformation, consistent with chondrosarcoma. We believe this clearly shows progression and subsequent malignant transformation Secondly, the duration of clinical signs in the case for over 2 years (26 months) prior to putative malignant transformation is greater than the reported median survival times for dogs with nasal chondrosarcoma 16 additionally making a missed initial diagnosis unlikely. Finally, bone invasion and destruction typically occur early in the development of sinonasal neoplasia 17, but in the current case, only occurred 2 years after initial imaging. This is supportive of progression or malignant transformation, with bone invasion thought to occur early in the disease process with nasosinal neoplasia 17.
In conclusion, whilst the malignant transformation of nasal polyps or secondary development of nasal neoplasia after chronic inflammation is likely to be relatively rare, this potential complication should be considered by the veterinary surgeon when managing a dog with inflammatory polyps, and the clinician should be vigilant for evidence of malignant transformation.
Conflict of Interest
None declared.
Authorship
JAS: Reviewed clinical case and drafted the manuscript. AIW: Extensively involved with the case management. AWS: Pathologist, revised the manuscript. PFW: Radiologist, involved with the image acquisition interpretation and revision of the manuscript. CSM: involved with case management and manuscript review.
Acknowledgments
The authors acknowledge the technical assistance of Mr. Kane Wilson and Ms. Melanie Burns in obtaining the images and Associate Professor Jennifer Charles for pathology review.
Clinical Case Reports 2018; 6(5): 821–826
References
- 1. Cohn, L. A. 2014. Canine nasal disease. Vet. Clin. North Am. Small Anim. Pract. 44:75–89. [DOI] [PubMed] [Google Scholar]
- 2. Meler, E. , Dunn M., and Lecuyer M.. 2008. A retrospective study of canine persistent nasal disease: 80 cases (1998‐2003). Can. Vet. J. 49:71–76. [PMC free article] [PubMed] [Google Scholar]
- 3. Tasker, S. , Knottenbelt C. M., Munro E. A., Stonehewer J., Simpson J. W., and Mackin A. J.. 1999. Aetiology and diagnosis of persistent nasal disease in the dog: a retrospective study of 42 cases. J. Small Anim. Pract. 40:473–478. [DOI] [PubMed] [Google Scholar]
- 4. Plickert, H. D. , Tichy A., and Hirt R. A.. 2014. Characteristics of canine nasal discharge related to intranasal diseases: a retrospective study of 105 cases. J. Small Anim. Pract. 55:145–152. [DOI] [PubMed] [Google Scholar]
- 5. Lobetti, R. G. 2009. A retrospective study of chronic nasal disease in 75 dogs. J. S. Afr. Vet. Assoc. 80:224–228. [DOI] [PubMed] [Google Scholar]
- 6. Holt, D. E. , and Goldschmidt M. H.. 2011. Nasal polyps in dogs: five cases (2005 to 2011). J. Small Anim. Pract. 52:660–663. [DOI] [PubMed] [Google Scholar]
- 7. Fletcher, D. J. , Snyder J. M., Messinger J. S., Chiu A. G., and Vite C. H.. 2006. Ventricular pneumocephalus and septic meningoencephalitis secondary to dorsal rhinotomy and nasal polypectomy in a dog. J. Am. Vet. Med. Assoc. 229:240–245. [DOI] [PubMed] [Google Scholar]
- 8. Rimmer, J. , Fokkens W., Chong L. Y., and Hopkins C.. 2014. Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst. Rev. (12):Cd006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma, R. , Lakhani R., Rimmer J., and Hopkins C.. 2014. Surgical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst. Rev. (11):Cd006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martinez‐Devesa, P. , and Patiar S.. 2011. Oral steroids for nasal polyps. Cochrane Database Syst. Rev. (7):Cd005232. [DOI] [PubMed] [Google Scholar]
- 11. Kalish, L. , Snidvongs K., Sivasubramaniam R., Cope D., and Harvey R. J.. 2012. Topical steroids for nasal polyps. Cochrane Database Syst. Rev. (12):Cd006549. [DOI] [PubMed] [Google Scholar]
- 12. de la Cruz Mera, A. , Sanchez Lopez M. J., Merino Royo E., and Requena L.. 1990. Premalignant changes in nasal and sinus polyps: a retrospective 10 year study (1979‐1988). J. Laryngol. Otol. 104:210–212. [DOI] [PubMed] [Google Scholar]
- 13. Hasegawa, M. , Nasu M., Ohki M., Sugiuchi Y., and Watanabe I.. 1988. Malignant transformation of nasal polyp. Case report. Arch. Otolaryngol. Head Neck Surg. 114:336–337. [DOI] [PubMed] [Google Scholar]
- 14. Hartmann, K. , Day M. J., Thiry E., Lloret A., Frymus T., Addie D., et al. 2015. Feline injection‐site sarcoma: ABCD guidelines on prevention and management. J. Feline Med. Surg. 17:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klenoff, B. H. , and Goodman M. L.. 1977. Mesenchymal cell atypicality in inflammatory polyps. J. Laryngol. Otol. 91:751–756. [DOI] [PubMed] [Google Scholar]
- 16. Ehrhart, N. P. , Ryan S. D., and Fan T. M.. 2013. Chapter 24 Pp. 463–503 in Withrow S. J., Vail D. M. and Page R. L., eds. Withrow & MacEwen's small animal clinical oncology, 5th edn. Elsevier/Saunders, St Louis, MO. [Google Scholar]
- 17. Turek, M. M. , and Lana S. E.. 2013. Chapter 23 Section B Pp. 435–451 in Withrow S. J., Vail D. M. and Page R. L., eds. Withrow & MacEwen's small animal clinical oncology, 5th edn. Elsevier/Saunders, St Louis, MO. [Google Scholar]


