Abstract
Mannose-capped lipoarabinomannan (ManLAM), present in all members of the Mycobacterium tuberculosis complex and in other pathogenic Mycobacterium spp, is a high molecular mass amphipathic lipoglycan with a defined critical role in mycobacterial survival during infection. In particular, ManLAM is well-characterized for its importance in providing M. tuberculosis a safe portal of entry to phagocytes, regulating the intracellular trafficking network, as well as immune responses of infected host cells. These ManLAM immunological characteristics are thought to be linked to the subtle but unique and well-defined structural characteristics of this molecule, including but not limited to the degree of acylation, the length of the D-mannan and D-arabinan cores, the length of the mannose caps, as well as the presence of other acidic constituents such as succinates, lactates and/or malates, and also the presence of 5-methylthioxylosyl. The impact of all these structural features on ManLAM spatial conformation and biological functions during M. tuberculosis infection is still uncertain. In this review, we dissect the relationship between ManLAM structure and biological function addressing how this relationship determines M. tuberculosis interactions with host cells, and how it aids this exceptional pathogen during the course of infection.
Keywords: tuberculosis, Mycobacterium tuberculosis, mannose-capped lipoarabinomannan, host cell response, biological functions
Mycobacterium tuberculosis mannose-capped lipoarabinomannan: relationship between structure and biological function.
INTRODUCTION
Mannose-capped lipoarabinomannan (ManLAM), present in all members of the Mycobacterium tuberculosis complex and in other pathogenic Mycobacterium spp. (M. leprae, M. avium, M. marinum, M. kansasii and M. xenopi), is a high molecular mass amphipathic lipoglycan with a defined critical role in mycobacterial survival during infection. In particular, ManLAM is well-characterized for its importance in providing M. tuberculosis a safe portal of entry to phagocytes, regulating the intracellular trafficking network, as well as immune responses of infected host cells. These ManLAM immunological characteristics are thought to be linked to the subtle but unique and well-defined structural characteristics of this molecule, including but not limited to the degree of acylation, the length of the D-mannan and D-arabinan cores, the length of the mannose caps, as well as the presence of other acidic constituents such as succinates, lactates and/or malates, and also the presence of 5-methylthioxylosyl. The impact of all these structural features on ManLAM spatial conformation and biological functions during M. tuberculosis infection is still uncertain.
In this review, we dissect the relationship between ManLAM structure and biological function addressing how this relationship determines M. tuberculosis interactions with host cells, and how it aids this exceptional pathogen during the course of infection. It is important to note that the majority of ManLAM properties and influence described below are concluded from cell free studies with purified ManLAM or ManLAM fractions, with only a few verified using M. tuberculosis infections, and more importantly M. tuberculosis strains lacking ManLAM. This is noted in Table 1.
Table 1.
Major ManLAM biological functions as determined by free assays, by M. tuberculosis infections using laboratory strains/clinical isolates, or demonstrated using partial or full M. tuberculosis ManLAM mutants; and their attribution to a specific phase of infection.
| Major ManLAM biological functionsa | Attributed during which phase of infectionb | Demonstrated by free assays | Demonstrated by M. tuberculosis infections using laboratory strains or clinical isolates | Directly demonstrated by partial or full M. tuberculosis ManLAM knockouts (KO) |
|---|---|---|---|---|
| Mediating M. tuberculosis binding to PRRs (i.e. the MR, DC-SIGN, TLRs, others) | - Initial infection (alveolar space) | Yes | Yes: By using receptor ligand competitors, Ag-blocking assays, host cells lacking the receptor studied during M. tuberculosis infections in vitro | No: Using BCG pimE and capA-KO mutants (not able to make PIM6f and Man-caps in ManLAM) showed limited role of ManLAM in binding to DC-SIGN in DCs |
| - Within granulomas, cavities (active TB) | ||||
| Mediating M. tuberculosis binding to soluble collectins | - Initial infection (Alveolar space) | Yes | Yes: By direct binding to collectins and in competition assays during M. tuberculosis infections in vitro | ND |
| - In cavities | ||||
| Induction of pro-inflammatory response via TLRs | - Initial infection | Yes | ND | ND |
| - Reactivation in granuloma and cavities | ||||
| Induction of an anti-inflammatory response | - Initial infection (Alveolar space) | Yes | Yes: Indirectly by using M. tuberculosis and MR-deficient host cells | No: A cap-less LAM M. tuberculosis mutant stimulates similar immune response than the wild-type strain |
| - Reactivation in granuloma and cavities | Yes: Indirectly by using M. tuberculosis and DC-SIGN-deficient or DC-SIGN overexpressing host cells (in vitro) or mouse models (in vivo) | |||
| Driving phagosome maturation arrest, and this is via the MR | - Initial infection within host cells (i.e. AMs) | Yes | Yes: By using MR ligand competitors and Ag-blocking assays during M. tuberculosis infections in vitro | No: MR knockout mice do not have altered resistance to M. tuberculosis infection in terms of survival and bacterial control |
| Blocking the oxidative response | - Initial infection within host cells, granuloma and active TB cavities | Yes | ND | No: A cap-less LAM M. tuberculosis mutant stimulated similar immune responses than the wild-type strain |
| Stimulating CD1b-restricted T cells | - In granuloma formation and active TB cavities | Yes | Yes: IFN-γ-producing ManLAM-CD1b-restricted T cells in bronchoalveolar lavage of donors with latent M. tuberculosis infection | ND |
| Blocking of apoptosis | - Initial infection within host cells and granuloma formation | Yes | Yes: Blocking M. tuberculosis-induced Ca2+-depending apoptosis; Inhibiting Bcl2 | Yes: A quantitative decrease of ManLAM on the M. tuberculosis cell wall of the pimB-KO strain increases bacterial induced cell death |
| Directly stimulating CD4 T cells | - In granuloma formation, latency, and active TB cavities | Yes | ND | ND |
| Directly stimulating B cells | - Granuloma formation, latency, reactivation, active TB | Yes | ND | ND |
| Ab production | - Latency, reactivation, cavities in active TB | Yes | Yes: Abs isolated from TB patients and in experiments where anti-LAM Abs prevent M. tuberculosis dissemination | ND |
| Yes: In BCG vaccination studies in vivo | ||||
| Affecting M. tuberculosis survival within host cells in vitro | N/A | Yes | Yes: Indirectly using anti-ManLAM Abs followed by M. tuberculosis infection or using specific receptor deficient host cells | No: The lack of the mannose caps in M. tuberculosis did not affect its interaction with macrophages in vitro |
| Affecting M. tuberculosis survival in vivo | N/A | N/A | N/A | No: The lack of the mannose cap in M. tuberculosis did not affect its virulence in mice |
aFor references see text; N/A: not applicable; ND: not determined.
bThese are authors’ speculations, and thus these are not fully based on experimental data.
ManLAM structure
Mycobacterium tuberculosis ManLAM is a heterogeneous lipoglycan composed of a phosphatidyl-myo-inositol (PI) anchor, a carbohydrate core and various mannose-capping motifs. ManLAM is thought to be biosynthetically related to phosphatidyl-myo-inositol mannosides (PIMs) and lipomannan (LM) (Besra et al.1997). What distinguishes ManLAM from LM is the presence of an immunodominant D-arabinan core that extends from a common mannan core in an as yet undefined manner. If we look closer at the unique M. tuberculosis ManLAM structure, its carbohydrate core consists of two very well differentiated polymers, a D-mannan and a D-arabinan (Fig. 1). The D-mannan structure consists of a linear α(1→6) linked mannopyranosyl backbone linked to a PI anchor, with a variable number of single mannose substitutions at its C-2 positions (Fig. 1). The D-mannan size and the degree of single mannose branching vary among M. tuberculosis strains (Torrelles 2012). Recently, it was suggested that this mannosyl backbone carries only one arabinan chain near the middle (Kaur et al.2014); however, the exact linkage between the arabinan polymer and the D-mannan core is still not determined. The D-arabinan core is based on a rare α-D-arabinofuranose (Araf) backbone, and consists of a branched linear α(1→5) linked Araf. Branching residues carry an additional α(1→3) linked Araf. At its non-reducing end, we can find two arrangements or motifs, a branched hexaarabinofuranoside (Ara6) defined as [β-D-Araf-(1→2)-α-D-Araf-(1→]2-3 and 5-α-D-Araf-(1→5)-α-D-Araf, and a linear tetraarabinofuranoside (Ara4) defined as β-D-Araf-(1→2)-α-D-Araf-(1→5)-α-D-Araf-(1→5)-α-D-Araf (Chatterjee and Khoo 1998) (Fig. 1). For slow-growing mycobacteria like M. tuberculosis, M. leprae, M. bovis and M. bovis BCG (the vaccine strain), some of these terminal arabinan motifs are further crowned at their terminal β-D-Araf C-5 position with a single Manp, a dimannoside (α-D-Manp-(1→2)-α-D-Manp) or a trimannoside (α-D-Manp-(1→2)-α-D-Manp-(1→2)-α-D-Manp) (Chatterjee and Khoo 1998) (Fig. 1). These mannooligosaccharides linked to β-Araf termini define ManLAM. The mannose cap units can be located in both tetra- and hexaarafuranosyl motifs of the arabinan non-reducing terminal (Chatterjee et al.1993). However, we must specify that not all of these motifs are mannose-capped in ManLAM. Several studies have shown that a dimannoside cap is the most frequently found in both linear (Ara4) and branched (Ara6) termini defining the Man2Ara4 and Man4Ara6 motifs (Chatterjee et al.1993). However, for both Ara4 and Ara6, all combinations of mannose caps have been defined, e.g. Man(1 to 3)Ara4 and Man[(1 to 3)x2]Ara6. This degree of mannose capping in ManLAM varies according to the M. tuberculosis strain studied, with fully virulent M. tuberculosis laboratory strains having ∼70% capping (Chatterjee et al.1992). For example, M. tuberculosis Erdman ManLAM is the most mannose-capped when compared to ManLAM from M. tuberculosis H37Rv and H37Ra (reviewed in Torrelles and Schlesinger 2010). Moreover, the number of mannose residues per cap is also variable even within ManLAM from the same M. tuberculosis strain (Nigou, Gilleron and Puzo 2003).
Figure 1.
Schematic visualization of the structure of ManLAM. Representation of ManLAM structure and its different mannose-cap motifs. ManLAM has an MPI anchor, and α-mannan and α-arabinan domains. The α-arabinan domain is decorated by α(1→2) mannose caps of different length providing uniqueness to this molecule present in all pathogenic Mycobacterium spp. described to date. For the mannose caps of ManLAM the most common displays found are Man2Ara4 and Man4Ara6. In the case of Man4Ara6 is commonly found as branched Ara6 substituted with two α(1→2) mannoses per branch. Numbers in mannose caps indicate the number of mannoses per each mannose-cap arrangement presented.
Studying the Ara:Man ratio of purified M. tuberculosis ManLAM molecules provides useful information about the structural variability of this molecule linked to its differential biological functions (Schlesinger et al.1996; Torrelles et al.2004; 2008; 2012). Indeed, the ManLAM Ara:Man ratio is considered normal at ∼1.0 (Khoo, Tang and Chatterjee 2001), where M. tuberculosis laboratory strains M. tuberculosis H37Ra, H37Rv and Erdman have an Ara:Man ratio of 0.95, 1.03 and 1.14, respectively (Khoo, Tang and Chatterjee 2001; Torrelles et al.2004; 2008). This Ara:Man ratio variability is further accentuated in ManLAMs from drug-resistant and/or hypervirulent strains of M. tuberculosis, such as HN878 (at 0.98, personal communication), HN1554 (at 1.02), CSU20 (at 1.17), HN885 (at 1.18) and CSU19 (at 1.44) among others (Khoo, Tang and Chatterjee 2001; Torrelles et al.2004; 2008,; 2012). Although these differences in the Ara:Man ratio may not be considered significant, they provide an indication for the ManLAM overall arabinan and mannan domains size (Shi, JB and Chatterjee 2008). However, interpretation of Ara:Man ratio values may mislead. As an example, ManLAMs from some clinical isolates have a higher Ara:Man ratio, suggesting a larger arabinan domain when compared to M. tuberculosis H37Rv, but in reality these have a smaller arabinan domain (Khoo, Tang and Chatterjee 2001; Torrelles et al.2004; 2008; 2012). This is explained by the decrease in mannose capping in these ManLAMs and/or a lesser degree of mannose substitutions in their mannan domains, which accentuates the Ara:Man ratio. Overall, M. tuberculosis strains with a ManLAM with smaller arabinan but high Ara:Man ratio are shown to have limited phagocytosis, faster intracellular growth within macrophages (Torrelles et al.2008), and in some instances, these ManLAMs can activate and induce the proliferation of CD1b-restricted T cells (Torrelles et al.2004).
The presence of additional substitutions on ManLAM with potential implications to its biological function has been reported by several authors. Early, Hunter et al. showed the existence of succinates and lactates (Hunter, Gaylord and Brennan 1986), although the presence of the latter has not been verified in subsequent studies. Using nuclear magnetic resonance spectroscopy (NMR), Delmas et al. located succinic groups (1–4 per molecule) in the C-2 of the 3,5-α-D-Araf and/or 5-α-D-Araf residues of ManLAM in different M. bovis BCG sub-strains (Delmas et al.1997). Later, studies by Chatterjee and colleagues analyzed the content of succinates in ManLAMs from different M. tuberculosis complex species and strains, and showed that M. leprae, M. tuberculosis H37Rv, and a M. tuberculosis drug-resistant strain (CSU20) also had succinates; where, ManLAM from H37Rv, CSU20, and M. leprae had an average number of 2, 4 and 7 succinates, respectively (Torrelles et al.2004). The biological importance of ManLAM succinates during mycobacterial infection is still unknown, but, as per the case of M. leprae, it is noteworthy that bacteria isolated from tissues have higher content of succinates in their ManLAMs. Several attempts have been made correlating the presence of succinates to the biological function of ManLAM (Torrelles et al.2004). Indeed, the presence of succinates in ManLAM is shown to be indirectly proportional to the ability of ManLAM to stimulate CD1-restricted T cells (Torrelles et al.2004, 2012). Contrary to this, the presence of succinates in the biosynthetically related LM gave opposite results, where the high content of succinates in LM resulted in better stimulation of CD1-restricted T cell clones (Torrelles et al.2011). Thus, the structure-spatial-function relationship for ManLAM and its biosynthetically related LM and PIMs with CD1-restricted T cell responses is still uncertain. In this instance, it is important to consider the chemo-lability of succinates under physiological conditions, and as such, their implications in ManLAM biological functions may not be reliable. Other non-acylated substituents are found in ManLAM making its structure unique. Treumann et al. using NMR found a new terminal sugar crowning some of the caps of M. tuberculosis ManLAM (Treumann et al.2002). This sugar was later defined as a 5-deoxy-5-methylthio-α-xylofuranosyl (MTX), and could be involved in M. tuberculosis–host interactions undermining the effects of the host cell reactive oxygen species by adding to the antioxidant properties of ManLAM (Turnbull et al.2004).
In addition to the mannose caps, the presence of additional acyl groups, the size and branching pattern of the D-Mannan and D-Arabinan cores, the Ara:Man ratio, the heterogeneity that characterizes ManLAM also occurs through the number, the location and the nature of the fatty acids esterifying its PI anchor. ManLAM PI anchor fatty acids are also thought to play an important role in defining ManLAM spatial conformation and position within the cell wall. The ManLAM PI anchor consists of an ns-glycerol 3-phospho-(1-D-myo-inositol) unit. The O-linked D-mannan core extents from the C-6 position of the myo-inositol, and its C-2 position is substituted by a single α-D-mannopyranosyl residue (Chatterjee and Khoo 1998). The ns-glycerol contains the characteristic fatty acids described for ManLAM; palmitic (16:0) and tuberculostearic (TBST) acids, at positions 1 and 2, respectively (Hunter et al.1986). However, traces of myristic (14:0), heptadecanoic (17:0), stearic (18:1), 10-methyl-heptadecanoic, 12-O-(methoxypropioryl)-12-hydroxy-stearic and 12-hydroxy-TBST acids can be also found (Leopold and Fischer 1993; Nigou et al.1997). The overall number of fatty acids vary and it seems to be strain dependent. This can go from one (lyso-forms) to four fatty acids. An average of three fatty acids is the most commonly found, specifically in positions 1 and 2 of the ns-glycerol and position C-6 of the single Manp unit linked to C-2 of the myo-inositol (Khoo et al.1995). However, this cannot be generalized, as tetra-acylated ManLAM forms are also described as the most common molecular form for some M. tuberculosis strains (Torrelles et al.2004).
Related to its biological function, ManLAM orientation in the M. tuberculosis cell wall is still unresolved. There are several hypotheses, an accepted one being that ManLAM is anchored by its lipidic anchor into the plasma membrane, projecting through the thickness of the cell wall so that its terminal arabinose or mannose-capped arabinose motifs are accessible on the bacterial cell surface (Gaylord and Brennan 1987; McNeil and Brennan 1991; Chatterjee and Khoo 1998; Pitarque et al.2008). This is supported by electron microscope observations where ManLAM seems to be mainly located in the bacterium poles/tips and within cell wall surface depressions (Kang et al.2005; Hett and Rubin 2008; Torrelles 2012), by the harsh physical and chemical conditions needed to extract ManLAM from the M. tuberculosis cell wall (Pitarque et al.2008; Shi, JB and Chatterjee 2008), that ManLAM has been detected associated to the plasma membrane fraction (Mehrotra et al.1999; Pitarque et al.2008) and by the fact that many steps in the biosynthesis of ManLAM are plasma membrane-associated (Besra et al.1997; Pitarque et al.2008; Kaur et al.2009).
Due to the thickness of the M. tuberculosis cell wall, other views situate ManLAM in the periplasmic space anchored on the plasma membrane below the peptidoglycan-arabinogalactan-mycolic acid complex (Pitarque et al.2008; Dhiman et al.2011), or directly interacting via its PI anchor with the mycolic acid layer and with other cell wall associated lipids (Rastogi 1991; Pitarque et al.2008). Another interesting point of view is the proposal that ManLAM has a non-permanent location in the cell wall, where it is considered a secreted molecule in transit through the envelope (Chatterjee and Khoo 1998; Pitarque et al.2008). Indeed, the existence of secreted non-PI containing mannose-capped arabinomannan in the so-called capsular/outer material associated with the M. tuberculosis cell wall surface has been shown (Lemassu and Daffe 1994). Other studies by Puzo and colleagues, subdivided ManLAM into two different types, the parietal and the cellular ManLAMs. Both ManLAMs had similar core structure but presented remarkable differences in the degree of acylation and mannose capping (Gilleron et al.2000). The fact that the parietal ManLAM can be obtained without cell disruption supports the concept of two different cell wall locations for this molecule. Thus, ManLAM may be firmly, but not covalently, anchored to the M. tuberculosis plasma membrane during its biosynthesis in the periplasmic space (cellular ManLAM). Later, ManLAM could be transferred passively or actively to the cell wall surface where it could interact with mycolic acids adopting a spatial conformation that favors the exposure of its mannose caps on the cell wall surface (parietal ManLAM). Finally, ManLAM could lose its PI anchor to form the mannose-capped AM (outer material AM). Importantly, structural changes in ManLAM significantly compromise the cell wall integrity of M. tuberculosis leading to increased antibiotic sensitivity and attenuated infectivity (Fukuda et al.2013). This fact is based on experimentally derived mutants, not naturally occurring mutants. Detailed information about ManLAM biosynthesis pathway(s) can be found elsewhere (Kaur et al.2009), and the biological function of M. tuberculosis ManLAM is discussed in detail below.
Mycobacterium tuberculosis ManLAM-host interface
Mycobacterium tuberculosis infection occurs by airborne transmission, where bacilli travel through the respiratory tract and are deposited in the alveolar spaces of the lungs (Torrelles and Schlesinger 2017). An accepted view is that M. tuberculosis is rather ‘static’ during the initial infection, without inducing an immune response as it is taken up by non-activated alveolar macrophages. However, another point of view is that M. tuberculosis infection is rather ‘dynamic’ (Torrelles and Schlesinger 2017). Indeed, when M. tuberculosis is initially deposited in the terminal bronchioles and alveoli, as well as following release from lysed macrophages and in cavities in active TB disease, M. tuberculosis bacilli are bathed in the lung mucosa. The lung mucosa contains homeostatic and antimicrobial hydrolytic enzymes (van Golde 1985; Hawgood and Poulain 2001), which alter the M. tuberculosis cell wall (Arcos et al.2011; 2015; 2017; Scordo et al.2017; Moliva et al.2018), including the removal of two major M. tuberculosis virulence factors, ManLAM and TDM (Arcos et al.2011) both with important biological properties including blocking the phagosome maturation process (Kang et al.2005; Axelrod et al.2008). Effects of lung mucosa hydrolases on the M. tuberculosis cell wall also produce the release of cell wall fragments into the lung milieu (Arcos et al.2011). These fragments contain ManLAM. Overall, these ManLAM-containing fragments are bioactive, but non-cytotoxic, regulate the function of phagocytes (macrophages and neutrophils), and thus are capable of modulating the immune response contributing to the control of M. tuberculosis infection (Arcos et al.2017; Scordo et al.2017). The dynamic relationship between M. tuberculosis and its surface molecules with the host environment results into two distinct scenarios. First, ManLAM remaining attached to the cell wall of M. tuberculosis, and second ManLAM (or fragments of it) being ‘free’ in solution within the lung mucosa, with different outcomes as discussed below. This fact of ManLAM potentially being free within the lung mucosa and able to interact with host cells before, at the same time, or after these get infected by M. tuberculosis, provides support to the relevance of the bacterial cell free assay studies performed to date attributing the biological functions of ManLAM as depicted below and in Table 1.
As M. tuberculosis reaches the alveolar space through its pathway to infection, the initial recognition, association and uptake of M. tuberculosis by host cells are quite complex and involves alveolar resident cells and many of their surface receptors. The concept of studying how a single specific receptor contributes to M. tuberculosis recognition and subsequent uptake is critical to our basic understanding of the pathway(s) that this bacillus exploits to gain entrance into host cells modulating their immune response. However, we cannot forget that during infection multiple host receptors may recognize one M. tuberculosis bacillus at the same time, or multiple bacilli are recognized by different receptors at the same time, which can generate a completely different outcome. In this context, the outcome of a microbe–host interaction is normally beneficial for the host cell, triggering an innate immune response that initially should allow for control of infection. However, there are pathogens such as M. tuberculosis that exploit phagocytic receptors for their advantage, gaining entry subverting the innate immune response and generating a unique niche leading to a pathway of intracellular survival. Here, we will describe the host phagocytic and signaling receptors involved in M. tuberculosis ManLAM recognition and the subsequent trafficking and inflammatory response (Table 1).
Phagocytic receptors
The encounter of M. tuberculosis with the host cell triggers the phagocytosis process, defined as recognition and uptake via the formation of a phagocytic cup that will develop into a phagosome. The phagocytic process depends on two important factors; one is the surface receptor repertoire present on the host cell (which is host cell dependent), and the constitution of the cell wall of M. tuberculosis (which is species/strain dependent). The first contact between the host cell and M. tuberculosis is within the lung alveolar space. When M. tuberculosis deposits into the alveolar space, resident phagocytes called alveolar macrophages (AMs), and structural alveolar epithelial cells together with recruited monocytes, dendritic cells, neutrophils, lymphocytes and fibroblasts represent the array of immune cells that participate in host defense. Known phagocytic receptors involved in M. tuberculosis recognition by the host are as follows: C-type lectin receptors (CLRs) including the mannose receptor (MR) and the dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN); and complement receptors (CRs). Mycobacterium tuberculosis uptake by these receptors leads to the formation of an M. tuberculosis containing phagosome with different outcomes. Mycobacterium tuberculosis ManLAM has been described to interact with both the MR in AMs and with DC-SIGN in dendritic cells.
The mannose receptor (CD206; MRC1/MRC2)
Usually macrophages use the homeostatic MR to remove host-derived highly N-mannosylated glycoproteins typically generated during inflammation from the circulation (Martinez-Pomares et al.2001). This process is thought to be effective without triggering unnecessary innate immune responses. Several studies, however, have shown that M. tuberculosis can take advantage of this process. Schlesinger and colleagues have suggested that M. tuberculosis is capable of using its surface-located highly mannosylated cell wall components to associate with the macrophage MR and gain entry to survive within macrophages. In this context, the mannosylated M. tuberculosis ManLAM and higher order PIMs (i.e. PIM5–6) are shown to associate with the MR through their α(1→2) mannosylated motifs located in their non-reducing end (Schlesinger, Hull and Kaufman 1994; Schlesinger et al.1996; Torrelles, Azad and Schlesinger 2006). Specifically, the MR recognizes the α(1→2) mono-, di- and tri-mannosyl caps of ManLAM, where the dimannosyl cap is the most recognized (Schlesinger, Hull and Kaufman 1994; Schlesinger et al.1996). This recognition is also applicable to the terminal α(1→2)-Man of PIM5f and the terminal α(1→2)-di-mannoside of PIM6f (Torrelles, Azad and Schlesinger 2006) (for more details see Torrelles et al.2008; Torrelles and Schlesinger 2010; Lugo-Villarino et al.2011; Sasindran and Torrelles 2011).
Recognition of ManLAM and its related molecules PIM5f and PIM6f on the M. tuberculosis cell surface by the MR leads to a pathway of intracellular survival within the host by blocking phagosome fusion with lysosomes and further acidification (Vieira et al.2001; Kang et al.2005; Torrelles, Azad and Schlesinger 2006). Specifically, the ability of M. tuberculosis to drive phagosome maturation arrest relies on membrane-associated lipids that serve as tags, recruiting and guiding the membrane trafficking machinery. One such lipid is phosphatidylinositol 3-phosphate (PI3P). PI3P is required for phagosome maturation (Fratti et al.2001), and its formation and accumulation is shown to be blocked by ManLAM leading to phagosome maturation arrest (Fratti et al.2003; Vergne, Chua and Deretic 2003; Schlesinger et al.2008). Indeed, ManLAM and higher order PIMs mimic mammalian cell phosphatidylinositol and phosphoinositides, and have potential to interfere with phosphoinositide interconversions (e.g. PI3P generation) on mycobacterial phagosomes, specially ManLAM as it is shown to directly intercalate into the phagosomal membrane (Torrelles and Schlesinger 2010). The key role of ManLAM disrupting PI3P production leading to mycobacterial phagosome maturation arrest is further potentiated by the secreted protein, SapM (Saleh and Belisle 2000; Vergne et al.2005), which has a strong PI3P phosphatase activity, and thus, also participates in M. tuberculosis phagosome maturation block (Saleh and Belisle 2000; Vergne et al.2005; Puri, Reddy and Tyagi 2013). The relative contribution of ManLAM vs. SapM in disrupting PI3P generation is still unknown. PI3P only accumulates in phagosomes containing killed M. tuberculosis (Vergne et al.2005), where SapM is inactive (Vergne et al.2005), and ManLAM is significantly reduced in the cell wall of heat-killed or γ-irradiated M. tuberculosis (Kang et al.2005). Finally, lower order PIMs (i.e. PIM2) also play an important role in blocking phagosome maturation, as these promote fusion between M. tuberculosis containing phagosomes and early endosomes (Vergne et al.2004), which it is thought to provide nutrients to M. tuberculosis.
The MR recognition of M. tuberculosis also reduces macrophage microbicidal activities by limiting the generation of pro-inflammatory cytokines, the oxidative response (nitric oxide and oxygen radicals) and by blocking M. tuberculosis-induced Ca2+-depending apoptosis (reviewed in Torrelles et al.2008). ManLAM-dependent inhibition of macrophage apoptosis is also mediated by the upregulation of the antiapoptotic B-cell CLL/lymphoma 2 (Bcl2) family member A1 (Halder et al.2015). Indeed, a quantitative decrease of ManLAM on the M. tuberculosis cell wall increases the rate of bacterial-induced human macrophage cell death (Torrelles et al.2009). The role of ManLAM as an immunosuppressive epitope of M. tuberculosis is further supported by the production of IL-37 by human type II alveolar epithelial cells via upregulating the TLR2/p38 or ERK1/2 pathway, providing further evidence for the contribution of ManLAM in establishing persistence of M. tuberculosis (Huang et al.2015).
Overall, there is controversy in the capacity of ManLAM to generate a pro-inflammatory or anti-inflammatory response. Indeed, ManLAM is shown to induce opposing immune responses to M. tuberculosis depending on structural diversity and experimental variations, such as differences in the protocols used for ManLAM isolation and for in vitro experiments including immune cell types and procedures to isolate them (reviewed in great detail in Kallenius et al.2016). In general, M. tuberculosis ManLAM binding to the MR induces an anti-inflammatory response led by the generation of IL-10 and TGF-β limiting the production of inflammatory cytokines such as TNF, IL-6, IL-1β and IL-12 (Astarie-Dequeker et al.1999; Nigou et al.2001; Chieppa et al.2003). This complex cytokine production network is partially mediated by the transcription regulatory factor peroxisome proliferator-activated receptor-gamma (PPAR-γ). Indeed, ManLAM and/or virulent M. tuberculosis engagement of the MR upregulates PPAR-γ leading to a simultaneous production of IL-8 (or CXCL-8), expression of COX2 (cycloxygenase 2) and production of prostaglandin 2 (PGE2) (Rajaram et al.2010). At the same time, ManLAM and/or M. tuberculosis engagement of the MR downregulates TNF production via PPAR-γ [66]. The relationship of ManLAM with PPAR-γ is further confirmed by studies using the aptamer ZXL1. ZXL1 specifically binds to ManLAM acting as an antagonist inhibiting ManLAM immunosuppression of DCs (Pan et al.2017). ZXL1 can also enhance IL-1β and IL-12, decrease IL-10 production and induce nitric oxide synthase expression, all linked to ZXL1 capacity to inhibit PPAR-γ expression (Pan et al.2017). Subsequent gene expression profiling of macrophages exposed to ManLAM indicates that PPARγ signaling likely influences important cellular functions such as phagocytosis, cytoskeleton remodeling, cell survival and autophagy (Halder et al.2015). Subsequent bioinformatic analyses also indicate that ManLAM regulates the signal transducer and activator of transcription (STAT)-5α (which at the same time modulates cell survival and myeloid cell development Smithgall et al.2000) in a PPARγ-dependent manner, suggesting that this could determine the fate of the host cell after M. tuberculosis infection (Halder et al.2015). The biological relevance of the ManLAM/MR pathway in vivo needs to be further determined, as studies have shown that the MR knockout mouse does not have altered resistance to M. tuberculosis infection in terms of survival, control of bacterial clearance, lung inflammation, granuloma formation, and cytokine and chemokine expression (Court et al.2010).
Another M. tuberculosis mannosylated lipoglycan, the LM, induces high levels of the miRNA miR-125b, which binds to the 3΄ UTR region of TNF mRNA and destabilizes its transcription, thus acting as a potent inhibitor of TNF biosynthesis in human macrophages (Rajaram et al.2011).
DC-SIGN (CD209)
Mycobacterium tuberculosis and its cell wall components α-glucan, ManLAM, LM and PIMs can bind DC-SIGN on DCs (Geijtenbeek et al.2003; Tailleux et al.2003; Driessen et al.2009; Ehlers 2010; Geurtsen et al.2009). As the MR does, DC-SIGN also recognizes the mannose caps of ManLAM and the non-reducing terminus of PIM5f and PIM6f, but contrary to the affinity of the MR to recognize the α(1→2)-Man linkage, DC-SIGN has not this limitation and can thus also recognize the α(1→6)-Man linkage present in PIM2f and LM (Torrelles, Azad and Schlesinger 2006). Conversely, studies using BCG pimE and capA-KO mutants (not able to make PIM6f and Man-caps in ManLAM) showed a limited role of ManLAM in binding to DC-SIGN in DCs (Driessen et al.2009; Torrelles et al.2008; Lugo-Villarino et al.2011). Although limited, this recognition and binding leads to bacterial killing by acidification of M. tuberculosis-containing phagosomes (Geijtenbeek et al.2003). The importance of ManLAM/LM/PIMs engagement to DC-SIGN and subsequent triggering of the innate immune response remains unclear. Initial in vitro studies directly using M. tuberculosis, or its cell wall components engaging DC-SIGN resulted in production of the anti-inflammatory cytokine IL-10 (Ehlers 2010). Subsequent in vivo studies using transgenic mice expressing human DC-SIGN or mice expressing human DC-SIGN homologues (Park et al.2001; McGreal, Miller and Gordon 2005; Powlesland et al.2006) determined that DC-SIGN engagement dampens pro-inflammation limiting tissue damage, and promoting host protection (Wieland et al.2007; Schaefer et al.2008; Tanne et al.2009). However, studies using a M. marimun strain lacking cell surface mannosylated components indicated that the ManLAM/PIMs engagement with DC-SIGN did not seem to regulate cytokine secretion (Appelmelk et al.2008).
Complement receptors
These receptors are described on the surface of all mononuclear phagocytes, and CR1, CR3 and CR4 have been implicated in M. tuberculosis phagocytosis (Fenton, Riley and Schlesinger 2005). Although there are no studies directly or indirectly linking ManLAM association with CRs, other M. tuberculosis mannosylated cell wall components, such as lower order PIMs such as PIM2, and some polysaccharides can directly bind the CR3 lectin domain mediating M. tuberculosis uptake by macrophages (Cywes et al.1997; Hoppe et al.1997; Villeneuve et al.2005). However, the role of CR3 in M. tuberculosis pathogenesis is still controversial as in vitro and in vivo studies using wild-type and CR3-deficient mice showed no differences in bacterial burden and lung pathology (Hu et al.2000). Less attention has been focused on the potential of ManLAM being recognized by the CR4 lectin domain, which together with the MR, is highly expressed on AMs and other cells involved in M. tuberculosis uptake (Hirsch et al.1994; Zaffran, Zhang and Ellner 1998; Schlesinger et al.2008).
Signaling receptors
In addition to the phagocytosis process, M. tuberculosis binds to and signals through specific signaling receptors located on the host cell surface and/or cytosol. In this instance, M. tuberculosis interacts with both Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like (NODs) receptors.
Toll-like receptors
TLRs are pattern recognition receptors (PRRs) expressed on many cell types but their function on phagocytes sensing the environment is especially important (reviewed in Kawai and Akira 2010). On phagocytes such as macrophages, TLRs are either surface expressed (i.e. TLR2 and -4) or in intracellular compartments (i.e. TLR8 and TLR9) (Kawai and Akira 2010). TLRs that are important in triggering immunity against M. tuberculosis infection include TLR-2 (alone or as a heterodimer associated with TLR1 or TLR6), TLR9 and probably TLR4 (Harding and Boom 2010). TLR2 alone or dimerized with TLR1 or TLR6 triggers a strong pro-inflammatory response by recognizing M. tuberculosis lower and higher order PIMs, LM, trehalose dimycolate and the 19-KDa lipoglycoprotein (reviewed in Torrelles 2012). Mycobacterium tuberculosis ManLAM interacts with TLR2 less than LAM from other mycobacteria, i.e. M. smegmatis phosphatidyl-myo-inositol capped LAM (or PI-LAM, formally defined as AraLAM in some publications). The number of fatty acids may play a role in how M. tuberculosis mannosylated cell wall components interact with TLR2. This was specifically observed for LM, where triacylated but not diacylated forms of LM acted as a TLR2 agonist (Quesniaux et al.2004; Gilleron et al.2006). Indeed, M. tuberculosis lipoprotein LprG binds triacylated forms of ManLAM, LM and PIMs translocating these molecules to the cell surface and thus, facilitating their recognition by TLR2 (Drage et al.2010) and, in particular for ManLAM, aiding its recognition by other receptors involved in M. tuberculosis intracellular survival (Gaur et al.2014; Shukla et al.2014). The LprG/ManLAM complex release from the M. tuberculosis cell wall to some degree depends on the degree of O-mannosylation of LprG (Alonso et al.2017).
Although the stimulation of most TLRs on phagocytes induces production of pro-inflammatory cytokines such as TNF, IL-6, IL-1β, IL-12 via activation of NF-κB (nuclear factor kappa-light chain-enhancer of activated B cell) and MAPK (mitogen-activated protein kinase) signaling pathways, signaling through TLR2 is more complex, where the type and intensity of the inflammatory response observed via TLR2 depends on the M. tuberculosis ligand(s) and, of course, the host cell studied (Underhill et al.1999; Thoma-Uszynski et al.2001). In this regard, whether LAM has a greater influence on the TLR2 signaling pathway than M. tuberculosis lipoproteins or other possible TLR2 ligands is a question that still needs to be addressed. The role of TLR4 in M. tuberculosis infection is unclear as only a few mannosylated M. tuberculosis ligands for TLR4 have been described (i.e. LM) (Doz et al.2007).
Cytosolic receptor: NOD2
Cytosolic regulators known as NOD receptors (Franchi et al.2008) are known to participate in the induction of pro-inflammation during M. tuberculosis infection. In this regard, Nod2, found in epithelial cells and antigen presenting cells, regulates the pro-inflammatory response by responding to M. tuberculosis peptidoglycan components such as muramyl dipeptide (Sirard et al.2007; Franchi et al.2008; Brooks et al.2011). How M. tuberculosis uses ManLAM to control host cytosolic receptors is undetermined; however, both ManLAM and Nod2 have been found associated with intracellular vesicles (Torrelles and Schlesinger 2010; Brooks et al.2011). Thus, a role for Nod2 in triggering pro-inflammation may depend on vesicular fusion events controlled by ManLAM during M. tuberculosis phagocytosis and phagosomal maturation (Sasindran and Torrelles 2011).
Other receptors
Other immunoreceptor tyrosine-based activation motif-coupled CLRs that are associated to ManLAM recognition are Dectin-2 (CLEC6A/Clec4n) and SIGNR3 (–/Cd209d). Dectin-2 is mainly described as the recognition receptor for α-mannan of fungal pathogens (McGreal et al.2006). Studies using ManLAM stimulation of DCs showed production of the pro-inflammatory cytokine IL-2 and the regulatory cytokine IL-10 through Dectin-2. However, this was not observed through Mincle, despite both receptors using the same FcRγ chain as a signaling subunit (Yonekawa et al.2014). Engagement of ManLAM with Dectin-2 and subsequent IL-2 and IL-10 production was further verified in DCs lacking Dectin-2, which failed to produce both IL-2 and IL-10 (Yonekawa et al.2014). Moreover, antigen-presenting cells stimulated with ManLAM are able to activate T cells isolated from TB patients, and this phenomenon was reversed by anti-Dectin-2 antibodies, confirming the role of Dectin-2 in recognizing ManLAM. SIGNR3 is the mouse DC-SIGN homolog present in lung cells capable of recognizing M. tuberculosis and ManLAM, triggering TNF and IL-6 production in an NF-κB– and Raf1–ERK-dependent pathway (Tanne et al.2009).
The dendritic cell immunoactivating receptor (DCAR) is reportedly involved in M. tuberculosis mannosylated cell wall components recognition. Although DCAR is not yet described to directly recognize ManLAM, this receptor coupling with FcγR recognizes triacylated and tetraacylated lower order PIMs (i.e. Ac1PIM2 and Ac2PIM2, respectively) (Toyonaga et al.2016). DCAR is expressed in lung monocyte populations and after PIM stimulation induces the secretion of the chemoattractant CCL-2 (MCP-1). Importantly, DCAR-infected mice showed increase bacterial burden, which was explained by an impaired production of IFN-γ by T cells (Ishikawa, Mori and Yamasaki 2017).
Soluble immunomodulators of the lung mucosa, such as surfactant protein -A and -D, and mannose-binding lectin, and their associate specific receptors can directly or indirectly assist in M. tuberculosis recognition by the host via ManLAM. SP-A can upregulate the MR on the macrophage surface (Beharka et al.2002), increasing chances of M. tuberculosis recognition by host cells through the ManLAM/MR pathway. SP-D can also bind and agglutinate M. tuberculosis bacilli (Ferguson et al.1999) leading them to a trafficking vesicular network that results in phagosome-lysosome fusion and bacterial killing (Ferguson et al.2006). ManLAM can associate with the mannose-binding lectin (Swierzko et al.2016), but not ficolins, activating the complement system, where in the particular case of M. leprae, ManLAM is defined as the dominant complement activator (Bahia El Idrissi et al.2015). Soluble immunomodulators of the lung mucosa contribution in M. tuberculosis pathogenesis is discussed in detail elsewhere (Torrelles et al.2008; Torrelles and Schlesinger 2010; Sasindran and Torrelles 2011).
Finally, M. tuberculosis ManLAM binding to lactosylceramide-enriched lipids rafts in the plasma membrane stimulates the phagocytosis process, but at the same time can inhibit the association of Src family kinase Hck with lactosylceramide-enriched lipid rafts in phagosomal membranes, effectively preventing phagosome-lysosome vesicle fusions (Nakayama et al.2016). Interestingly, ManLAM also binds to the soluble collectin CL-LK, which serum levels in active TB patients inversely correlates to the magnitude of the disease (Troegeler et al.2015). Conversely, ManLAM strongly stimulates the production of pentraxin 3 (Garlanda et al.2002), which levels are directly correlated to severity of TB disease (Mortaz et al.2015). Other receptors involved in the recognition of M. tuberculosis being independent of ManLAM are Mincle (also known as CLEC4E), Fcγ-receptor, Dectin-1, CD14 and scavenger receptor-A (or MARCO) (Sasindran and Torrelles 2011).
Mycobacterium tuberculosis ManLAM recognition by T cells
In addition to PRRs present on host cells, mycobacterial lipoglycans in the form of LAM and its structural variants can be recognized by T cells in the context of the non-polymorphic MHC molecule CD1. Humans have several CD1 molecules (group 1 with CD1a, CD1b, CD1c, CD1e and group 2 with CD1d). CD1 molecules are expressed on innate CD1-restricted T cells and NK T cells (Wilson and Delovitch 2003). Upon recognition of mycobacterial lipids, CD1-restricted T cells can become activated and proliferate, secreting Th1 type inflammatory cytokines (i.e. IFN-γ).
The integrity of the phosphatidyl-myo-inositol anchor is crucial for LAM biological activity, in particular for its presentation to CD4/CD8 double-negative T cells in the context of human CD1b molecules. The hydrophobic CD1 pocket binds the acyl chains of LAM relatively non-specifically, thereby positioning the LAM hydrophilic domains for highly specific interactions with T cell receptors. Thus, ManLAM structural heterogeneity plays an important role in its capacity to stimulate CD1-restricted T cells. As an example, CD1b-restricted T cell lines derived from a skin biopsy of a cutaneous lesion of a leprosy patient when cultured with ManLAM primed antigen presenting cells could strongly recognize ManLAM from M. leprae and from a drug-resistant M. tuberculosis strain (CSU20), but did not readily recognize ManLAM from the laboratory strain M. tuberculosis H37Rv (Torrelles et al.2004). A comparative structural study indicated that the number of succinyl residues present in ManLAM play an important role in the capacity of this molecule to stimulate CD1b-restricted T cells (Torrelles et al.2004). Further studies separated the heterogeneity of ManLAM into three different isoforms based on their fatty acid number, and into eight different isoforms based their isoelectric point (Torrelles et al.2012). Indeed, the specific ManLAM isoform pI 5.8 was uniquely able to activate and induce proliferation of CD1b-restrited T cells via antigen presenting cell CD1 presentation (Torrelles et al.2012). Subsequent analyses determined that this LAM isoform pI 5.8 is smaller and highly acylated with respect to the other isoforms studied. Indeed, there are nine positions that potentially could be acylated at the ManLAM PI anchor. To date the most acylated ManLAM PI anchor described in the literature is a tetra-acylated form, where the fatty acids are located in positions C1 and C2 of the ns-glycerol, in the position C6 of the α(1→2)-D-Manp and in the position C3 of the myo-inositol. Thus, there are five hydroxyl groups in the MPI anchor that may be acylated in the case of ManLAM isoform pI 5.8. We cannot rule out, however, that there are additional acylations in the D-mannan and D-arabinan cores of ManLAM. The observation that ManLAM separates into eight isoforms, LM separates into only two isoforms and PIMs produce a diffuse band in 2D-SDS-PAGE indicates that the specific ManLAM attributes driving pI separation could be located in the D-arabinan (Torrelles et al.2012).
Contrary to other CD1 molecules, CD1e is found in the cytosol, accumulating during the DC maturation process (Ishikawa, Mori and Yamasaki 2017). Although CD1e does not directly present Ag to T cells, this molecule regulates Ag-presentation by others CD1 molecules. In particular, cytosolic CD1e is involved in the PIM6 processing to PIM2, which is subsequently presented by CD1b (de la Salle et al.2005). An important discussion point suggested by others (Ishikawa, Mori and Yamasaki 2017) is the fact that C-type lectins and CD1-restricted T-cell receptor specificity for mannosylated cell wall components including ManLAM, LM and PIMs is overlapping, and thus both may compete for the same ligand and/or synergistically interact in building the immune response during M. tuberculosis infection.
Mycobacterium tuberculosis ManLAM can also directly inhibit CD4 + T-cell activation by inserting into T cell membranes or indirectly within M. tuberculosis-derived bacterial vesicles, putting these cells into a stage of anergy (Mahon et al.2012; Karim et al.2017). Indeed, ManLAM has been found associated with T cells in vivo (Athman et al.2017), and inhibiting T cell responses by inducing global changes in the CD4 + T-cell proteome affecting Akt-mTOR signaling, resulting in broad functional impairment of CD4 + T-cell activation beyond inhibition of proximal T-cell receptor-CD3 signaling (Karim et al.2017). The importance of ManLAM in regulating T cell responses is further stressed in human studies, studying bronchoalveolar lavage T cell populations obtained from donors with latent M. tuberculosis infection and active TB (Busch et al.2016). Bronchoalveolar lavage cells from donors with latent M. tuberculosis infection limited bacterial growth more efficiently than those in patients with active TB. A subset of IFN-γ-producing ManLAM-CD1b-restricted T cells co-expressed cytotoxic molecules perforin, granulysin and granzyme B was found responsible for the limited M. tuberculosis growth (Busch et al.2016).
Mycobacterium tuberculosis ManLAM recognition by B cells and antibody production
Few studies have addressed the direct impact of M. tuberculosis ManLAM on B cells, with the most persuasive finding that liposomes containing MLCwA (M. leprae total cell wall antigen), ManLAM and other immunomodulators were able to upregulate the expression of CD40 on B cells of lepromatous leprosy patients, which could drive the generation of more protective isotyping antibodies to various M. leprae antigens (Chattree et al.2008). Vaccination studies of cattle with BCG also show the generation of reactive IgG1 and IgG2 B cell responses to BCG ManLAM, which were colostral transferred to neo-natal calves (Foote et al.2007). Finally, in vitro studies using bone marrow cells also indicate that ManLAM can stimulate the early production of IL-4 in CD19 + /B220 + precursor cells, presumed to be pre-B cells producing IL-4 constitutively and whose frequency is rapidly and markedly upregulated by ManLAM (Collins, Schaible and Kaufmann 1998). As IL-4 plays a key role in the Th1/Th2 T cell dichotomy, ManLAM is hypothesized to influence hematopoiesis by rapidly inducing IL-4 secretion in the bone marrow (Collins, Schaible and Kaufmann 1998).
Of particular interest is the uniqueness of antibodies that develop against ManLAM in order to reduce its immunosuppressive function during infection (Perley et al.2014) and its role in limiting phagosome-lysosome fusion (Kumar et al.2015), as biomarkers for the M. tuberculosis complex infection (Sarkar et al.2014), and as effectors to modify the course of M. tuberculosis infection (Glatman-Freedman et al.2000). Early studies using Ab against ManLAM already demonstrated their capacity to revert ManLAM immunosuppressed T cell responses (Moreno, Mehlert and Lamb 1988), as well as their capacity to prevent dissemination of M. tuberculosis infection (Costello et al.1992). More recently, the production of highly specific anti-ManLAM aptamers showed promising results in inhibiting in vivo M. tuberculosis infection using mouse and non-human primate models (Pan et al.2014).
In the TB diagnostics field, there is significant focus on the generation of antibodies to detect ManLAM in whole blood, serum, urine, saliva, sputum and even in tears and milk, to further develop and/or optimize point-of-care tests for human and animal TB disease (Chan et al.2015; Crawford et al.2017; Paris et al.2017). The importance of ManLAM in TB diagnosis is discussed in detail elsewhere (Achkar et al.2011; Sarkar et al.2014).
CONCLUSIONS
Due to the complexity of the cell wall of M. tuberculosis, we need to consider the essentiality of ManLAM. A summary of ManLAM functions that are linked to its specific motifs is described in Fig. 2. An attempt to bring forth a unified view of ManLAM’s true or most significant contribution to pathogenesis during the course of an M. tuberculosis infection is in Table 1. In this regard, there is a concern that some of these attributed functions to ManLAM are based on studies solely dependent on bacterial cell free assays and do not consider other potential ligands of this bacterium that can synergize with or reduce the influence of ManLAM during infection.
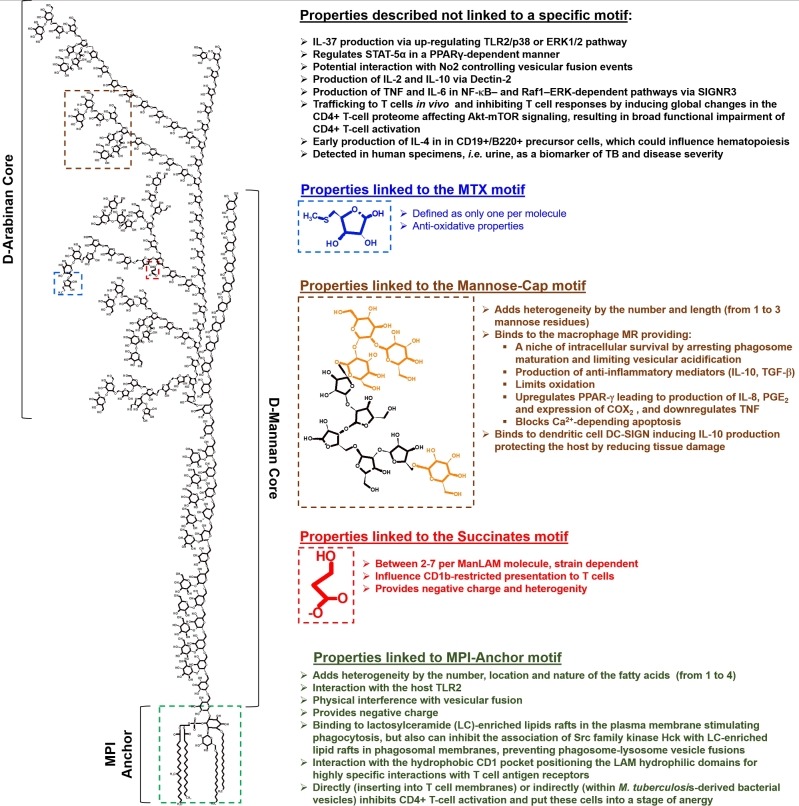
Figure 2.
Relationship between M. tuberculosis ManLAM structure and biological function. ManLAM is described as a tripartite structure composed of an MPI anchor (green square), and D-mannan and D-arabinan cores. The MPI anchor is the main driver of the heterogeneity that characterizes ManLAM. The immunodominant D-arabinan core contains particular motifs giving ManLAM unique biological functions to interfering with the host cell response to M. tuberculosis infection. These motifs are the mannose-cap units (brown square), succinate residues (red square) and the MTX residue (blue square). Other properties of ManLAM have been also elucidated; however, these have not yet been linked to any specific motif.
The studies described above allow us to speculate that ManLAM is an important virulence factor for M. tuberculosis primarily through modulating phagocyte function. In order to confirm this, efforts have been directed into creating isogenic mutants of M. tuberculosis deficient in the partial or full production of ManLAM. In this instance, and contrary to expected, M. tuberculosis strains deficient in the biosynthesis of the mannose cap of ManLAM do not change virulence in mice or in interaction with macrophages in vitro (Afonso-Barroso et al.2012). Thus, the prediction that the mannose cap of ManLAM would influence M. tuberculosis survival does not hold true in vivo (Afonso-Barroso et al.2012). It is obvious that studies using live capless LAM mutant M. tuberculosis yield data conflicting with those obtained with purified ManLAM, indicating some redundancy of the role of ManLAM (Afonso-Barroso et al.2012). Other studies using mycobacterial mutants drive attention towards the importance of the mannan domain branching of LAM in mycobacterial pathogenesis (Stoop et al.2013). However, we may not obtain the real answer by just depleting partially or fully the presence of ManLAM from the M. tuberculosis cell wall, as we need to consider the rearrangement that the M. tuberculosis cell wall may undergo upon the absence of ManLAM. The lack of ManLAM may also significantly alter the cytotoxic properties of other M. tuberculosis cell wall components. Finally, we will need to consider evaluating the constitution of the M. tuberculosis cell wall during infection. Indeed, exposure of M. tuberculosis to the human lung mucosa for less than 15 min removes ∼65% of ManLAM molecules from the bacterial surface. These ManLAM molecules are released into the lung milieu together with other whole and/or fragmented cell wall components, which when in contact with phagocytes, downregulate the phagocyte pro-inflammatory response, but at the same time increases the effectiveness of the intracellular phagocyte to kill M. tuberculosis (reducing M tuberculosis association with macrophages by ∼80% and increasing P-L fusion events by ∼35%) (Arcos et al.2017; Scordo et al.2017). Another additional factor to consider is how the cell wall differs among M. tuberculosis strains, in particular for ManLAM. Mycobacterium tuberculosis clinical isolates deficient in ManLAM and PIMs surface exposure present higher quantities of triglycerides, phenolic glycolipid and phthiocerol dimycocerosates in their cell walls (Torrelles et al.2008). These M. tuberculosis strains have reduced phagocytosis but a faster bacterial intracellular growth rate in human macrophages (Torrelles et al.2008). Thus, the TB clinical spectrum is not only dictated by the host but could also be related to the cell surface abundance/exposure of specific cell wall adherence factors defined by each phylogenetically related M. tuberculosis strain (Torrelles et al.2008; Torrelles and Schlesinger 2010). Multiple passages of M. tuberculosis through the host could also influence its phenotypic and genotypic adaptations to the host environment(s). In particular, it will be essential to answer the question of what the cell wall of drug-resistant M. tuberculosis strains looks like. Studies performed using transmission electron and atomic force microscopy determined drug-resistant strains [extensive (XDR) and extreme (XXDR) drug resistant strains] having a rougher cell surface and thicker cell wall with tubular extensions compared to susceptible strains (Velayati et al.2009, 2010). Drug-resistant strains have their cell walls overpopulated with triglycerides (personal communication); thus, it is plausible to question any relationship between the relative abundance of a specific hydrophobic lipid on the M. tuberculosis cell wall and drug resistance, a question that remains unanswered.
FUNDING
This work was supported by the National Institutes of Health (AI073856 and AI093570 to JBT, and AG051428 to JT and JBT).
Conflict of interest. None declare.
REFERENCES
- Achkar JM, Lawn SD, Moosa MY et al. Adjunctive tests for diagnosis of tuberculosis: serology, ELISPOT for site-specific lymphocytes, urinary lipoarabinomannan, string test, and fine needle aspiration. J Infect Dis 2011;204(Suppl 4):S1130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso-Barroso A, Clark SO, Williams A et al. Lipoarabinomannan mannose caps do not affect mycobacterial virulence or the induction of protective immunity in experimental animal models of infection and have minimal impact on in vitro inflammatory responses. Cell Microbiol 2013;15:660–74. [DOI] [PubMed] [Google Scholar]
- Alonso H, Parra J, Malaga W et al. Protein O-mannosylation deficiency increases LprG-associated lipoarabinomannan release by Mycobacterium tuberculosis and enhances the TLR2-associated inflammatory response. Sci Rep 2017;7:7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelmelk BJ, den DJ, Driessen NN et al. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell Microbiol 2008;10:930–44. [DOI] [PubMed] [Google Scholar]
- Arcos J, Diangelo L, Scordo J et al. Lung Mucosa Lining fluid modification of Mycobacterium tuberculosis to reprogram human neutrophil killing mechanisms. J Infect Dis 2015;212:948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcos J, Sasindran SJ, Fujiwara N et al. Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection. J Immunol 2011;187:372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcos J, Sasindran SJ, Moliva JI et al. Mycobacterium tuberculosis cell wall released fragments by the action of the human lung mucosa modulate macrophages to control infection in an IL-10-dependent manner. Mucosal Immunol 2017;10:1248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarie-Dequeker C, N’Diaye EN, Le Cabec V et al. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect Immun 1999;67:469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athman JJ, Sande OJ, Groft SG et al. Mycobacterium tuberculosis membrane vesicles inhibit T cell activation. J Immunol 2017;198:2028–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod S, Oschkinat H, Enders J et al. Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell Microbiol 2008;10:1530–45. [DOI] [PubMed] [Google Scholar]
- Bahia El Idrissi N, Das PK, Fluiter K et al. M. leprae components induce nerve damage by complement activation: identification of lipoarabinomannan as the dominant complement activator. Acta Neuropathol 2015;129:653–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beharka AA, Gaynor CD, Kang BK et al. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol 2002;169:3565–73. [DOI] [PubMed] [Google Scholar]
- Besra GS, Morehouse CB, Rittner CM et al. Biosynthesis of mycobacterial lipoarabinomannan. J Biol Chem 1997;272:18460–6. [DOI] [PubMed] [Google Scholar]
- Brooks MN, Rajaram MV, Azad AK et al. NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell Microbiol 2011;13:402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch M, Herzmann C, Kallert S et al. Lipoarabinomannan-responsive polycytotoxic T cells are associated with protection in human tuberculosis. Am J Respir Crit Care Med 2016;194:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CE, Gotze S, Seah GT et al. The diagnostic targeting of a carbohydrate virulence factor from M. tuberculosis. Sci Rep 2015;5:10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D, Khoo K-H. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology 1998;8:113–20. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Khoo K-H, McNeil MR et al. Structural definition of the non-reducing termini of mannose-capped LAM from Mycobacterium tuberculosis through selective enzymatic degradation and fast atom bombardment-mass spectrometry. Glycobiology 1993;3:497–506. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Lowell K, Rivoire B et al. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J Biol Chem 1992;267:6234–9. [PubMed] [Google Scholar]
- Chattree V, Khanna N, Bisht V et al. Inhibition of apoptosis, activation of NKT cell and upregulation of CD40 and CD40L mediated by M. leprae antigen(s) combined with Murabutide and Trat peptide in leprosy patients. Mol Cell Biochem 2008;309:87–97. [DOI] [PubMed] [Google Scholar]
- Chieppa M, Bianchi G, Doni A et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol 2003;171:4552–60. [DOI] [PubMed] [Google Scholar]
- Collins HL, Schaible UE, Kaufmann SH. Early IL-4 induction in bone marrow lymphoid precursor cells by mycobacterial lipoarabinomannan. J Immunol 1998;161:5546–54. [PubMed] [Google Scholar]
- Costello AM, Kumar A, Narayan V. et al. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans R Soc Trop Med Hyg 1992;86:686–92. [DOI] [PubMed] [Google Scholar]
- Court N, Vasseur V, Vacher R et al. Partial redundancy of the pattern recognition receptors, scavenger receptors, and C-type lectins for the long-term control of Mycobacterium tuberculosis infection. J Immunol 2010;184:7057–70. [DOI] [PubMed] [Google Scholar]
- Crawford ACL, Laurentius LB, Mulvihill TS et al. Detection of the tuberculosis antigenic marker mannose-capped lipoarabinomannan in pretreated serum by surface-enhanced Raman scattering. Analyst 2017;142:186–96. [DOI] [PubMed] [Google Scholar]
- Cywes C, Hoppe HC, Daffe M et al. Nonopsonic binding of Mycobacterium tuberculosis to complement receptor type 3 is mediated by capsular polysaccharides and is strain dependent. Infect Immun 1997;65:4258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Salle H, Mariotti S, Angenieux C et al. Assistance of microbial glycolipid antigen processing by CD1e. Science 2005;310:1321–4. [DOI] [PubMed] [Google Scholar]
- Delmas C, Gilleron M, Brando T et al. Comparative structural study of the mannosylated-lipoarabinomannans from Mycobacterium bovis BCG vaccine strains: characterization and localization of succinates. Glycobiology 1997;7:811–7. [DOI] [PubMed] [Google Scholar]
- Dhiman RK, Dinadayala P, Ryan GJ et al. Lipoarabinomannan localization and abundance during growth of Mycobacterium smegmatis. J Bacteriol 2011;193:5802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doz E, Rose S, Nigou J et al. Acylation determines the Toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of Pro-inflammatory Cytokines by Mycobacterial Lipomannan. J Biol Chem 2007;282:26014–25. [DOI] [PubMed] [Google Scholar]
- Drage MG, Tsai HC, Pecora ND et al. Mycobacterium tuberculosis lipoprotein LprG (Rv1411c) binds triacylated glycolipid agonists of Toll-like receptor 2. Nat Struct Mol Biol 2010;17:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen NN, Ummels R, Maaskant JJ et al. Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect Immun 2009;77:4538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S. DC-SIGN and mannosylated surface structures of Mycobacterium tuberculosis: a deceptive liaison. Eur J Cell Biol 2010;89:95–101. [DOI] [PubMed] [Google Scholar]
- Fenton MJ, Riley LW, Schlesinger LS. Receptor-Mediated recognition of Mycobacterium tuberculosis by host cells. In: Cole ST, Eisenach KD, McMurray DN, Jacobs WR Jr (eds). Tuberculosis and the Tubercle Bacillus. New York: ASM Press, 2005, 405–26. [Google Scholar]
- Ferguson JS, Martin JL, Azad AK et al. Surfactant protein D increases fusion of Mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect Immun 2006;74:7005–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JS, Voelker DR, McCormack FX et al. Surfactant protein D binds to Mycobacterium tuberculosis bacili and lipoarrabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol 1999;163:312–21. [PubMed] [Google Scholar]
- Foote MR, Nonnecke BJ, Beitz DC et al. Antigen-specific B-cell responses by neonatal calves after early vaccination. J Dairy Sci 2007;90:5208–17. [DOI] [PubMed] [Google Scholar]
- Franchi L, Park JH, Shaw MH et al. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol 2008;10:1–8. [DOI] [PubMed] [Google Scholar]
- Fratti RA, Backer JM, Gruenberg J et al. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol 2001;154:631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti RA, Chua J, Vergne I et al. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. P Natl Acad Sci USA 2003;100:5437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Matsumura T, Ato M et al. Critical roles for lipomannan and lipoarabinomannan in cell wall integrity of mycobacteria and pathogenesis of tuberculosis. mBio 2013;4:e00472–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Hirsch E, Bozza S et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 2002;420:182–6. [DOI] [PubMed] [Google Scholar]
- Gaur RL, Ren K, Blumenthal A et al. LprG-mediated surface expression of lipoarabinomannan is essential for virulence of Mycobacterium tuberculosis. PLoS Pathog 2014;10:e1004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord H, Brennan PJ. Leprosy and the leprosy bacillus: recent developments in characterization of antigens and immunology of the disease. Annu Rev Microbiol 1987;41:645–75. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Van Vliet SJ, Koppel EA et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med 2003;197:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurtsen J, Chedammi S, Mesters J et al. Identification of mycobacterial alpha-Glucan as a novel ligand for DC-SIGN: Involvement of mycobacterial capsular polysaccharides in host immune modulation. J Immunol 2009;183:5221–31. [DOI] [PubMed] [Google Scholar]
- Gilleron M, Bala L, Brando T et al. Mycobacterium tuberculosis H37Rv parietal and cellular lipoarabinomannans. J Biol Chem 2000;275:677–84. [DOI] [PubMed] [Google Scholar]
- Gilleron M, Nigou J, Nicolle D et al. The acylation state of mycobacterial lipomannans modulates innate immunity response through toll-like receptor 2. Chem Biol 2006;13:39–47. [DOI] [PubMed] [Google Scholar]
- Glatman-Freedman A, Mednick AJ, Lendvai N et al. Clearance and organ distribution of Mycobacterium tuberculosis lipoarabinomannan (LAM) in the presence and absence of LAM-binding immunoglobulin M. Infect Immun 2000;68:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder P, Kumar R, Jana K et al. Gene expression profiling of Mycobacterium tuberculosis Lipoarabinomannan-treated macrophages: A role of the Bcl-2 family member A1 in inhibition of apoptosis in mycobacteria-infected macrophages. IUBMB Life 2015;67:726–36. [DOI] [PubMed] [Google Scholar]
- Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol 2010;8:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawgood S, Poulain FR. The pulmonary collectins and surfactant metabolism. Annu Rev Physiol 2001;63:495–519. [DOI] [PubMed] [Google Scholar]
- Hett EC, Rubin EJ. Bacterial growth and cell division: a mycobacterial perspective. Microbiol Mol Biol Rev 2008;72:126–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch CS, Ellner JJ, Russell DG et al. Complement receptor-mediated uptake and tumor necrosis factor- alpha-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol 1994;152:743–53. [PubMed] [Google Scholar]
- Hoppe HC, De Wet JM, Cywes C et al. Identification of phosphatidylinositol mannoside as a mycobacterial adhesin mediating both direct and opsonic binding to nonphagocytic mammalian cells. Infect Immun 1997;65:3896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Mayadas-Norton T, Tanaka K et al. Mycobacterium tuberculosis infection in complement receptor 3-deficient mice. J Immunol 2000;165:2596–602. [DOI] [PubMed] [Google Scholar]
- Huang Z, Zhao GW, Gao CH et al. Mannose-capped lipoarabinomannan from Mycobacterium tuberculosis induces IL-37 production via upregulating ERK1/2 and p38 in human type II alveolar epithelial cells. Int J Clin Exp Med 2015;8:7279–87. [PMC free article] [PubMed] [Google Scholar]
- Hunter SW, Gaylord H, Brennan PJ. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem 1986;261:12345–51. [PubMed] [Google Scholar]
- Ishikawa E, Mori D, Yamasaki S. Recognition of mycobacterial lipids by immune receptors. Trends Immunol 2017;38:66–76. [DOI] [PubMed] [Google Scholar]
- Kallenius G, Correia-Neves M, Buteme H et al. Lipoarabinomannan, and its related glycolipids, induce divergent and opposing immune responses to Mycobacterium tuberculosis depending on structural diversity and experimental variations. Tuberculosis 2016;96:120–30. [DOI] [PubMed] [Google Scholar]
- Kang PB, Azad AK, Torrelles JB et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med 2005;202:560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim AF, Sande OJ, Tomechko SE et al. Proteomics and network analyses reveal inhibition of Akt-mTOR Signaling in CD4(+) T cells by Mycobacterium tuberculosis mannose-capped lipoarabinomannan. Proteomics 2017;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D, Angala SK, Wu SW et al. A single arabinan chain is attached to the phosphatidylinositol mannosyl core of the major immunomodulatory mycobacterial cell envelope glycoconjugate, lipoarabinomannan. J Biol Chem 2014;289:30249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D, Guerin ME, Skovierova H et al. Chapter 2: Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. Adv Appl Microbiol 2009;69:23–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010;11:373–84. [DOI] [PubMed] [Google Scholar]
- Khoo K-H, Dell A, Morris HR et al. Structural definition of acylated phosphatidylinositol mannosides from Mycobacterium tuberculosis: definition of a common anchor for lipomannan and lipoarabinomannan. Glycobiology 1995;5:117–27. [DOI] [PubMed] [Google Scholar]
- Khoo K-H, Tang J-B, Chatterjee D. Variation in mannose-capped terminal arabinan motifs of lipoarabinomannans from clinical isolates of Mycobacterium tuberculosis and Mycobacterium avium complex. J Biol Chem 2001;276:3863–71. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Singh P, Sinha S. Naturally produced opsonizing antibodies restrict the survival of Mycobacterium tuberculosis in human macrophages by augmenting phagosome maturation. Open Biol 2015;5:150171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemassu A, Daffe M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem J 1994;297(Pt 2):351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold K, Fischer W. Molecular analysis of the lipoglycans of Mycobacterium tuberculosis. Anal Biochem 1993;208:57–64. [DOI] [PubMed] [Google Scholar]
- Lugo-Villarino G, Hudrisier D, Tanne A et al. C-type lectins with a sweet spot for Mycobacterium tuberculosis. Eur J Microbiol Immunol 2011;1:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon RN, Sande OJ, Rojas RE et al. Mycobacterium tuberculosis ManLAM inhibits T-cell-receptor signaling by interference with ZAP-70, Lck and LAT phosphorylation. Cell Immunol 2012;275:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pomares L, Linehan SA, Taylor PR et al. Binding properties of the mannose receptor. Immunobiology 2001;204:527–35. [DOI] [PubMed] [Google Scholar]
- McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol 2005;17:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreal EP, Rosas M, Brown GD et al. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 2006;16:422–30. [DOI] [PubMed] [Google Scholar]
- McNeil MR, Brennan PJ. Structure, function and biogenesis of the cell envelope of mycobacteria in relation to bacterial physiology, pathogenesis and drug resistance; some thoughts and possibilities arising from recent structural information. Res Microbiol 1991;142:451–63. [DOI] [PubMed] [Google Scholar]
- Mehrotra J, Mittal A, Rastogi AK et al. Antigenic definition of plasma membrane proteins of bacillus Calmette-Guerin: predominant activation of human T cells by low-molecular-mass integral proteins. Scand J Immunol 1999;50:411–9. [DOI] [PubMed] [Google Scholar]
- Moliva JI, Hossfeld AP, Canan CH et al. Exposure to human alveolar lining fluid enhances Mycobacterium bovis BCG vaccine efficacy against Mycobacterium tuberculosis infection in a CD8(+) T-cell-dependent manner. Mucosal Immunol 2017Sep 20. doi: 10.1038/mi.2017.80. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C, Mehlert A, Lamb J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin Exp Immunol 1988;74:206–10. [PMC free article] [PubMed] [Google Scholar]
- Mortaz E, Adcock IM, Tabarsi P et al. Interaction of pattern recognition receptors with Mycobacterium tuberculosis. J Clin Immunol 2015;35:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Kurihara H, Morita YS et al. Lipoarabinomannan binding to lactosylceramide in lipid rafts is essential for the phagocytosis of mycobacteria by human neutrophils. Sci Signal 2016;9:ra101. [DOI] [PubMed] [Google Scholar]
- Nigou J, Gilleron M, Cahuzac B et al. The Phosphatidyl- myo -inositol anchor of the lipoarabinomannans from Mycobacterium bovis Bacillus Calmette Guérin. J Biol Chem 1997;272:23094–103. [DOI] [PubMed] [Google Scholar]
- Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: from structure to biosynthesis. Biochimie 2003;85:153–66. [DOI] [PubMed] [Google Scholar]
- Nigou J, Zelle-Rieser C, Gilleron M et al. Mannosylated Lipoarabinomannans inhibit IL-12 Production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol 2001;166:7477–85. [DOI] [PubMed] [Google Scholar]
- Pan Q, Wang Q, Sun X et al. Aptamer against mannose-capped lipoarabinomannan inhibits virulent Mycobacterium tuberculosis infection in mice and rhesus monkeys. Mol Ther 2014;22:940–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Yan J, Liu Q et al. A single-stranded DNA aptamer against mannose-capped lipoarabinomannan enhances anti-tuberculosis activity of macrophages through downregulation of lipid-sensing nuclear receptor peroxisome proliferator-activated receptor gamma expression. Microbiol Immunol 2017;61:92–102. [DOI] [PubMed] [Google Scholar]
- Paris L, Magni R, Zaidi F et al. Urine lipoarabinomannan glycan in HIV-negative patients with pulmonary tuberculosis correlates with disease severity. Sci Transl Med 2017;9:eaal2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CG, Takahara K, Umemoto E et al. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int Immunol 2001;13:1283–90. [DOI] [PubMed] [Google Scholar]
- Perley CC, Frahm M, Click EM et al. The human antibody response to the surface of Mycobacterium tuberculosis. PLoS One 2014;9:e98938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitarque S, Larrouy-Maumus G, Payre B et al. The immunomodulatory lipoglycans, lipoarabinomannan and lipomannan, are exposed at the mycobacterial cell surface. Tuberculosis 2008;88:560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powlesland AS, Ward EM, Sadhu SK et al. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem 2006;281:20440–9. [DOI] [PubMed] [Google Scholar]
- Puri RV, Reddy PV, Tyagi AK. Secreted acid phosphatase (SapM) of Mycobacterium tuberculosis is indispensable for arresting phagosomal maturation and growth of the pathogen in guinea pig tissues. PLoS One 2013;8:e70514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesniaux VJ, Nicolle DM, Torres D et al. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol 2004;172:4425–34. [DOI] [PubMed] [Google Scholar]
- Rajaram MV, Brooks MN, Morris JD et al. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol 2010;185:929–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram MV, Ni B, Morris JD et al. Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2 (MK2) and microRNA miR-125b. P Natl Acad Sci USA 2011;108:17408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N. Recent observations concerning structure and function relationships in the mycobacterial cell envelope: Elaboration of a model in terms of mycobacterial pathogenicity, virulence and drug-resistance. Res Microbiol 1991;142:464–76. [DOI] [PubMed] [Google Scholar]
- Saleh MT, Belisle JT. Secretion of an acid phosphatase (SapM) by Mycobacterium tuberculosis that is similar to eukaryotic acid phosphatases. J Bacteriol 2000;182:6850–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P, Biswas D, Sindhwani G et al. Application of lipoarabinomannan antigen in tuberculosis diagnostics: current evidence. Postgrad Med J 2014;90:155–63. [DOI] [PubMed] [Google Scholar]
- Sasindran SJ, Torrelles JB. Mycobacterium tuberculosis infection and inflammation: What is beneficial for the host and for the bacterium? Front Microbiol 2011;2:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Reiling N, Fessler C et al. Decreased pathology and prolonged survival of human DC-SIGN transgenic mice during mycobacterial infection. J Immunol 2008;180:6836–45. [DOI] [PubMed] [Google Scholar]
- Schlesinger LS, Azad AK, Torrelles JB et al. Determinants of phagocytosis, phagosome biogenesis and autophagy for Mycobacterium tuberculosis. In: Kaufmann SHE, Britton WJ (eds). Handbook of Tuberculosis. Immunology and Cell Biology. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co; KGaA, 2008, 1–22. [Google Scholar]
- Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol 1994;152:4070–9. [PubMed] [Google Scholar]
- Schlesinger LS, Kaufman TM, Iyer S et al. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol 1996;157:4568–75. [PubMed] [Google Scholar]
- Scordo J, Arcos J, HV K et al. Mycobacterium tuberculosis cell wall fragments released upon bacterial contact with the human lung mucosa alter the neutrophil response to infection. Front Immunol 2017;8:e307, eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, JB T, Chatterjee D. Lipoglycans of Mycobacterium tuberculosis: isolation, purification,and characterization. In: Parish TBrown CA. (eds). Mycobacteria Protocols, 2nd edn Totowa, NJ: Humana Press, 2008, 23–45. [DOI] [PubMed] [Google Scholar]
- Shukla S, Richardson ET, Athman JJ et al. Mycobacterium tuberculosis lipoprotein LprG binds lipoarabinomannan and determines its cell envelope localization to control phagolysosomal fusion. PLoS Pathog 2014;10:e1004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirard JC, Vignal C, Dessein R et al. Nod-like receptors: cytosolic watchdogs for immunity against pathogens. PLoS Pathog 2007;3:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithgall TE, Briggs SD, Schreiner S et al. Control of myeloid differentiation and survival by stats. Oncogene 2000;19:2612–8. [DOI] [PubMed] [Google Scholar]
- Stoop EJ, Mishra AK, Driessen NN et al. Mannan core branching of lipo(arabino)mannan is required for mycobacterial virulence in the context of innate immunity. Cell Microbiol 2013;15:2093–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierzko AS, Bartlomiejczyk MA, Brzostek A et al. Mycobacterial antigen 85 complex (Ag85) as a target for ficolins and mannose-binding lectin. Int J Med Microbiol 2016;306:212–21. [DOI] [PubMed] [Google Scholar]
- Tailleux L, Schwartz O, Herrmann JL et al. DC-SIGN Is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med 2003;197:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanne A, Ma B, Boudou F et al. A murine DC-SIGN homologue contributes to early host defense against Mycobacterium tuberculosis. J Exp Med 2009;206:2205–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma-Uszynski S, Stenger S, Takeuchi O et al. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 2001;291:1544–7. [DOI] [PubMed] [Google Scholar]
- Torrelles JB. Broadening our view about the role of Mycobacterium tuberculosis cell envelope components during infection: A battle for survival. In: Cardona PJ. (ed). Understanding Tuberculosis - Analyzing the Origin of Mycobacterium tuberculosis Pathogenicity. Rijeka, Croatia: Intech, 2012, 1–46. [Google Scholar]
- Torrelles JB, Azad AK, Henning LN et al. Role of C-type lectins in mycobacterial infections. Curr Drug Targets 2008;9:102–12. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol 2006;177:1805–16. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Desjardin LE, MacNeil J et al. Inactivation of Mycobacterium tuberculosis mannosyltransferase pimB reduces the cell wall lipoarabinomannan and lipomannan content and increases the rate of bacterial-induced human macrophage cell death. Glycobiology 2009;19:743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, Khoo KH, Sieling PA et al. Truncated structural variants of lipoarabinomannan in Mycobacterium leprae and an ethambutol-resistant strain of Mycobacterium tuberculosis. J Biol Chem 2004;279:41227–39. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Knaup R, Kolareth A et al. Identification of Mycobacterium tuberculosis clinical isolates with altered phagocytosis by human macrophages due to a truncated lipoarabinomannan. J Biol Chem 2008;283:31417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, Schlesinger LS. Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis 2010;90:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, Schlesinger LS. Integrating lung physiology, immunology, and tuberculosis. Trends Microbiol 2017;25:688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, Sieling PA, Arcos J et al. Structural differences in lipomannans from pathogenic and nonpathogenic mycobacteria that impact CD1b-restricted T cell responses. J Biol Chem 2011;286:35438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, Sieling PA, Zhang N et al. Isolation of a distinct Mycobacterium tuberculosis mannose-capped lipoarabinomannan isoform responsible for recognition by CD1b-restricted T cells. Glycobiology 2012;22:1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyonaga K, Torigoe S, Motomura Y et al. C-Type lectin receptor DCAR recognizes mycobacterial phosphatidyl-inositol mannosides to promote a Th1 response during infection. Immunity 2016;45:1245–57. [DOI] [PubMed] [Google Scholar]
- Treumann A, Xidong F, McDonnell L et al. 5-Methylthiopentose: a new substituent on lipoarabinomannan in Mycobacterium tuberculosis. J Mol Biol 2002;316:89–100. [DOI] [PubMed] [Google Scholar]
- Troegeler A, Lugo-Villarino G, Hansen S et al. Collectin CL-LK is a novel soluble pattern recognition receptor for Mycobacterium tuberculosis. PLoS One 2015;10:e0132692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull WB, Shimizu KH, Chatterjee D et al. Identification of the 5-methylthiopentosyl substituent in Mycobacterium tuberculosis Lipoarabinomannan. Angew Chem Int Ed 2004;43:3918–22. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Smith KD et al. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. P Natl Acad Sci USA 1999;96:14459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Golde LM. Synthesis of surfactant lipids in the adult lung. Annu Rev Physiol 1985;47:765–74. [DOI] [PubMed] [Google Scholar]
- Velayati AA, Farnia P, Ibrahim TA et al. Differences in cell wall thickness between resistant and nonresistant strains of Mycobacterium tuberculosis: using transmission electron microscopy. Chemotherapy 2009;55:303–7. [DOI] [PubMed] [Google Scholar]
- Velayati AA, Farnia P, Merza MA et al. New insight into extremely drug-resistant tuberculosis: Using atomic force microscopy. Eur Respir J 2010;36:1490–3. [DOI] [PubMed] [Google Scholar]
- Vergne I, Chua J, Deretic V. Tuberculosis Toxin Blocking phagosome maturation inhibits a novel Ca2+/Calmodulin-PI3K hVPS34 Cascade. J Exp Med 2003;198:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Chua J, Lee HH et al. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. P Natl Acad Sci USA 2005;102:4033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Fratti RA, Hill PJ et al. Mycobacterium tuberculosis phagosome maturation arrest: Mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol Biol Cell 2004;15:751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Botelho RJ, Rameh L et al. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J Cell Biol 2001;155:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve C, Gilleron M, Maridonneau-Parini I et al. Mycobacteria use their surface-exposed glycolipids to infect human macrophages through a receptor-dependent process. J Lipid Res 2005;46:475–83. [DOI] [PubMed] [Google Scholar]
- Wieland CW, Koppel EA, den DJ et al. Mice lacking SIGNR1 have stronger T helper 1 responses to Mycobacterium tuberculosis. Microbes Infect 2007;9:134–41. [DOI] [PubMed] [Google Scholar]
- Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol 2003;3:211–22. [DOI] [PubMed] [Google Scholar]
- Yonekawa A, Saijo S, Hoshino Y et al. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of Mycobacteria. Immunity 2014;41:402–13. [DOI] [PubMed] [Google Scholar]
- Zaffran Y, Zhang L, Ellner JJ. Role of CR4 in Mycobacterium tuberculosis-human macrophages binding and signal transduction in the absence of serum. Infect Immun 1998;66:4541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]